A Systematic Review of Extracellular Matrix-Related Alterations in Parkinson’s Disease
Abstract
1. Introduction
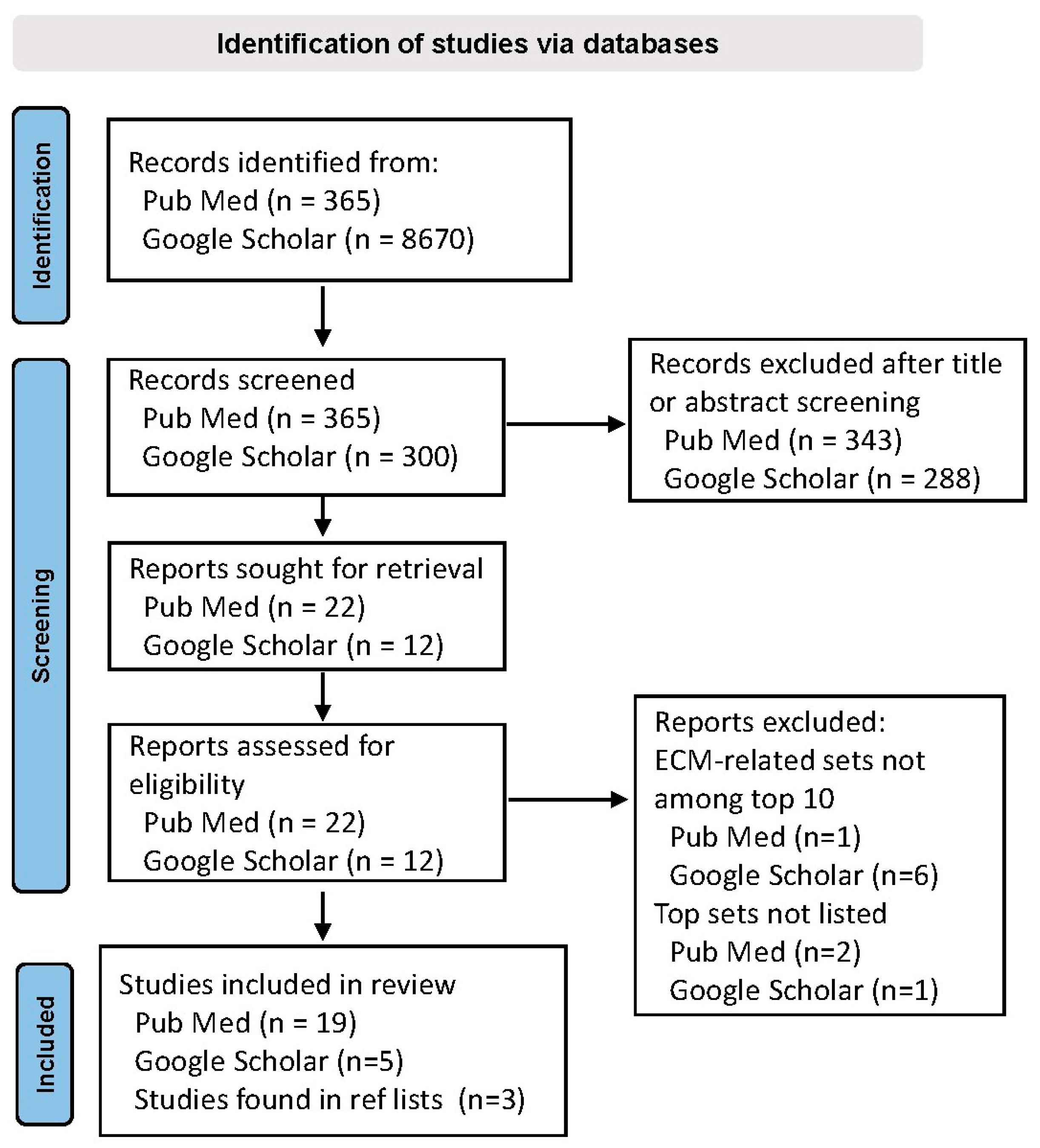
2. Materials and Methods
3. Results
3.1. Transcriptomics Studies
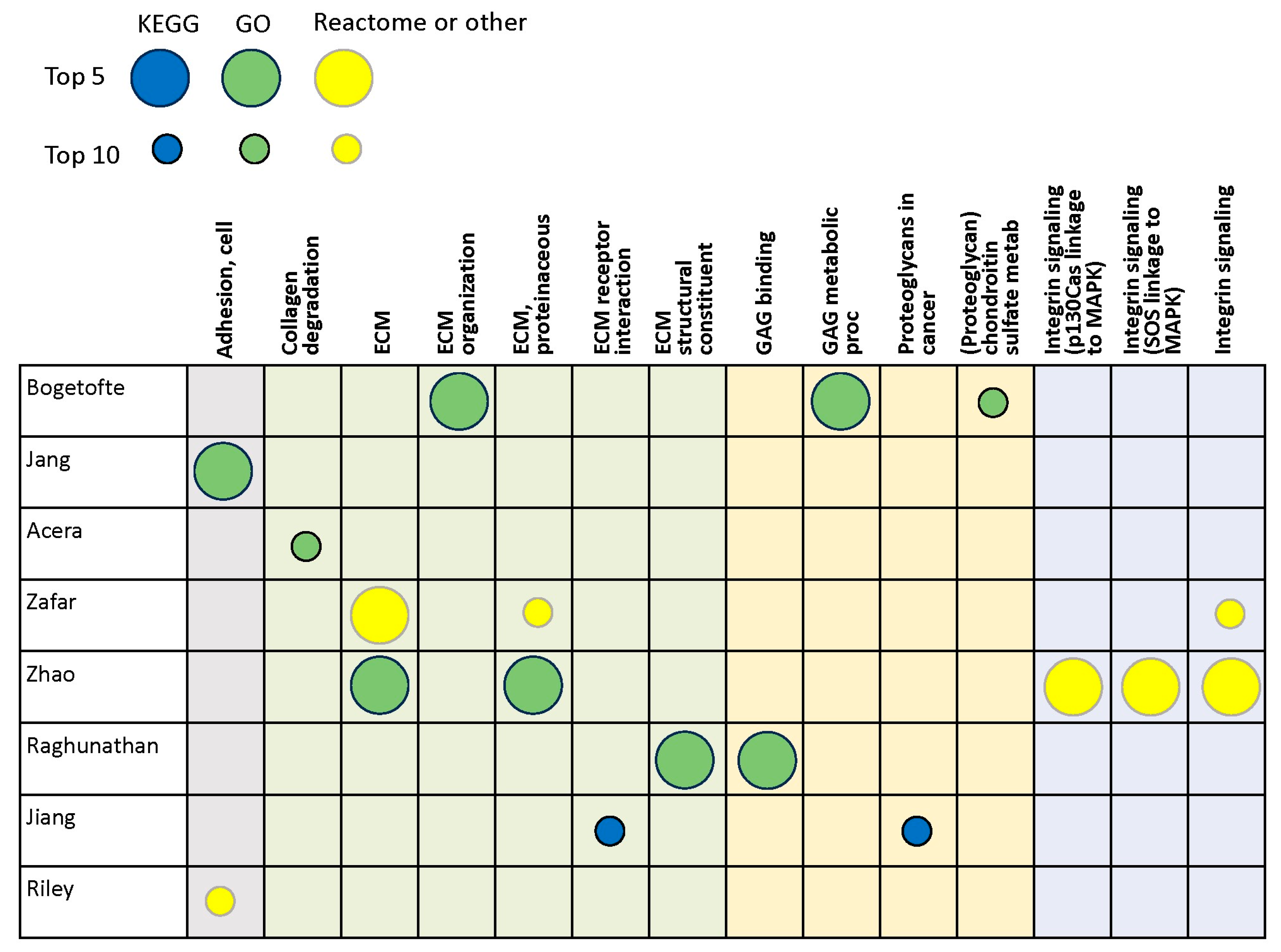
3.2. Proteomics Studies
3.3. Genomics Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Meredith, J.E., Jr.; Fazeli, B.; Schwartz, M.A. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 1993, 4, 953–961. [Google Scholar] [CrossRef]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Yayon, A.; Klagsbrun, M.; Esko, J.D.; Leder, P.; Ornitz, D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 1991, 64, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.W.; Fisher, C.E.; Perona-Wright, G.; Davies, J.A. Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J. Cell Sci. 2002, 115, 4495–4503. [Google Scholar] [CrossRef] [PubMed]
- Bespalov, M.M.; Sidorova, Y.A.; Tumova, S.; Ahonen-Bishopp, A.; Magalhães, A.C.; Kulesskiy, E.; Paveliev, M.; Rivera, C.; Rauvala, H.; Saarma, M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J. Cell Biol. 2011, 192, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Sorg, B.A.; Berretta, S.; Blacktop, J.M.; Fawcett, J.W.; Kitagawa, H.; Kwok, J.C.; Miquel, M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J. Neurosci. 2016, 36, 11459–11468. [Google Scholar] [CrossRef] [PubMed]
- Chaunsali, L.; Tewari, B.P.; Sontheimer, H. Perineuronal Net Dynamics in the Pathophysiology of Epilepsy. Epilepsy Curr. 2021, 21, 273–281. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Fyhn, M.; Jendelova, P.; Kwok, J.C.F.; Ruzicka, J.; Sorg, B.A. The extracellular matrix and perineuronal nets in memory. Mol. Psychiatry 2022, 27, 3192–3203. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Oohashi, T.; Pizzorusso, T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat. Rev. Neurosci. 2019, 20, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Pizzorusso, T.; Medini, P.; Berardi, N.; Chierzi, S.; Fawcett, J.W.; Maffei, L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 2002, 298, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cacquevel, M.; Saksida, L.M.; Bussey, T.J.; Schneider, B.L.; Aebischer, P.; Melani, R.; Pizzorusso, T.; Fawcett, J.W.; Spillantini, M.G. Perineuronal net digestion with chondroitinase restores memory in mice with tau pathology. Exp. Neurol. 2015, 265, 48–58. [Google Scholar] [CrossRef]
- Bruckner, G.; Morawski, M.; Arendt, T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience 2008, 151, 489–504. [Google Scholar] [CrossRef]
- Bekku, Y.; Oohashi, T. Neurocan contributes to the molecular heterogeneity of the perinodal ECM. Arch. Histol. Cytol. 2010, 73, 95–102. [Google Scholar] [CrossRef]
- Bonneh-Barkay, D.; Wiley, C.A. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009, 19, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santiago, R.; Esteve-Codina, A.; Fernández, M.; Valldeoriola, F.; Sanchez-Gómez, A.; Muñoz, E.; Compta, Y.; Tolosa, E.; Ezquerra, M.; Martí, M.J. Transcriptome analysis in LRRK2 and idiopathic Parkinson’s disease at different glucose levels. NPJ Parkinsons Dis. 2021, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, R.; Hogan, J.D.; Labadorf, A.; Myers, R.H.; Zaia, J. A glycomics and proteomics study of aging and Parkinson’s disease in human brain. Sci. Rep. 2020, 10, 12804. [Google Scholar] [CrossRef]
- Jung, S.Y.; Choi, J.M.; Rousseaux, M.W.C.; Malovannaya, A.; Kim, J.J.; Kutzera, J.; Wang, Y.; Huang, Y.; Zhu, W.; Maity, S.; et al. An Anatomically Resolved Mouse Brain Proteome Reveals Parkinson Disease-relevant Pathways. Mol. Cell Proteom. 2017, 16, 581–593. [Google Scholar] [CrossRef]
- Villadiego, J.; García-Swinburn, R.; García-González, D.; Lebrón-Galán, R.; Murcia-Belmonte, V.; García-Roldán, E.; Suárez-Luna, N.; Nombela, C.; Marchena, M.; de Castro, F.; et al. Extracellular matrix protein anosmin-1 overexpression alters dopaminergic phenotype in the CNS and the PNS with no pathogenic consequences in a MPTP model of Parkinson’s disease. Brain Struct. Funct. 2023, 228, 907–920. [Google Scholar] [CrossRef]
- Khan, A.H.; Lee, L.K.; Smith, D.J. Single-cell analysis of gene expression in the substantia nigra pars compacta of a pesticide-induced mouse model of Parkinson’s disease. Transl. Neurosci. 2022, 13, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Kim, Y.S.; Bok, E.; Yune, T.Y.; Maeng, S.; Jin, B.K. MMP-3 contributes to nigrostriatal dopaminergic neuronal loss, BBB damage, and neuroinflammation in an MPTP mouse model of Parkinson’s disease. Mediators Inflamm. 2013, 2013, 370526. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Joh, T.H. Matrix metalloproteinases, new insights into the understanding of neurodegenerative disorders. Biomol. Ther. 2012, 20, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Herrero, M.-T.; Di Pentima, M.; Gomez, A.; Lombardi, L.; Ros, C.M.; De Pablos, V.; Fernandez-Villalba, E.; De Stefano, M.E. Metalloproteinase-9 contributes to inflammatory glia activation and nigro-striatal pathway degeneration in both mouse and monkey models of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism. Brain Struct. Funct. 2015, 220, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, G.; Sehgal, A.; Bhardwaj, S.; Singh, S.; Buhas, C.; Judea-Pusta, C.; Uivarosan, D.; Munteanu, M.A.; Bungau, S. Multifaceted Role of Matrix Metalloproteinases in Neurodegenerative Diseases: Pathophysiological and Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 1413. [Google Scholar] [CrossRef] [PubMed]
- Rosh, I.; Tripathi, U.; Hussein, Y.; Rike, W.A.; Djamus, J.; Shklyar, B.; Manole, A.; Houlden, H.; Winkler, J.; Gage, F.H.; et al. Synaptic dysfunction and extracellular matrix dysregulation in dopaminergic neurons from sporadic and E326K-GBA1 Parkinson’s disease patients. NPJ Parkinsons Dis. 2024, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Akrioti, E.; Karamitros, T.; Gkaravelas, P.; Kouroupi, G.; Matsas, R.; Taoufik, E. Early Signs of Molecular Defects in iPSC-Derived Neural Stems Cells from Patients with Familial Parkinson’s Disease. Biomolecules 2022, 12, 876. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Lau, S.; Manole, A.; Rosh, I.; Percia, M.M.; Ben Ezer, R.; Shokhirev, M.N.; Qiu, F.; Schafer, S.; Mansour, A.A.; et al. Reduced synaptic activity and dysregulated extracellular matrix pathways in midbrain neurons from Parkinson’s disease patients. NPJ Parkinsons Dis. 2022, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, S.M.J.; Swart, P.; Womersely, J.S.; Ovenden, E.S.; van den Heuvel, L.L.; McGregor, N.W.; Meier, S.; Bardien, S.; Abrahams, S.; Tromp, G.; et al. RNA-seq analysis of gene expression profiles in posttraumatic stress disorder, Parkinson’s disease and schizophrenia identifies roles for common and distinct biological pathways. Discov. Ment. Health 2022, 2, 6. [Google Scholar] [CrossRef]
- Cho, E.; Park, J.; Kim, K.; Kim, M.G.; Cho, S.R. Reelin Alleviates Mesenchymal Stem Cell Senescence and Reduces Pathological alpha-Synuclein Expression in an In Vitro Model of Parkinson’s Disease. Genes 2021, 12, 1066. [Google Scholar] [CrossRef]
- Yang, M.; Wu, X.Q.; Ding, C.B.; Zhang, G.F.; Li, M.; Lv, L.N.; Li, Y.H.; Sun, D.W.; Zhao, J.J. Weighted gene co-expression network analysis identifies specific modules and hub genes related to Parkinson’s disease. Neuroreport 2021, 32, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.E.; Wessely, F.; Connor-Robson, N.; Rinaldi, F.; Vowles, J.; Browne, C.; Evetts, S.G.; Hu, M.T.; Cowley, S.A.; Webber, C.; et al. RNA sequencing reveals MMP2 and TGFB1 downregulation in LRRK2 G2019S Parkinson’s iPSC-derived astrocytes. Neurobiol. Dis. 2019, 129, 56–66. [Google Scholar] [CrossRef] [PubMed]
- González-Casacuberta, I.; Moren, C.; Juarez-Flores, D.L.; Esteve-Codina, A.; Sierra, C.; Catalan-Garcia, M.; Guitart-Mampel, M.; Tobias, E.; Milisenda, J.C.; Pont-Sunyer, C.; et al. Transcriptional alterations in skin fibroblasts from Parkinson’s disease patients with parkin mutations. Neurobiol. Aging 2018, 65, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Liu, X.; Chen, J. Microarray Analysis of the Molecular Mechanism Involved in Parkinson’s Disease. Parkinsons Dis. 2018, 2018, 1590465. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.; Prieto, C.; Sierra, M.; Sanchez-Juan, P.; Gonzalez-Aramburu, I.; Sanchez-Quintana, C.; Berciano, J.; Combarros, O.; Sainz, J. Comparative blood transcriptome analysis in idiopathic and LRRK2 G2019S-associated Parkinson’s disease. Neurobiol. Aging 2016, 38, 214.e1–214.e5. [Google Scholar] [CrossRef] [PubMed]
- Riley, B.E.; Gardai, S.J.; Emig-Agius, D.; Bessarabova, M.; Ivliev, A.E.; Schule, B.; Alexander, J.; Wallace, W.; Halliday, G.M.; Langston, J.W.; et al. Systems-based analyses of brain regions functionally impacted in Parkinson’s disease reveals underlying causal mechanisms. PLoS ONE 2014, 9, e102909. [Google Scholar] [CrossRef] [PubMed]
- Durrenberger, P.F.; Grunblatt, E.; Fernando, F.S.; Monoranu, C.M.; Evans, J.; Riederer, P.; Reynolds, R.; Dexter, D.T. Inflammatory Pathways in Parkinson’s Disease; A BNE Microarray Study. Parkinsons Dis. 2012, 2012, 214714. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, C.; Lin, Q.; Huang, J. Identification of key pathways and transcription factors related to Parkinson disease in genome wide. Mol. Biol. Rep. 2012, 39, 10881–10887. [Google Scholar] [CrossRef] [PubMed]
- Edwards, Y.J.; Beecham, G.W.; Scott, W.K.; Khuri, S.; Bademci, G.; Tekin, D.; Martin, E.R.; Jiang, Z.; Mash, D.C.; Ffrench-Mullen, J.; et al. Identifying consensus disease pathways in Parkinson’s disease using an integrative systems biology approach. PLoS ONE 2011, 6, e16917. [Google Scholar] [CrossRef]
- Grunblatt, E.; Mandel, S.; Jacob-Hirsch, J.; Zeligson, S.; Amariglo, N.; Rechavi, G.; Li, J.; Ravid, R.; Roggendorf, W.; Riederer, P.; et al. Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome, heat shock protein, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicle trafficking genes. J. Neural Transm. 2004, 111, 1543–1573. [Google Scholar] [CrossRef]
- Mandel, S.; Grunblatt, E.; Riederer, P.; Amariglio, N.; Jacob-Hirsch, J.; Rechavi, G.; Youdim, M.B. Gene expression profiling of sporadic Parkinson’s disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Ann. N. Y. Acad. Sci. 2005, 1053, 356–375. [Google Scholar] [PubMed]
- Bogetofte, H.; Ryan, B.J.; Jensen, P.; Schmidt, S.I.; Vergoossen, D.L.E.; Barnkob, M.B.; Kiani, L.N.; Chughtai, U.; Heon-Roberts, R.; Caiazza, M.C.; et al. Post-translational proteomics platform identifies neurite outgrowth impairments in Parkinson’s disease GBA-N370S dopamine neurons. Cell Rep. 2023, 42, 112180. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Pletnikova, O.; Troncoso, J.C.; Pantelyat, A.Y.; Dawson, T.M.; Rosenthal, L.S.; Na, C.H. Mass Spectrometry-Based Proteomics Analysis of Human Substantia Nigra From Parkinson’s Disease Patients Identifies Multiple Pathways Potentially Involved in the Disease. Mol. Cell Proteom. 2023, 22, 100452. [Google Scholar]
- Acera, A.; Gomez-Esteban, J.C.; Murueta-Goyena, A.; Galdos, M.; Azkargorta, M.; Elortza, F.; Ruzafa, N.; Ibarrondo, O.; Pereiro, X.; Vecino, E. Potential Tear Biomarkers for the Diagnosis of Parkinson’s Disease-A Pilot Study. Proteomes 2022, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Noor, A.; Younas, N.; Shafiq, M.; Schmitz, M.; Wurster, I.; Brockmann, K.; Gasser, T.; Zerr, I. SWATH Mass Spectrometry-Based CSF Proteome Profile of GBA-Linked Parkinson’s Disease Patients. Int. J. Mol. Sci. 2022, 23, 14166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Zhang, J.; Yang, G. Plasma proteome profiling using tandem mass tag labeling technology reveals potential biomarkers for Parkinson’s disease: A preliminary study. Proteom. Clin. Appl. 2022, 16, e2100010. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Rong, C.; Ke, R.; Meng, S.; Yan, X.; Ke, H.; Wu, S. Differential proteomic analysis of serum exosomes reveals alterations in progression of Parkinson disease. Medicine 2019, 98, e17478. [Google Scholar] [CrossRef] [PubMed]
- Sandor, C.; Honti, F.; Haerty, W.; Szewczyk-Krolikowski, K.; Tomlinson, P.; Evetts, S.; Millin, S.; Keane, T.; McCarthy, S.A.; Durbin, R.; et al. Whole-exome sequencing of 228 patients with sporadic Parkinson’s disease. Sci. Rep. 2017, 7, 41188. [Google Scholar] [CrossRef]
- Hu, Y.; Deng, L.; Zhang, J.; Fang, X.; Mei, P.; Cao, X.; Lin, J.; Wei, Y.; Zhang, X.; Xu, R. A Pooling Genome-Wide Association Study Combining a Pathway Analysis for Typical Sporadic Parkinson’s Disease in the Han Population of Chinese Mainland. Mol. Neurobiol. 2016, 53, 4302–4318. [Google Scholar] [CrossRef]
- O’Dushlaine, C.; Kenny, E.; Heron, E.A.; Segurado, R.; Gill, M.; Morris, D.W.; Corvin, A. The SNP ratio test: Pathway analysis of genome-wide association datasets. Bioinformatics 2009, 25, 2762–2763. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Jellinger, K.A. Synuclein deposition and non-motor symptoms in Parkinson disease. J. Neurol. Sci. 2011, 310, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.; Lim, S.; Lee, S.; Kim, S. Biomedical knowledge graph learning for drug repurposing by extending guilt-by-association to multiple layers. Nat. Commun. 2023, 14, 3570. [Google Scholar] [CrossRef]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.N.; Chew, E.G.Y.; Chung, S.J.; Peng, R.; Blauwendraat, C.; Nalls, M.A.; Mok, K.Y.; Satake, W.; Toda, T.; Chao, Y.; et al. Identification of Risk Loci for Parkinson Disease in Asians and Comparison of Risk Between Asians and Europeans: A Genome-Wide Association Study. JAMA Neurol. 2020, 77, 746–754. [Google Scholar] [CrossRef]
- Chang, D.; Nalls, M.A.; Hallgrimsdottir, I.B.; Hunkapiller, J.; van der Brug, M.; Cai, F.; International Parkinson’s Disease Genomics Consortium; 23andMe Research Team; Kerchner, G.A.; Ayalon, G.; et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 2017, 49, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.S.; Sawalha, A.H. Glycoprotein nonmetastatic melanoma protein B: A key mediator and an emerging therapeutic target in autoimmune diseases. FASEB J. 2020, 34, 8810–8823. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, U.; Rosh, I.; Ben Ezer, R.; Nayak, R.; Chowdhary, A.; Djamus, J.; Manole, A.; Haulden, H.; Gage, F.H.; Stern, S. Upregulated extracellular matrix-related genes and impaired synaptic activity in dopaminergic and hippocampal neurons derived from Parkinson’s disease patients with PINK1 and PARK2 mutations. bioRxiv 2022. [Google Scholar] [CrossRef]
- Rosh, I.; Tripathi, U.; Hussein, Y.; Rike, W.A.; Manole, A.; Cordeiro, D.; Houlden, H.; Winkler, J.; Gage, F.; Stern, S. Synaptic dysfunction and dysregulation of extracellular matrix-related genes in dopaminergic neurons derived from Parkinson’s disease sporadic patients and with GBA1 mutations. bioRxiv 2023. [Google Scholar] [CrossRef]
- Rike, W.A.; Stern, S. Proteins and Transcriptional Dysregulation of the Brain Extracellular Matrix in Parkinson’s Disease: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7435. [Google Scholar] [CrossRef]
- Teves, J.M.Y.; Bhargava, V.; Kirwan, K.R.; Corenblum, M.J.; Justiniano, R.; Wondrak, G.T.; Anandhan, A.; Flores, A.J.; Schipper, D.A.; Khalpey, Z.; et al. Parkinson’s Disease Skin Fibroblasts Display Signature Alterations in Growth, Redox Homeostasis, Mitochondrial Function, and Autophagy. Front. Neurosci. 2017, 11, 737. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Browser. Extracelullar Matrix Organization. Available online: https://www.informatics.jax.org/vocab/gene_ontology/GO:0030198# (accessed on 15 October 2023).
- Liu, C.Z.; Guo, D.S.; Ma, J.J.; Dong, L.R.; Chang, Q.Q.; Yang, H.Q.; Liang, K.K.; Li, X.H.; Yang, D.W.; Fan, Y.Y.; et al. Correlation of matrix metalloproteinase 3 and matrix metalloproteinase 9 levels with non-motor symptoms in patients with Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 889257. [Google Scholar] [CrossRef] [PubMed]
- Downs, M.; Sethi, M.K.; Raghunathan, R.; Layne, M.D.; Zaia, J. Matrisome changes in Parkinson’s disease. Anal. Bioanal. Chem. 2022, 414, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.; Aroso, M.; Rocha, S.; Ferreira, R.; Vitorino, R.; Gomez-Lazaro, M. Bioinformatic analysis of the human brain extracellular matrix proteome in neurodegenerative disorders. Eur. J. Neurosci. 2021, 53, 4016–4033. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Singh, M.K.; Garg, R.K.; Pant, K.K.; Khattri, S. Evaluation of peripheral matrix metalloproteinase-1 in Parkinson’s disease: A case-control study. Int. J. Neurosci. 2014, 124, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; De Jong, G.I.; de Vos, R.A.; Jansen Steur, E.N.; Luiten, P.G. Pathological features of cerebral cortical capillaries are doubled in Alzheimer’s disease and Parkinson’s disease. Acta Neuropathol. 2000, 100, 395–402. [Google Scholar] [CrossRef]
- Downs, M.; Zaia, J.; Sethi, M.K. Mass spectrometry methods for analysis of extracellular matrix components in neurological diseases. Mass. Spectrom. Rev. 2023, 42, 1848–1875. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.; Pandini, C.; Messa, L.; Launi, R.; Barzaghini, B.; Zangaglia, R.; Raimondi, M.T.; Gagliardi, S.; Cereda, C.; Zuccotti, G.V.; et al. alpha-Synuclein antisense transcript SNCA-AS1 regulates synapses- and aging-related genes suggesting its implication in Parkinson’s disease. Aging Cell 2021, 20, e13504. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, D.; Yuan, Y.; Chen, W. Gene Set Enrichment Analysis and Genetic Experiment Reveal Changes in Cell Signaling Pathways Induced by alpha-Synuclein Overexpression. Biomedicines 2023, 11, 263. [Google Scholar] [CrossRef]
- Mehra, S.; Sahay, S.; Maji, S.K. alpha-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef]
- Riederer, P.; Berg, D.; Casadei, N.; Cheng, F.; Classen, J.; Dresel, C.; Jost, W.; Krüger, R.; Müller, T.; Reichmann, H.; et al. alpha-Synuclein in Parkinson’s disease: Causal or bystander? J. Neural Transm. 2019, 126, 815–840. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Chalapathi, A.V.; Balagurunathan, K. Investigating the Roles of Heparan Sulfate Structures in Alpha-Synuclein Aggregation in Cell Culture Models. Methods Mol. Biol. 2022, 2303, 807–820. [Google Scholar] [PubMed]
- Mehra, S.; Ghoshn, D.; Kumar, R.; Mondal, M.; Gadhe, L.G.; Das, S.; Anoop, A.; Jha, N.N.; Jacob, R.S.; Chatterjee, D.; et al. Glycosaminoglycans have variable effects on alpha-synuclein aggregation and differentially affect the activities of the resulting amyloid fibrils. J. Biol. Chem. 2018, 293, 12975–12991. [Google Scholar] [CrossRef] [PubMed]
- Maiza, A.; Chantepie, S.; Vera, C.; Fifre, A.; Huynh, M.B.; Stettler, O.; Ouidja, M.O.; Papy-Garcia, D. The role of heparan sulfates in protein aggregation and their potential impact on neurodegeneration. FEBS Lett. 2018, 592, 3806–3818. [Google Scholar] [CrossRef] [PubMed]
- Ihse, E.; Yamakado, H.; van Wijk, X.M.; Lawrence, R.; Esko, J.D.; Masliah, E. Cellular internalization of alpha-synuclein aggregates by cell surface heparan sulfate depends on aggregate conformation and cell type. Sci. Rep. 2017, 7, 9008. [Google Scholar] [CrossRef] [PubMed]
- Adulla, A.; Patel, U.; Ashok, A.; Katiyar, P.; Kaulakis, M.; Kritikos, A.E.; Pillai, S.; Lee, H.; Lindner, E.; Rhee, D.J.; et al. Alpha-Synuclein modulates fibronectin expression in the trabecular meshwork independent of TGFbeta2. Exp. Eye Res. 2023, 226, 109351. [Google Scholar] [CrossRef] [PubMed]
- Paiva, I.; Jain, G.; Lazaro, D.F.; Jercic, K.G.; Hentrich, T.; Kerimoglu, C.; Pinho, R.; Szegő, M.; Burkhardt, S.; Capece, V.; et al. Alpha-synuclein deregulates the expression of COL4A2 and impairs ER-Golgi function. Neurobiol. Dis. 2018, 119, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Cohlberg, J.A.; Li, J.; Uversky, V.N.; Fink, A.L. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from alpha-synuclein in vitro. Biochemistry 2002, 41, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Gao, J.; Hou, L.; Wang, L.; Zhang, F.; Sun, F.; Zhang, T.; Xu, P.; Shi, Z.; Hu, F.; et al. Neuroprotective effect of chondroitin sulfate on SH-SY5Y cells overexpressing wild-type or A53T mutant alpha-synuclein. Mol. Med. Rep. 2017, 16, 8721–8728. [Google Scholar] [CrossRef]
- Tsuboi, K.; Grzesiak, J.J.; Bouvet, M.; Hashimoto, M.; Masliah, E.; Shults, C.W. Alpha-synuclein overexpression in oligodendrocytic cells results in impaired adhesion to fibronectin and cell death. Mol. Cell Neurosci. 2005, 29, 259–268. [Google Scholar] [CrossRef]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef]
- Yamada, K.; Iwatsubo, T. Extracellular alpha-synuclein levels are regulated by neuronal activity. Mol. Neurodegener. 2018, 13, 9. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Romero-Ramos, M. Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front. Cell Neurosci. 2018, 12, 247. [Google Scholar] [CrossRef]
- Surguchev, A.A.; Surguchov, A. Integrins-A missing link in synuclein’s pathogenic mechanism. J. Neurosci. Res. 2019, 97, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Bao, X.; Zang, C.; Yang, H.; Sun, F.; Che, Y.; Wu, X.; Li, S.; Zhang, D.; Wang, Q. Integrin CD11b mediates alpha-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2018, 14, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Englund, E.; Holton, J.L.; Soulet, D.; Hagell, P.; Lees, A.J.; Lashley, T.; Quinn, N.P.; Rehncrona, S.; Björklund, A.; et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008, 14, 501–503. [Google Scholar] [CrossRef]
- DeWitt, D.A.; Richey, P.L.; Praprotnik, D.; Silver, J.; Perry, G. Chondroitin sulfate proteoglycans are a common component of neuronal inclusions and astrocytic reaction in neurodegenerative diseases. Brain Res. 1994, 656, 205–209. [Google Scholar] [CrossRef]
- Lehri-Boufala, S.; Ouidja, M.O.; Barbier-Chassefiere, V.; Henault, E.; Raisman-Vozari, R.; Garrigue-Antar, L.; Papy-Garcia, D.; Morin, C. New roles of glycosaminoglycans in alpha-synuclein aggregation in a cellular model of Parkinson disease. PLoS ONE 2015, 10, e0116641. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Sun, Y.; Lv, S.; Xia, W.; Zhao, K.; Xu, Q.; Zhao, Q.; He, L.; Le, W.; Wang, Y.; et al. Heparin induces alpha-synuclein to form new fibril polymorphs with attenuated neuropathology. Nat. Commun. 2022, 13, 4226. [Google Scholar] [CrossRef]
- Nikolaus, S.; Antke, C.; Muller, H.W. In vivo imaging of synaptic function in the central nervous system: I. Movement disorders and dementia. Behav. Brain Res. 2009, 204, 1–31. [Google Scholar] [CrossRef]
- Sousa, V.L.; Bellani, S.; Giannandrea, M.; Yousuf, M.; Valtorta, F.; Meldolesi, J.; Chieregatti, E. {alpha}-synuclein and its A30P mutant affect actin cytoskeletal structure and dynamics. Mol. Biol. Cell 2009, 20, 3725–3739. [Google Scholar] [CrossRef] [PubMed]
- Dankovich, T.M.; Rizzoli, S.O. The Synaptic Extracellular Matrix: Long-Lived, Stable, and Still Remarkably Dynamic. Front. Synaptic Neurosci. 2022, 14, 854956. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, N.; Klingenberg, S.; Schweiger, S.; Gerber, S. Common Factors in Neurodegeneration: A Meta-Study Revealing Shared Patterns on a Multi-Omics Scale. Cells 2020, 9, 2642. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nie, Y.; Yu, J. An Effective Method to Identify Shared Pathways and Common Factors among Neurodegenerative Diseases. PLoS ONE 2015, 10, e0143045. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sakaguchi, M.; Sabit, H.; Tamai, S.; Ichinose, T.; Tanaka, S.; Kinoshita, M.; Uchida, Y.; Ohtsuki, S.; Nakada, M. COL1A2 inhibition suppresses glioblastoma cell proliferation and invasion. J. Neurosurg. 2023, 138, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.; Keon, M.; Liu, B.; Su, Z.; Saksena, N.K. Panoramic Visualization of Circulating MicroRNAs Across Neurodegenerative Diseases in Humans. Mol. Neurobiol. 2019, 56, 7380–7407. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C. Biochemistry of Parkinson’s disease with special reference to the dopaminergic systems. Mol. Neurobiol. 1994, 9, 135–142. [Google Scholar] [CrossRef]
- Aguila, J.; Cheng, S.; Kee, N.; Cao, M.; Wang, M.; Deng, Q.; Hedlund, E. Spatial RNA Sequencing Identifies Robust Markers of Vulnerable and Resistant Human Midbrain Dopamine Neurons and Their Expression in Parkinson’s Disease. Front. Mol. Neurosci. 2021, 14, 699562. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Thalhammer, A.; Cingolani, L.A. Cell adhesion and homeostatic synaptic plasticity. Neuropharmacology 2014, 78, 23–30. [Google Scholar] [CrossRef]
- Dityatev, A.; Seidenbecher, C.I.; Schachner, M. Compartmentalization from the outside: The extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010, 33, 503–512. [Google Scholar] [CrossRef]
- Soles, A.; Selimovic, A.; Sbrocco, K.; Ghannoum, F.; Hamel, K.; Moncada, E.L.; Gilliat, S.; Cvetanovic, M. Extracellular Matrix Regulation in Physiology and in Brain Disease. Int. J. Mol. Sci. 2023, 24, 7049. [Google Scholar] [CrossRef]
- Schulz-Schaeffer, W.J. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010, 120, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Plowey, E.D.; Chu, C.T. Synaptic dysfunction in genetic models of Parkinson’s disease: A role for autophagy? Neurobiol. Dis. 2011, 43, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ng, X.Y.; Cao, M. Dysfunction of synaptic endocytic trafficking in Parkinson’s disease. Neural Regen. Res. 2024, 19, 2649–2660. [Google Scholar] [CrossRef] [PubMed]
- Dityatev, A.; Schachner, M.; Sonderegger, P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 2010, 11, 735–746. [Google Scholar] [CrossRef]
- Mehdi, S.J.; Rosas-Hernandez, H.; Cuevas, E.; Lantz, S.M.; Barger, S.W.; Sarkar, S.; Paule, M.G.; Ali, S.F.; Imam, S.Z. Protein Kinases and Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 1585. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, K. LRRK2-targeted Parkinson disease drug advances into phase III. Nat. Rev. Drug Discov. 2023, 22, 3–5. [Google Scholar] [CrossRef]
- Chapman, M.A. Interactions between cell adhesion and the synaptic vesicle cycle in Parkinson’s disease. Med. Hypotheses 2014, 83, 203–207. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, L.; Liu, H.; Yang, J.; Yu, F.; Cui, C.; Huang, D. A Diagnostic Model for Parkinson’s Disease Based on Anoikis-Related Genes. Mol. Neurobiol. 2024, 61, 3641–3656. [Google Scholar] [CrossRef]
- Pantaleo, E.; Monaco, A.; Amoroso, N.; Lombardi, A.; Bellantuono, L.; Urso, D.; Giudice, C.L.; Picardi, E.; Tafuri, B.; Nigro, S.; et al. A Machine Learning Approach to Parkinson’s Disease Blood Transcriptomics. Genes 2022, 13, 727. [Google Scholar] [CrossRef]
- Uehara, Y.; Ueno, S.I.; Amano-Takeshige, H.; Suzuki, S.; Imamichi, Y.; Fujimaki, M.; Ota, N.; Murase, T.; Inoue, T.; Saiki, S.; et al. Non-invasive diagnostic tool for Parkinson’s disease by sebum RNA profile with machine learning. Sci. Rep. 2021, 11, 18550. [Google Scholar] [CrossRef]
- Odumpatta, R.; Arumugam, M. Integrative Analysis of Gene Expression and Regulatory Network Interaction Data Reveals the Protein Kinase C Family of Serine/Threonine Receptors as a Significant Druggable Target for Parkinson’s Disease. J. Mol. Neurosci. 2021, 71, 466–480. [Google Scholar] [CrossRef]
- Hu, L.; Dong, M.X.; Huang, Y.L.; Lu, C.Q.; Qian, Q.; Zhang, C.C.; Xu, X.M.; Liu, Y.; Chen, G.H.; Wei, Y.D. Integrated Metabolomics and Proteomics Analysis Reveals Plasma Lipid Metabolic Disturbance in Patients With Parkinson’s Disease. Front. Mol. Neurosci. 2020, 13, 80. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, S.; Li, J.; Xiao, J.; Li, X.; Yang, J.; Lu, D.; Wang, Y. Comprehensive analysis of core genes and key pathways in Parkinson’s disease. Am. J. Transl. Res. 2020, 12, 5630–5639. [Google Scholar]
- Moni, M.A.; Rana, H.K.; Islam, M.B.; Ahmed, M.B.; Xu, H.; Hasan, M.A.M.; Lei, Y.; Quinn, J.M. A computational approach to identify blood cell-expressed Parkinson’s disease biomarkers that are coordinately expressed in brain tissue. Comput. Biol. Med. 2019, 113, 103385. [Google Scholar] [CrossRef]
- Dong, N.; Zhang, X.; Liu, Q. Identification of therapeutic targets for Parkinson’s disease via bioinformatics analysis. Mol. Med. Rep. 2017, 15, 731–735. [Google Scholar] [CrossRef]
- Rezaei-Tavirani, M.; Zamanian-Azodi, M.; Rajabi, S.; Masoudi-Nejad, A.; Rostami-Nejad, M.; Rahmatirad, S. Protein Clustering and Interactome Analysis in Parkinson and Alzheimer’s Diseases. Arch. Iran. Med. 2016, 19, 101–109. [Google Scholar]
- Dumitriu, A.; Golji, J.; Labadorf, A.T.; Gao, B.; Beach, T.G.; Myers, R.H.; Longo, K.A.; Latourelle, J.C. Integrative analyses of proteomics and RNA transcriptomics implicate mitochondrial processes, protein folding pathways and GWAS loci in Parkinson disease. BMC Med. Genom. 2016, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, R.; Banica, M.; Roncaglia, P.; Robotti, E.; Finaurini, S.; Vlachouli, C.; Antonutti, L.; Iorio, F.; Carissimo, A.; Cattaruzza, T.; et al. Blood transcriptomics of drug-naive sporadic Parkinson’s disease patients. BMC Genom. 2015, 16, 876. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Chen, X.; Dai, D.; Zou, C.; Wu, X.; Chen, J. Bioinformatic analysis of microRNA expression in Parkinson’s disease. Mol. Med. Rep. 2015, 11, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Licker, V.; Cote, M.; Lobrinus, J.A.; Rodrigo, N.; Kovari, E.; Hochstrasser, D.F.; Turck, N.; Sanchez, J.C.; Burkhard, P.R. Proteomic profiling of the substantia nigra demonstrates CNDP2 overexpression in Parkinson’s disease. J. Proteom. 2012, 75, 4656–4667. [Google Scholar] [CrossRef] [PubMed]
- Botta-Orfila, T.; Sanchez-Pla, A.; Fernandez, M.; Carmona, F.; Ezquerra, M.; Tolosa, E. Brain transcriptomic profiling in idiopathic and LRRK2-associated Parkinson’s disease. Brain Res. 2012, 1466, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Jin, J.; Wang, Y.; Beyer, R.P.; Kitsou, E.; Albin, R.L.; Gearing, M.; Pan, C.; Zhang, J. Mortalin: A protein associated with progression of Parkinson disease? J. Neuropathol. Exp. Neurol. 2008, 67, 117–124. [Google Scholar] [CrossRef]
- Ferraro, F.; Fevga, C.; Bonifati, V.; Mandemakers, W.; Mahfouz, A.; Reinders, M. Correcting Differential Gene Expression Analysis for Cyto-Architectural Alterations in Substantia Nigra of Parkinson’s Disease Patients Reveals Known and Potential Novel Disease-Associated Genes and Pathways. Cells 2022, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Kurvits, L.; Lattekivi, F.; Reimann, E.; Kadastik-Eerme, L.; Kasterpalu, K.M.; Koks, S.; Taba, P.; Planken, A. Transcriptomic profiles in Parkinson’s disease. Exp. Biol. Med. 2021, 246, 584–595. [Google Scholar] [CrossRef]
- Licker, V.; Turck, N.; Kovari, E.; Burkhardt, K.; Cote, M.; Surini-Demiri, M.; Lobrinus, J.A.; Sanchez, J.C.; Burkhard, P.R. Proteomic analysis of human substantia nigra identifies novel candidates involved in Parkinson’s disease pathogenesis. Proteomics 2014, 14, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cerdan, A.; Andreu, Z.; Hidalgo, M.R.; Grillo-Risco, R.; Catala-Senent, J.F.; Soler-Saez, I.; Neva-Alejo, A.; Gordillo, F.; de la Iglesia-Vayá, M.; García-García, F. Unveiling sex-based differences in Parkinson’s disease: A comprehensive meta-analysis of transcriptomic studies. Biol. Sex. Differ. 2022, 13, 68. [Google Scholar] [CrossRef]
- Riboldi, G.M.; Vialle, R.A.; Navarro, E.; Udine, E.; de Paiva Lopes, K.; Humphrey, J.; Allan, A.; Parks, M.; Henderson, B.; Astudillo, K.; et al. Transcriptome deregulation of peripheral monocytes and whole blood in GBA-related Parkinson’s disease. Mol. Neurodegener. 2022, 17, 52. [Google Scholar] [CrossRef]


| Authors, Year | Tissue | Method |
|---|---|---|
| Transcriptomics Studies | ||
| Rosh et al., 2024 [26] | iPSC-derived DA neurons | RNA sequencing |
| Akrioti et al., 2022 [27] | iPSC-derived neural progenitor cells | RNA sequencing |
| Stern et al., 2022 [28] | iPSC-derived DA neurons | RNA sequencing |
| Hemmings et al., 2022 [29] | Blood | RNA sequencing |
| Cho et al., 2021 [30] | MSCs from adipose tissue | RNA sequencing |
| Fernandez-Santiago et al., 2021 [17] | Dermal fibroblasts | RNA sequencing |
| Yang et al., 2021 [31] | Blood | RNA sequencing |
| Booth et al., 2019 [32] | iPSC-derived, midbrain-patterned astrocytes generated from skin | RNA sequencing |
| Gonzalez-Cascuberta et al., 2018 [33] | Dermal fibroblasts | RNA sequencing |
| Tan et al., 2018 [34] | Blood | Microarray |
| Infante et al., 2016 [35] | Whole blood | RNA sequencing |
| Riley et al., 2014 [36] | Substantia nigra, striatum, cortex | Microarray |
| Durrenberger et al., 2012 [37] | Substantia nigra | Microarray |
| Zhang et al., 2012 [38] | Substantia nigra | Microarray |
| Edwards et al., 2011 [39] | Dorsal motor nucleus of vagus, locus coeruleus, substantia nigra, putamen, insula | Microarray |
| Grunblatt et al., 2004 [40]; Mandel et al., 2005 [41] | Substantia nigra, pars compacta | Microarray |
| Proteomics studies | ||
| Bogetofte et al., 2023 [42] | iPSC-derived DA neurons | LC/MS |
| Jang et al., 2023 [43] | Substantia nigra | LC/MS |
| Acera et al., 2022 [44] | Tears | LC/MS |
| Zafar et al., 2022 [45] | Cerebrospinal fluid | LC/MS |
| Zhao et al., 2022 [46] | Plasma | LC/MS |
| Raghunathan et al., 2020 [18] | Prefrontal cortex | LC/MS |
| Jiang et al., 2019 [47] | Serum exosomes | LC/MS |
| Riley et al., 2014 [36] | Striatum, cortex | LC/MS |
| Genomics studies | ||
| Sandor et al., 2017 [48] | Not reported | Whole exome sequencing |
| Hu et al., 2016 [49] | White blood cells | GWAS |
| Edwards et al., 2011 [39] | Not reported | GWAS |
| O’Dushlaine et al., 2009 [50] | Not reported | GWAS/SNP ratio test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapman, M.A.; Sorg, B.A. A Systematic Review of Extracellular Matrix-Related Alterations in Parkinson’s Disease. Brain Sci. 2024, 14, 522. https://doi.org/10.3390/brainsci14060522
Chapman MA, Sorg BA. A Systematic Review of Extracellular Matrix-Related Alterations in Parkinson’s Disease. Brain Sciences. 2024; 14(6):522. https://doi.org/10.3390/brainsci14060522
Chicago/Turabian StyleChapman, Mary Ann, and Barbara A. Sorg. 2024. "A Systematic Review of Extracellular Matrix-Related Alterations in Parkinson’s Disease" Brain Sciences 14, no. 6: 522. https://doi.org/10.3390/brainsci14060522
APA StyleChapman, M. A., & Sorg, B. A. (2024). A Systematic Review of Extracellular Matrix-Related Alterations in Parkinson’s Disease. Brain Sciences, 14(6), 522. https://doi.org/10.3390/brainsci14060522






