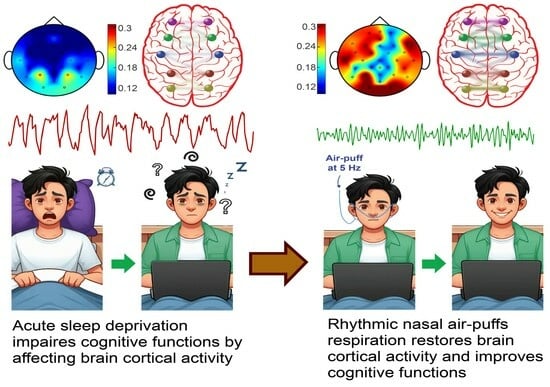
Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Questionnaires
2.3.1. Pittsburgh Sleep Quality Questionnaire
2.3.2. Cognitive Disability Questionnaire
2.4. Applying Nasal Air-Puffs
2.5. Numerical Stroop Test (NST)
2.6. EEG Recording and Pre-Processing
2.7. Signal Processing
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Cognitive Performance in NST
3.3. EEG Power
3.4. Signals Complexity
3.5. Intra-DMN Functional Connectivity
3.5.1. Cross-Correlation
3.5.2. Coherence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrara, M.; de Gennaro, L. How much sleep do we need? Sleep Med. Rev. 2001, 5, 155–179. [Google Scholar] [CrossRef]
- Sejnowski, T.J.; Destexhe, A. Why do we sleep? Brain Res. 2000, 886, 208–223. [Google Scholar] [CrossRef]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- Landolt, H.-P.; Holst, S.C.; Sousek, A.; Bassetti, C.; Dogas, Z.; Peigneux, P. Effects of Acute and Chronic Sleep Deprivation; European Sleep Research Society: Regensburg, Germany, 2014; ISBN 9781119038931. [Google Scholar]
- Sanches, I.; Teixeira, F.; dos Santos, J.M.; Ferreira, A.J. Effects of Acute Sleep Deprivation Resulting from Night Shift Work on Young Doctors. Acta Med. Port. 2015, 28, 457–462. [Google Scholar] [CrossRef]
- Orzeł-Gryglewska, J. Consequences of sleep deprivation. Int. J. Occup. Med. Environ. Health 2010, 23, 95–114. [Google Scholar] [CrossRef]
- Corsi-Cabrera, M.; Ramos, J.; Arce, C.; Guevara, M.A.; Ponce-de León, M.; Lorenzo, I. Changes in the waking EEG as a consequence of sleep and sleep deprivation. Sleep 1992, 15, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Duan, W.; Lei, X. Impaired Coupling of the Brain’s Default Network During Sleep Deprivation: A Resting-State EEG Study. Nat. Sci. Sleep 2020, 12, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-T.; Hsiao, Y.-T.; Yi, P.-L.; Chang, F.-C. Deep Brain Stimulation Increases Seizure Threshold by Altering REM Sleep and Delta Powers During NREM Sleep. Front. Neurol. 2020, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Kurtis, M.M.; Rajah, T.; Delgado, L.F.; Dafsari, H.S. The effect of deep brain stimulation on the non-motor symptoms of Parkinson's disease: A critical review of the current evidence. NPJ Parkinsons. Dis. 2017, 3, 16024. [Google Scholar] [CrossRef]
- Diekelmann, S. Sleep for cognitive enhancement. Front. Syst. Neurosci. 2014, 8, 46. [Google Scholar] [CrossRef]
- Luber, B.; Steffener, J.; Tucker, A.; Habeck, C.; Peterchev, A.V.; Deng, Z.-D.; Basner, R.C.; Stern, Y.; Lisanby, S.H. Extended remediation of sleep deprived-induced working memory deficits using fMRI-guided transcranial magnetic stimulation. Sleep 2013, 36, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Dalong, G.; Jiyuan, L.; Ying, Z.; Lei, Z.; Yanhong, H.; Yongcong, S. Transcranial direct current stimulation reconstructs diminished thalamocortical connectivity during prolonged resting wakefulness: A resting-state fMRI pilot study. Brain Imaging Behav. 2020, 14, 278–288. [Google Scholar] [CrossRef] [PubMed]
- McIntire, L.K.; McKinley, R.A.; Goodyear, C.; McIntire, J.P. The Effects of Anodal Transcranial Direct Current Stimulation on Sleep Time and Efficiency. Front. Hum. Neurosci. 2020, 14, 357. [Google Scholar] [CrossRef]
- McIntire, L.K.; McKinley, R.A.; Goodyear, C.; Nelson, J. A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimul. 2014, 7, 499–507. [Google Scholar] [CrossRef] [PubMed]
- McIntire, L.K.; McKinley, R.A.; Nelson, J.M.; Goodyear, C. Transcranial direct current stimulation versus caffeine as a fatigue countermeasure. Brain Stimul. 2017, 10, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- San-Juan, D.; Mas, R.N.M.; Gutiérrez, C.; Morales, J.; Díaz, A.; Quiñones, G.; Galindo, A.K.; Baigts, L.A.; Ximenez-Camilli, C.; Anschel, D. Effect of the anodal transcranial direct current electrical stimulation on cognition of medical residents with acute sleep deprivation. Sleep Sci. 2022, 15, 89–96. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, A.; Scarpelli, S.; Gorgoni, M.; Alfonsi, V.; Annarumma, L.; Giannini, A.M.; Ferrara, M.; Ferlazzo, F.; Rossini, P.M.; de Gennaro, L. Bilateral Theta Transcranial Alternating Current Stimulation (tACS) Modulates EEG Activity: When tACS Works Awake It Also Works Asleep. Nat. Sci. Sleep 2019, 11, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.D.; Sengupta, S.; Chitnis, S.; Amara, A.W. Deep Brain Stimulation and Sleep-Wake Disturbances in Parkinson Disease: A Review. Front. Neurol. 2018, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.W.; Watts, R.L.; Walker, H.C. The effects of deep brain stimulation on sleep in Parkinson's disease. Ther. Adv. Neurol. Disord. 2011, 4, 15–24. [Google Scholar] [CrossRef]
- Herrero Babiloni, A.; Bellemare, A.; Beetz, G.; Vinet, S.-A.; Martel, M.O.; Lavigne, G.J.; de Beaumont, L. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: A systematic review. Sleep Med. Rev. 2021, 55, 101381. [Google Scholar] [CrossRef]
- Grosmaitre, X.; Santarelli, L.C.; Tan, J.; Luo, M.; Ma, M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat. Neurosci. 2007, 10, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Ito, A.; LaFever, B.J. Subpopulations of Projection Neurons in the Olfactory Bulb. Front. Neural Circuits 2020, 14, 561822. [Google Scholar] [CrossRef]
- Yanovsky, Y.; Ciatipis, M.; Draguhn, A.; Tort, A.B.L.; Brankačk, J. Slow oscillations in the mouse hippocampus entrained by nasal respiration. J. Neurosci. 2014, 34, 5949–5964. [Google Scholar] [CrossRef]
- Zelano, C.; Jiang, H.; Zhou, G.; Arora, N.; Schuele, S.; Rosenow, J.; Gottfried, J.A. Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function. J. Neurosci. 2016, 36, 12448–12467. [Google Scholar] [CrossRef] [PubMed]
- Juventin, M.; Ghibaudo, V.; Granget, J.; Amat, C.; Courtiol, E.; Buonviso, N. Respiratory influence on brain dynamics: The preponderant role of the nasal pathway and deep slow regime. Pflugers Arch. 2023, 475, 23–35. [Google Scholar] [CrossRef]
- Karalis, N.; Sirota, A. Breathing coordinates cortico-hippocampal dynamics in mice during offline states. Nat. Commun. 2022, 13, 467. [Google Scholar] [CrossRef]
- Herrero, J.L.; Khuvis, S.; Yeagle, E.; Cerf, M.; Mehta, A.D. Breathing above the brain stem: Volitional control and attentional modulation in humans. J. Neurophysiol. 2018, 119, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Piarulli, A.; Zaccaro, A.; Laurino, M.; Menicucci, D.; de Vito, A.; Bruschini, L.; Berrettini, S.; Bergamasco, M.; Laureys, S.; Gemignani, A. Ultra-slow mechanical stimulation of olfactory epithelium modulates consciousness by slowing cerebral rhythms in humans. Sci. Rep. 2018, 8, 6581. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liu, Y.; Wang, L.; Li, B.; Xu, F. Activity Patterns Elicited by Airflow in the Olfactory Bulb and Their Possible Functions. J. Neurosci. 2017, 37, 10700–10711. [Google Scholar] [CrossRef]
- Ito, J.; Roy, S.; Liu, Y.; Cao, Y.; Fletcher, M.; Lu, L.; Boughter, J.D.; Grün, S.; Heck, D.H. Whisker barrel cortex delta oscillations and gamma power in the awake mouse are linked to respiration. Nat. Commun. 2014, 5, 3572. [Google Scholar] [CrossRef]
- Baghdadi, G.; Kamarajan, C.; Hadaeghi, F. Editorial: Role of brain oscillations in neurocognitive control systems. Front. Syst. Neurosci. 2023, 17, 1182496. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Kann, M.; Wang, Q. Neuromodulation of Neural Oscillations in Health and Disease. Biology 2023, 12, 371. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Farrahi Moghaddam, J.; Nakhaee, N.; Sheibani, V.; Garrusi, B.; Amirkafi, A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath. 2012, 16, 79–82. [Google Scholar] [CrossRef]
- Nejati, V. Cognitive Abilities Questionnaire: Development and Evaluation of Psychometric Properties. ICSS 2013, 15, 11–19. [Google Scholar]
- Fakhari, A.; Azizi, H.; Mostofi, M.; Sadeghpour, S.; Farahbakhsh, M. Validity and Reliability of the Iranian Rapid Assessment for Psychiatric Illness Screening Instrument (IRA-PISI) in Primary Health Care. Iran. J. Psychiatry Behav. Sci. 2022, 16, e112790. [Google Scholar] [CrossRef]
- Zielinski, M.C.; Tang, W.; Jadhav, S.P. The role of replay and theta sequences in mediating hippocampal-prefrontal interactions for memory and cognition. Hippocampus 2020, 30, 60–72. [Google Scholar] [CrossRef]
- Beldzik, E.; Domagalik, A.; Froncisz, W.; Marek, T. Dissociating EEG sources linked to stimulus and response evaluation in numerical Stroop task using Independent Component Analysis. Clin. Neurophysiol. 2015, 126, 914–926. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Huang, Z. Brief history and development of electrophysiological recording techniques in neuroscience. In Signal Processing in Neuroscience; Springer: Singapore, 2016; pp. 1–10. [Google Scholar]
- Zappasodi, F.; Olejarczyk, E.; Marzetti, L.; Assenza, G.; Pizzella, V.; Tecchio, F. Fractal dimension of EEG activity senses neuronal impairment in acute stroke. PLoS ONE 2014, 9, e100199. [Google Scholar] [CrossRef]
- Susmáková, K.; Krakovská, A. Discrimination ability of individual measures used in sleep stages classification. Artif. Intell. Med. 2008, 44, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Approach to an irregular time series on the basis of the fractal theory. Phys. D Nonlinear Phenom. 1988, 31, 277–283. [Google Scholar] [CrossRef]
- Salimi, M.; Javadi, A.-H.; Nazari, M.; Bamdad, S.; Tabasi, F.; Parsazadegan, T.; Ayene, F.; Karimian, M.; Gholami-Mahtaj, L.; Shadnia, S.; et al. Nasal Air Puff Promotes Default Mode Network Activity in Mechanically Ventilated Comatose Patients: A Noninvasive Brain Stimulation Approach. Neuromodulation 2022, 25, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Zeydabadinezhad, M.; Horn, C.C.; Mahmoudi, B. Quantifying the effects of vagus nerve stimulation on gastric myoelectric activity in ferrets using an interpretable machine learning approach. PLoS ONE 2023, 18, e0295297. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J. Fractals and the analysis of waveforms. Comput. Biol. Med. 1988, 18, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Jahdali, H. The consequences of sleep deprivation on cognitive performance. Neurosciences 2023, 28, 91–99. [Google Scholar] [CrossRef]
- Yoo, S.-S.; Hu, P.T.; Gujar, N.; Jolesz, F.A.; Walker, M.P. A deficit in the ability to form new human memories without sleep. Nat. Neurosci. 2007, 10, 385–392. [Google Scholar] [CrossRef]
- Sagaspe, P.; Sanchez-Ortuno, M.; Charles, A.; Taillard, J.; Valtat, C.; Bioulac, B.; Philip, P. Effects of sleep deprivation on Color-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain Cogn. 2006, 60, 76–87. [Google Scholar] [CrossRef]
- Hudson, A.N.; van Dongen, H.P.A.; Honn, K.A. Sleep deprivation, vigilant attention, and brain function: A review. Neuropsychopharmacology 2020, 45, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Alhola, P.; Polo-Kantola, P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567. [Google Scholar] [PubMed]
- Wu, J.; Zhou, Q.; Li, J.; Chen, Y.; Shao, S.; Xiao, Y. Decreased resting-state alpha-band activation and functional connectivity after sleep deprivation. Sci. Rep. 2021, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Chee, M.W.L.; Zhou, J. Functional connectivity and the sleep-deprived brain. Prog. Brain Res. 2019, 246, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.; Yao, Y.; Pan, Y.; Sun, Y. Sleep deprivation disturbed regional brain activity in healthy subjects: Evidence from a functional magnetic resonance-imaging study. Neuropsychiatr. Dis. Treat. 2016, 12, 801–807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Durbin, R.P. Letter: Acid secretion by gastric mucous membrane. Am. J. Physiol. 1975, 229, 1726. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, V.; Chuah, Y.M.L.; Huettel, S.A.; Chee, M.W.L. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 2007, 30, 603–609. [Google Scholar] [CrossRef]
- Mullin, B.C.; Phillips, M.L.; Siegle, G.J.; Buysse, D.J.; Forbes, E.E.; Franzen, P.L. Sleep deprivation amplifies striatal activation to monetary reward. Psychol. Med. 2013, 43, 2215–2225. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Balkin, T.J.; Wesensten, N.J. Impaired decision making following 49 h of sleep deprivation. J. Sleep Res. 2006, 15, 7–13. [Google Scholar] [CrossRef]
- Olson, E.A.; Weber, M.; Rauch, S.L.; Killgore, W.D.S. Daytime Sleepiness Is Associated with Reduced Integration of Temporally Distant Outcomes on the Iowa Gambling Task. Behav. Sleep Med. 2016, 14, 200–211. [Google Scholar] [CrossRef]
- Biskamp, J.; Bartos, M.; Sauer, J.-F. Organization of prefrontal network activity by respiration-related oscillations. Sci. Rep. 2017, 7, 45508. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.T.; Peace, S.T.; Cleland, T.A. Properties and mechanisms of olfactory learning and memory. Front. Behav. Neurosci. 2014, 8, 238. [Google Scholar] [CrossRef]
- Zald, D.H.; Pardo, J.V. Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation. Proc. Natl. Acad. Sci. USA 1997, 94, 4119–4124. [Google Scholar] [CrossRef]
- Bhattarai, J.P.; Etyemez, S.; Jaaro-Peled, H.; Janke, E.; Leon Tolosa, U.D.; Kamiya, A.; Gottfried, J.A.; Sawa, A.; Ma, M. Olfactory modulation of the medial prefrontal cortex circuitry: Implications for social cognition. Semin. Cell Dev. Biol. 2022, 129, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Feurer, C.; Jimmy, J.; Bhaumik, R.; Duffecy, J.; Medrano, G.R.; Ajilore, O.; Shankman, S.A.; Langenecker, S.A.; Craske, M.G.; Phan, K.L.; et al. Anterior cingulate cortex activation during attentional control as a transdiagnostic marker of psychotherapy response: A randomized clinical trial. Neuropsychopharmacology 2022, 47, 1350–1357. [Google Scholar] [CrossRef]
- Alvites, R.; Caine, A.; Cherubini, G.B.; Prada, J.; Varejão, A.S.P.; Maurício, A.C. The Olfactory Bulb in Companion Animals-Anatomy, Physiology, and Clinical Importance. Brain Sci. 2023, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Ghazvineh, S.; Salimi, M.; Nazari, M.; Garousi, M.; Tabasi, F.; Dehdar, K.; Salimi, A.; Jamaati, H.; Mirnajafi-Zadeh, J.; Arabzadeh, E.; et al. Rhythmic air-puff into nasal cavity modulates activity across multiple brain areas: A non-invasive brain stimulation method to reduce ventilator-induced memory impairment. Respir. Physiol. Neurobiol. 2021, 287, 103627. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Li, C.-T.; Juan, C.-H. A review of critical brain oscillations in depression and the efficacy of transcranial magnetic stimulation treatment. Front. Psychiatry 2023, 14, 1073984. [Google Scholar] [CrossRef]
- Jiang, Y.; Jessee, W.; Hoyng, S.; Borhani, S.; Liu, Z.; Zhao, X.; Price, L.K.; High, W.; Suhl, J.; Cerel-Suhl, S. Sharpening Working Memory with Real-Time Electrophysiological Brain Signals: Which Neurofeedback Paradigms Work? Front. Aging Neurosci. 2022, 14, 780817. [Google Scholar] [CrossRef]
- Guan, A.; Wang, S.; Huang, A.; Qiu, C.; Li, Y.; Li, X.; Wang, J.; Wang, Q.; Deng, B. The role of gamma oscillations in central nervous system diseases: Mechanism and treatment. Front. Cell. Neurosci. 2022, 16, 962957. [Google Scholar] [CrossRef]
- Rojas-Líbano, D.; Del Wimmer Solar, J.; Aguilar-Rivera, M.; Montefusco-Siegmund, R.; Maldonado, P.E. Local cortical activity of distant brain areas can phase-lock to the olfactory bulb's respiratory rhythm in the freely behaving rat. J. Neurophysiol. 2018, 120, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mai, X.; Liu, C. The default mode network and social understanding of others: What do brain connectivity studies tell us. Front. Hum. Neurosci. 2014, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Garcés, P.; Angel Pineda-Pardo, J.; Canuet, L.; Aurtenetxe, S.; López, M.E.; Marcos, A.; Yus, M.; Llanero-Luque, M.; Del-Pozo, F.; Sancho, M.; et al. The Default Mode Network is functionally and structurally disrupted in amnestic mild cognitive impairment—A bimodal MEG-DTI study. Neuroimage Clin. 2014, 6, 214–221. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Sun, F.; Xu, Y.; Xu, F.; Wang, S.; Wang, X. Altered neuromagnetic activity in default mode network in childhood absence epilepsy. Front. Neurosci. 2023, 17, 1133064. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Rojas-Líbano, D.; Kay, L.M. Olfactory system gamma oscillations: The physiological dissection of a cognitive neural system. Cogn. Neurodyn. 2008, 2, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Lepousez, G.; Nissant, A.; Bryant, A.K.; Gheusi, G.; Greer, C.A.; Lledo, P.-M. Olfactory learning promotes input-specific synaptic plasticity in adult-born neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 13984–13989. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Takeuchi, Y.; Wang, J.; Gellért, L.; Barcsai, L.; Pedraza, L.K.; Nagy, A.J.; Kozák, G.; Nakai, S.; Kato, S.; et al. Reinstating olfactory bulb-derived limbic gamma oscillations alleviates depression-like behavioral deficits in rodents. Neuron 2023, 111, 2065–2075.e5. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998, 55, 257–332. [Google Scholar] [CrossRef]
- Carmichael, S.T.; Price, J.L. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1995, 363, 615–641. [Google Scholar] [CrossRef]








| Groups | n | Gender | Age (Years Old) | Weight (kg) | ||
|---|---|---|---|---|---|---|
| Male (n) | Female (n) | |||||
| Control | 8 | 3 | 5 | 27.50 ± 1.33 | 72 ± 9.34 | |
| Treatment | Subgroup 1 | 7 | 4 | 3 | 27.86 ± 1.908 | 75.57 ± 11.62 |
| Subgroup 2 | 6 | 3 | 3 | 26.67 ± 1.726 | 74.33 ± 8.441 | |
| Subgroup 1 + Subgroup 2 | 13 | 7 | 6 | 27.31 ± 1.26 | 75.00 ± 7.08 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riazi, H.; Nazari, M.; Raoufy, M.R.; Mirnajafi-Zadeh, J.; Shojaei, A. Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation. Brain Sci. 2024, 14, 378. https://doi.org/10.3390/brainsci14040378
Riazi H, Nazari M, Raoufy MR, Mirnajafi-Zadeh J, Shojaei A. Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation. Brain Sciences. 2024; 14(4):378. https://doi.org/10.3390/brainsci14040378
Chicago/Turabian StyleRiazi, Hanieh, Milad Nazari, Mohammad Reza Raoufy, Javad Mirnajafi-Zadeh, and Amir Shojaei. 2024. "Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation" Brain Sciences 14, no. 4: 378. https://doi.org/10.3390/brainsci14040378
APA StyleRiazi, H., Nazari, M., Raoufy, M. R., Mirnajafi-Zadeh, J., & Shojaei, A. (2024). Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation. Brain Sciences, 14(4), 378. https://doi.org/10.3390/brainsci14040378