Investigating Dyslexia through Diffusion Tensor Imaging across Ages: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Compilation
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Data Analysis
3. Results
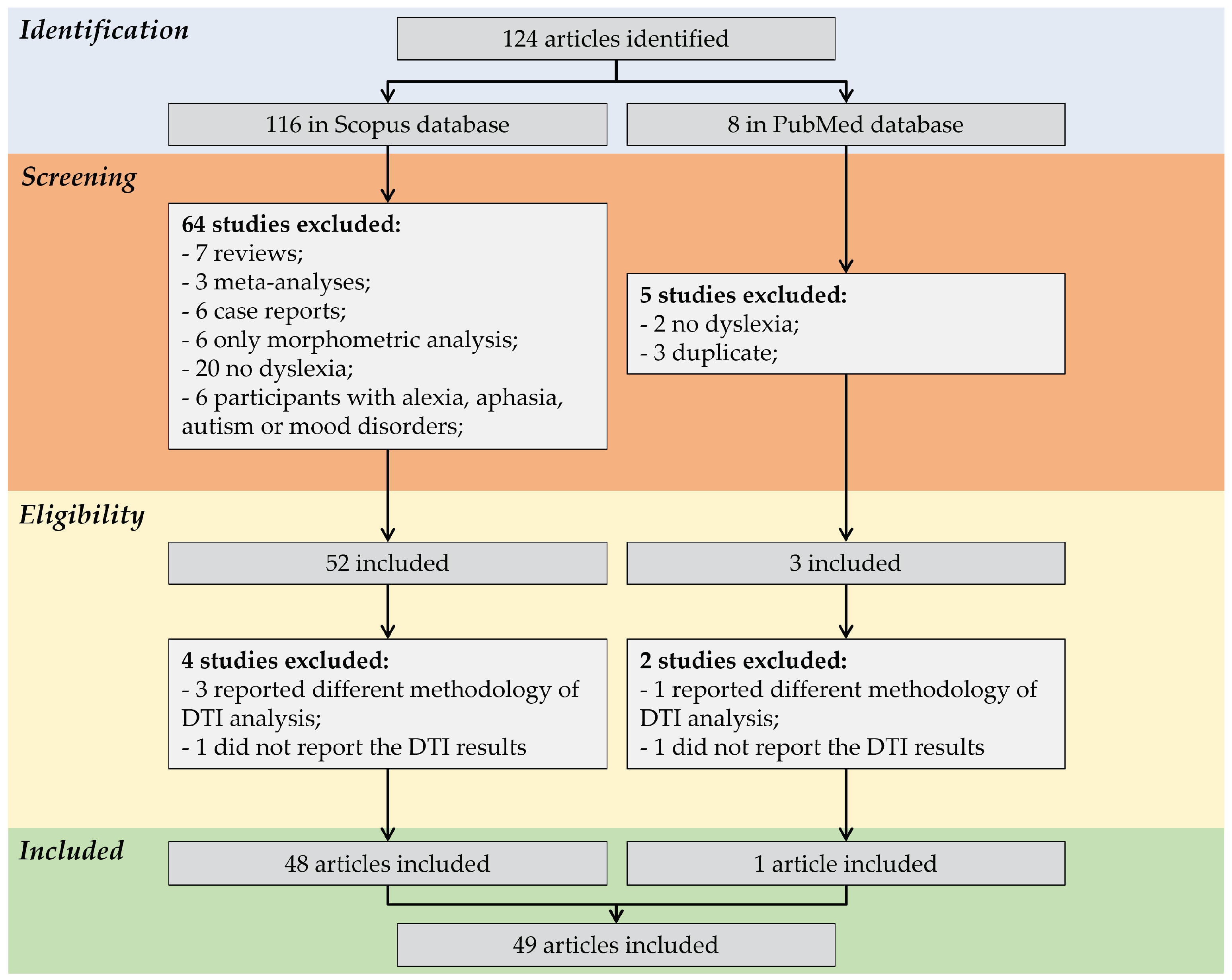
3.1. Overview of the Screening Process of the Included Studies
3.2. Demographic and Neuropsychological Characteristics of Studied Dyslexia Subjects
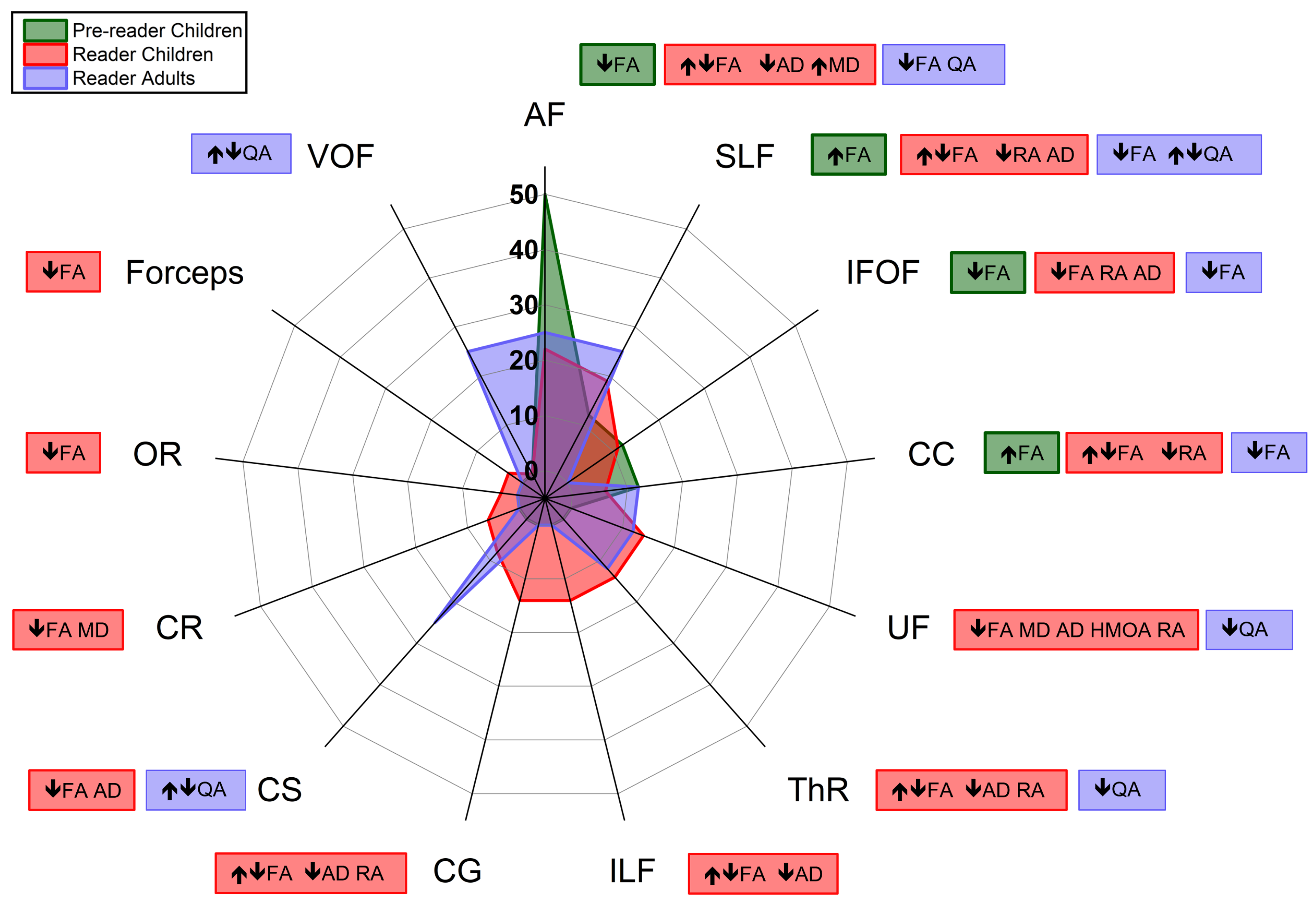
3.3. Brain Structural Connectivity Characteristics on Acquisition, Process, and Outcomes of Dyslexia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Rev.; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Peterson, R.L.; Pennington, B.F. Developmental dyslexia. Lancet 2012, 379, 1997–2007. [Google Scholar] [CrossRef]
- Peterson, R.L.; Pennington, B.F. Developmental dyslexia. Annu. Rev. Clin. Psychol. 2015, 11, 283–307. [Google Scholar] [CrossRef]
- Peterson, R.L.; McGrath, L.M.; Willcutt, E.G.; Keenan, J.M.; Olson, R.K.; Pennington, B.F. How specific are learning disabilities? J. Learn. Disabil. 2021, 54, 466–483. [Google Scholar] [CrossRef]
- Habib, M. The neurological basis of developmental dyslexia and related disorders: A reappraisal of the temporal hypothesis, twenty years on. Brain Sci. 2021, 11, 708. [Google Scholar] [CrossRef]
- Majeed, N.M.; Hartanto, A.; Tan, J.J. Developmental dyslexia and creativity: A meta-analysis. Dyslexia 2021, 27, 187–203. [Google Scholar] [CrossRef]
- Di Folco, C.; Guez, A.; Peyre, H.; Ramus, F. Epidemiology of developmental dyslexia: A comparison of DSM-5 and ICD-11 criteria. MedRxiv 2020, 1–34. [Google Scholar] [CrossRef]
- Roitsch, J.; Watson, S.M. An overview of dyslexia: Definition, characteristics, assessment, identification, and intervention. Sci. J. Educ. 2019, 7. [Google Scholar] [CrossRef]
- Van Hecke, W.; Emsell, L.; Sunaert, S. Diffusion Tensor Imaging: A Practical Handbook; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. In Vivo 2002, 15, 435–455. [Google Scholar] [CrossRef]
- Steven, A.J.; Zhuo, J.; Melhem, E.R. Diffusion kurtosis imaging: An emerging technique for evaluating the microstructural environment of the brain. Am. J. Roentgenol. 2014, 202, W26–W33. [Google Scholar] [CrossRef]
- Huisman, T. Diffusion-weighted and diffusion tensor imaging of the brain, made easy. Cancer Imaging 2010, 10, S163. [Google Scholar] [CrossRef]
- Vandermosten, M.; Boets, B.; Wouters, J.; Ghesquière, P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012, 36, 1532–1552. [Google Scholar] [CrossRef]
- Moreau, D.; Stonyer, J.E.; McKay, N.S.; Waldie, K.E. No evidence for systematic white matter correlates of dyslexia: An activation likelihood estimation meta-analysis. Brain Res. 2018, 1683, 36–47. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Zuk, J.; Dunstan, J.; Norton, E.; Yu, X.; Ozernov-Palchik, O.; Wang, Y.; Hogan, T.P.; Gabrieli, J.D.; Gaab, N. Multifactorial pathways facilitate resilience among kindergarteners at risk for dyslexia: A longitudinal behavioral and neuroimaging study. Dev. Sci. 2021, 24, e12983. [Google Scholar] [CrossRef]
- Yu, X.; Zuk, J.; Perdue, M.V.; Ozernov-Palchik, O.; Raney, T.; Beach, S.D.; Norton, E.S.; Ou, Y.; Gabrieli, J.D.; Gaab, N. Putative protective neural mechanisms in prereaders with a family history of dyslexia who subsequently develop typical reading skills. Hum. Brain Mapp. 2020, 41, 2827–2845. [Google Scholar] [CrossRef]
- Langer, N.; Peysakhovich, B.; Zuk, J.; Drottar, M.; Sliva, D.D.; Smith, S.; Becker, B.L.; Grant, P.E.; Gaab, N. White matter alterations in infants at risk for developmental dyslexia. Cereb. Cortex 2017, 27, 1027–1036. [Google Scholar] [CrossRef]
- Kraft, I.; Schreiber, J.; Cafiero, R.; Metere, R.; Schaadt, G.; Brauer, J.; Neef, N.E.; Müller, B.; Kirsten, H.; Wilcke, A.; et al. Predicting early signs of dyslexia at a preliterate age by combining behavioral assessment with structural MRI. Neuroimage 2016, 143, 378–386. [Google Scholar] [CrossRef]
- Vandermosten, M.; Vanderauwera, J.; Theys, C.; De Vos, A.; Vanvooren, S.; Sunaert, S.; Wouters, J.; Ghesquière, P. A DTI tractography study in pre-readers at risk for dyslexia. Dev. Cogn. Neurosci. 2015, 14, 8–15. [Google Scholar] [CrossRef]
- Van Der Auwera, S.; Vandermosten, M.; Wouters, J.; Ghesquière, P.; Vanderauwera, J. A three-time point longitudinal investigation of the arcuate fasciculus throughout reading acquisition in children developing dyslexia. NeuroImage 2021, 237, 118087. [Google Scholar] [CrossRef]
- Wang, Y.; Mauer, M.V.; Raney, T.; Peysakhovich, B.; Becker, B.L.; Sliva, D.D.; Gaab, N. Development of tract-specific white matter pathways during early reading development in at-risk children and typical controls. Cereb. Cortex 2017, 27, 2469–2485. [Google Scholar] [CrossRef]
- Vanderauwera, J.; Wouters, J.; Vandermosten, M.; Ghesquière, P. Early dynamics of white matter deficits in children developing dyslexia. Dev. Cogn. Neurosci. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Zhao, J.; Song, Z.; Zhao, Y.; de Schotten, M.T.; Altarelli, I.; Ramus, F. White matter connectivity in uncinate fasciculus accounts for visual attention span in developmental dyslexia. Neuropsychologia 2022, 177, 108414. [Google Scholar] [CrossRef]
- Meisler, S.L.; Gabrieli, J.D. A large-scale investigation of white matter microstructural associations with reading ability. NeuroImage 2022, 249, 118909. [Google Scholar] [CrossRef]
- Liu, T.; Thiebaut de Schotten, M.; Altarelli, I.; Ramus, F.; Zhao, J. Neural dissociation of visual attention span and phonological deficits in developmental dyslexia: A hub-based white matter network analysis. Hum. Brain Mapp. 2022, 43, 5210–5219. [Google Scholar] [CrossRef]
- Farah, R.; Glukhovsky, N.; Rosch, K.; Horowitz-Kraus, T. Structural white matter characteristics for working memory and switching/inhibition in children with reading difficulties: The role of the left superior longitudinal fasciculus. Netw. Neurosci. 2022, 6, 897–915. [Google Scholar] [CrossRef]
- Partanen, M.; Kim, D.H.; Rauscher, A.; Siegel, L.S.; Giaschi, D.E. White matter but not grey matter predicts change in reading skills after intervention. Dyslexia 2021, 27, 224–244. [Google Scholar] [CrossRef]
- Lou, C.; Cross, A.M.; Peters, L.; Ansari, D.; Joanisse, M.F. Rich-club structure contributes to individual variance of reading skills via feeder connections in children with reading disabilities. Dev. Cogn. Neurosci. 2021, 49, 100957. [Google Scholar] [CrossRef]
- Liu, T.; de Schotten, M.T.; Altarelli, I.; Ramus, F.; Zhao, J. Maladaptive compensation of right fusiform gyrus in developmental dyslexia: A hub-based white matter network analysis. Cortex 2021, 145, 57–66. [Google Scholar] [CrossRef]
- Koirala, N.; Perdue, M.V.; Su, X.; Grigorenko, E.L.; Landi, N. Neurite density and arborization is associated with reading skill and phonological processing in children. NeuroImage 2021, 241, 118426. [Google Scholar] [CrossRef]
- Huber, E.; Mezer, A.; Yeatman, J.D. Neurobiological underpinnings of rapid white matter plasticity during intensive reading instruction. NeuroImage 2021, 243, 118453. [Google Scholar] [CrossRef]
- Borghesani, V.; Wang, C.; Watson, C.; Bouhali, F.; Caverzasi, E.; Battistella, G.; Bogley, R.; Yabut, N.A.; Deleon, J.; Miller, Z.A.; et al. Functional and morphological correlates of developmental dyslexia: A multimodal investigation of the ventral occipitotemporal cortex. J. Neuroimaging 2021, 31, 962–972. [Google Scholar] [CrossRef]
- Vander Stappen, C.; Dricot, L.; Van Reybroeck, M. RAN training in dyslexia: Behavioral and brain correlates. Neuropsychologia 2020, 146, 107566. [Google Scholar] [CrossRef]
- El-Sady, S.; Mohammad, S.A.; Aboualfotouh Ahmed, K.; Khattab, A.N.; Nashaat, N.H.; Orabi, G.; Abdelraouf, E.R. Correlation between diffusion tensor imaging measures and the reading and cognitive performance of Arabic readers: Dyslexic children perspective. Neuroradiology 2020, 62, 525–531. [Google Scholar] [CrossRef]
- Wang, H.L.S.; Wang, N.Y.H.; Yeh, F.C. Specifying the diffusion MRI connectome in Chinese-speaking children with developmental dyslexia and auditory processing deficits. Pediatr. Neonatol. 2019, 60, 297–304. [Google Scholar] [CrossRef]
- Vanderauwera, J.; van Setten, E.R.; Maurits, N.M.; Maassen, B.A. The interplay of socio-economic status represented by paternal educational level, white matter structure and reading. PLoS ONE 2019, 14, e0215560. [Google Scholar] [CrossRef]
- Lou, C.; Duan, X.; Altarelli, I.; Sweeney, J.A.; Ramus, F.; Zhao, J. White matter network connectivity deficits in developmental dyslexia. Hum. Brain Mapp. 2019, 40, 505–516. [Google Scholar] [CrossRef]
- Lebel, C.; Benischek, A.; Geeraert, B.; Holahan, J.; Shaywitz, S.; Bakhshi, K.; Shaywitz, B. Developmental trajectories of white matter structure in children with and without reading impairments. Dev. Cogn. Neurosci. 2019, 36, 100633. [Google Scholar] [CrossRef]
- Banfi, C.; Koschutnig, K.; Moll, K.; Schulte-Körne, G.; Fink, A.; Landerl, K. White matter alterations and tract lateralization in children with dyslexia and isolated spelling deficits. Hum. Brain Mapp. 2019, 40, 765–776. [Google Scholar] [CrossRef]
- Žarić, G.; Timmers, I.; Gerretsen, P.; Fraga González, G.; Tijms, J.; van der Molen, M.W.; Blomert, L.; Bonte, M. Atypical white matter connectivity in dyslexic readers of a fairly transparent orthography. Front. Psychol. 2018, 9, 1147. [Google Scholar] [CrossRef]
- Yagle, K.; Richards, T.; Askren, K.; Mestre, Z.; Beers, S.; Abbott, R.; Nagy, W.; Boord, P.; Berninger, V. Relationships between eye movements during sentence reading comprehension, word spelling and reading, and DTI and fMRI connectivity in students with and without dysgraphia or dyslexia. J. Syst. Integr. Neurosci. 2017, 1–11. [Google Scholar] [CrossRef]
- Su, M.; Zhao, J.; de Schotten, M.T.; Zhou, W.; Gong, G.; Ramus, F.; Shu, H. Alterations in white matter pathways underlying phonological and morphological processing in Chinese developmental dyslexia. Dev. Cogn. Neurosci. 2018, 31, 11–19. [Google Scholar] [CrossRef]
- Christodoulou, J.A.; Murtagh, J.; Cyr, A.; Perrachione, T.K.; Chang, P.; Halverson, K.; Hook, P.; Yendiki, A.; Ghosh, S.; Gabrieli, J.D. Relation of white-matter microstructure to reading ability and disability in beginning readers. Neuropsychology 2017, 31, 508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; de Schotten, M.T.; Altarelli, I.; Dubois, J.; Ramus, F. Altered hemispheric lateralization of white matter pathways in developmental dyslexia: Evidence from spherical deconvolution tractography. Cortex 2016, 76, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Koerte, I.K.; Willems, A.; Muehlmann, M.; Moll, K.; Cornell, S.; Pixner, S.; Steffinger, D.; Keeser, D.; Heinen, F.; Kubicki, M.; et al. Mathematical abilities in dyslexic children: A diffusion tensor imaging study. Brain Imaging Behav. 2016, 10, 781–791. [Google Scholar] [CrossRef]
- Garcia-Zapirain, B.; Garcia-Chimeno, Y.; Saralegui, I.; Fernandez-Ruanova, B.; Martinez, R. Differences in effective connectivity between children with dyslexia, monocular vision and typically developing readers: A DTI study. Biomed. Signal Process. Control 2016, 23, 19–27. [Google Scholar] [CrossRef]
- Fernandez, V.G.; Juranek, J.; Romanowska-Pawliczek, A.; Stuebing, K.; Williams, V.J.; Fletcher, J.M. White matter integrity of cerebellar-cortical tracts in reading impaired children: A probabilistic tractography study. Brain Lang. 2016, 161, 45–56. [Google Scholar] [CrossRef]
- De Moura, L.M.; Cogo-Moreira, H.; De Ávila, C.R.B.; Pan, P.M.; Gadelha, A.; Moriyama, T.; Del Aquilla, M.A.; Hoexter, M.; Salum, G.A.; Picon, F.A.; et al. Children with poor reading skills at the word level show reduced fractional anisotropy in white matter tracts of both hemispheres. Brain Connect. 2016, 6, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.L.; Grabowski, T.J.; Boord, P.; Yagle, K.; Askren, M.; Mestre, Z.; Robinson, P.; Welker, O.; Gulliford, D.; Nagy, W.; et al. Contrasting brain patterns of writing-related DTI parameters, fMRI connectivity, and DTI–fMRI connectivity correlations in children with and without dysgraphia or dyslexia. Neuroimage Clin. 2015, 8, 408–421. [Google Scholar] [CrossRef]
- Marino, C.; Scifo, P.; Della Rosa, P.A.; Mascheretti, S.; Facoetti, A.; Lorusso, M.L.; Giorda, R.; Consonni, M.; Falini, A.; Molteni, M.; et al. The DCDC2/intron 2 deletion and white matter disorganization: Focus on developmental dyslexia. Cortex 2014, 57, 227–243. [Google Scholar] [CrossRef]
- Fan, Q.; Davis, N.; Anderson, A.W.; Cutting, L.E. Thalamo-cortical connectivity: What can diffusion tractography tell us about reading difficulties in children? Brain Connect. 2014, 4, 428–439. [Google Scholar] [CrossRef]
- Fan, Q.; Anderson, A.W.; Davis, N.; Cutting, L.E. Structural connectivity patterns associated with the putative visual word form area and children’ s reading ability. Brain Res. 2014, 1586, 118–129. [Google Scholar] [CrossRef]
- Hasan, K.M.; Molfese, D.L.; Walimuni, I.S.; Stuebing, K.K.; Papanicolaou, A.C.; Narayana, P.A.; Fletcher, J.M. Diffusion tensor quantification and cognitive correlates of the macrostructure and microstructure of the corpus callosum in typically developing and dyslexic children. NMR Biomed. 2012, 25, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Enzinger, C.; Kronbichler, M.; Schurz, M.; Reishofer, G.; Koschutnig, K.; Kargl, R.; Purgstaller, C.; Fazekas, F.; Fink, A. Distinct patterns of brain function in children with isolated spelling impairment: New insights. Neuropsychologia 2012, 50, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, F.; McCandliss, B.D.; Black, J.M.; Gantman, A.; Zakerani, N.; Hulme, C.; Lyytinen, H.; Whitfield-Gabrieli, S.; Glover, G.H.; Reiss, A.L.; et al. Neural systems predicting long-term outcome in dyslexia. Proc. Natl. Acad. Sci. USA 2011, 108, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, A.J.; Virtala, P.; Thiede, A.; Laasonen, M.; Kujala, T. Structural white matter connectometry of reading and dyslexia. NeuroImage 2021, 241, 118411. [Google Scholar] [CrossRef] [PubMed]
- Tschentscher, N.; Ruisinger, A.; Blank, H.; Díaz, B.; Von Kriegstein, K. Reduced structural connectivity between left auditory thalamus and the motion-sensitive planum temporale in developmental dyslexia. J. Neurosci. 2019, 39, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Wilson, A.J.; McKay, N.S.; Nihill, K.; Waldie, K.E. No evidence for systematic white matter correlates of dyslexia and dyscalculia. NeuroImage Clin. 2018, 18, 356–366. [Google Scholar] [CrossRef]
- Müller-Axt, C.; Anwander, A.; von Kriegstein, K. Altered structural connectivity of the left visual thalamus in developmental dyslexia. Curr. Biol. 2017, 27, 3692–3698. [Google Scholar] [CrossRef] [PubMed]
- Vandermosten, M.; Poelmans, H.; Sunaert, S.; Ghesquière, P.; Wouters, J. White matter lateralization and interhemispheric coherence to auditory modulations in normal reading and dyslexic adults. Neuropsychologia 2013, 51, 2087–2099. [Google Scholar] [CrossRef]
- Lebel, C.; Shaywitz, B.; Holahan, J.; Shaywitz, S.; Marchione, K.; Beaulieu, C. Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain Lang. 2013, 125, 215–222. [Google Scholar] [CrossRef]
- Vandermosten, M.; Boets, B.; Poelmans, H.; Sunaert, S.; Wouters, J.; Ghesquiere, P. A tractography study in dyslexia: Neuroanatomic correlates of orthographic, phonological and speech processing. Brain 2012, 135, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Liederman, J.; Hasan, K.M.; Lincoln, A.; Malmberg, B.; McLean, J., III; Papanicolaou, A. Diffusion tensor quantification of the relations between microstructural and macrostructural indices of white matter and reading. Hum. Brain Mapp. 2011, 32, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Mather, N.; Schneider, D. The Use of Cognitive Tests in the Assessment of Dyslexia. J. Intell. 2023, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Lumaca, M.; Brattico, E.; Vuust, P.; Kringelbach, M.; Bonetti, L. Dissociated brain functional connectivity of fast versus slow frequencies underlying individual differences in fluid intelligence: A DTI and MEG study. Sci. Rep. 2022, 12, 4746. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; Donnelly, P.M.; Rokem, A.; Yeatman, J.D. Rapid and widespread white matter plasticity during an intensive reading intervention. Nat. Commun. 2018, 9, 2260. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, C.R.; Muñoz Maniega, S.; Valdés Hernández, M.C.; Ballerini, L.; Barclay, G.; Taylor, A.M.; Russ, T.C.; Tucker-Drob, E.M.; Wardlaw, J.M.; Deary, I.J.; et al. Comparison of structural MRI brain measures between 1.5 and 3 T: Data from the Lothian Birth Cohort 1936. Hum. Brain Mapp. 2021, 42, 3905–3921. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.; Jensen, J.H.; Xuan, L.; Helpern, J.A. Estimation of the orientation distribution function from diffusional kurtosis imaging. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2008, 60, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Glenn, G.R.; Helpern, J.A.; Tabesh, A.; Jensen, J.H. Optimization of white matter fiber tractography with diffusional kurtosis imaging. NMR Biomed. 2015, 28, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Glenn, G.R.; Kuo, L.W.; Chao, Y.P.; Lee, C.Y.; Helpern, J.A.; Jensen, J.H. Mapping the orientation of white matter fiber bundles: A comparative study of diffusion tensor imaging, diffusional kurtosis imaging, and diffusion spectrum imaging. Am. J. Neuroradiol. 2016, 37, 1216–1222. [Google Scholar] [CrossRef]
- Barrio-Arranz, G.; de Luis-García, R.; Tristán-Vega, A.; Martín-Fernández, M.; Aja-Fernández, S. Impact of MR acquisition parameters on DTI scalar indexes: A tractography based approach. PLoS ONE 2015, 10, e0137905. [Google Scholar] [CrossRef]
- Bao, S.S.; Zhao, C.; Bao, X.X.; Rao, J.S.; Rao, J. Effect of Value on Imaging Quality for Diffusion Tensor Imaging of the Spinal Cord at Ultrahigh Field Strength. BioMed Res. Int. 2021, 2021, 4836804. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.P.; Zhong, Z.; Sun, Y.S.; Li, X.T.; Tang, L.; Zhou, X.J. Optimal selection of b-values for differential diagnosis of mediastinal lymph nodes using diffusion-weighted imaging. Heliyon 2023, 9, e16702. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, T.; Sartoretti, E.; Wyss, M.; Mannil, M.; van Smoorenburg, L.; Eichenberger, B.; Reischauer, C.; Alfieri, A.; Binkert, C.; Sartoretti-Schefer, S. Diffusion-weighted MRI of ischemic stroke at 3T: Value of synthetic b-values. Br. J. Radiol. 2021, 94, 20200869. [Google Scholar] [CrossRef] [PubMed]
- Kumpulainen, V.; Merisaari, H.; Copeland, A.; Silver, E.; Pulli, E.P.; Lewis, J.D.; Saukko, E.; Saunavaara, J.; Karlsson, L.; Karlsson, H.; et al. Effect of number of diffusion-encoding directions in diffusion metrics of 5-year-olds using tract-based spatial statistical analysis. Eur. J. Neurosci. 2022, 56, 4843–4868. [Google Scholar] [CrossRef]
- Hoefnagels, F.W.; de Witt Hamer, P.C.; Pouwels, P.J.; Barkhof, F.; Vandertop, W.P. Impact of gradient number and voxel size on diffusion tensor imaging tractography for resective brain surgery. World Neurosurg. 2017, 105, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, B.; Descoteaux, M.; Mori, S.; Leemans, A. Diffusion MRI fiber tractography of the brain. NMR Biomed. 2019, 32, e3785. [Google Scholar] [CrossRef] [PubMed]
- Schlaier, J.R.; Beer, A.L.; Faltermeier, R.; Fellner, C.; Steib, K.; Lange, M.; Greenlee, M.W.; Brawanski, A.T.; Anthofer, J.M. Probabilistic vs. deterministic fiber tracking and the influence of different seed regions to delineate cerebellar-thalamic fibers in deep brain stimulation. Eur. J. Neurosci. 2017, 45, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, D.; Reisert, M.; Dhollander, T.; Sunaert, S.; Suetens, P.; Maes, F. Global tractography of multi-shell diffusion-weighted imaging data using a multi-tissue model. Neuroimage 2015, 123, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Bodammer, N.; Kaufmann, J.; Kanowski, M.; Tempelmann, C. Eddy current correction in diffusion-weighted imaging using pairs of images acquired with opposite diffusion gradient polarity. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2004, 51, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Oldham, S.; Arnatkevičiūtė, A.; Smith, R.; Tiego, J.; Bellgrove, M.; Fornito, A. The efficacy of different preprocessing steps in reducing motion-related confounds in diffusion MRI connectomics. NeuroImage 2020, 222, 117252. [Google Scholar] [CrossRef]
| Ref. | Year | Country | Language | Group | N | Sex (F:M) | Age (Years) | Years of Education (or Level) | IQ | A Word Reading/Spelling | Pseudoword Reading | Text Reading | RAN | Phonological Awareness | Language | Attention | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zuk J, et al. [16] | 2021 | USA | English | TR FHD− TR FHD+ RD FHD+ | 39 18 17 | 21:18 7:11 9:8 | 5.5 ± 0.3 5.5 ± 0.3 5.7 ± 0.4 | Pre-Kindergarten; Kindergarten | Non-verbal (KBIT-2) | LWID (WRMT-R/NU and WRMT-R); Letter Sound Knowledge (YARC) | NR | NR | Objects; Colors; Letter | Elision; Blending Words (CTOPP) | Vocabulary Knowledge (PPVT-4); Sentence Comprehension (CELF-4); Speed Accuracy | NR | WM: Nonword Repetition (CTOPP), Sentence Repetition (GAPS) |
| Yu X, et al. [17] | 2020 | USA | English | TR FHD− TR FHD+ RD FHD+ | 34 35 12 | 16:18 17:18 4:8 | 5.4 ± 0.3 5.5 ± 0.4 5.8 ± 0.5 | The end of 1st grade to 4th/ grade | Non-verbal (KBIT-2) | WID (WRMT-R); SWE (TOWRE-2) | PDE (TOWRE-2); WA (WRMT-R) | NR | Objects; Colors | CTOPP | CELF-4 | NR | HLE |
| Langer N, et al. [18] | 2017 | USA | English | FHD+ FHD− | 14 18 | 7:7 10:8 | 0.9 ± 0.3 0.8 ± 0.3 | NR | NR | NA | NA | NA | NA | NA | Expressive and receptive language (MSEL) | NA | Gross and fine motor (MSEL); Visual reception (MSEL) |
| Kraft I, et al. [19] | 2016 | Germany | German | FHD+ FHD− | 25 28 | 11:14 12:16 | 5.7 ± 0.4 5.6 ± 0.4 | Kindergarten | Non-verbal | One minute word reading (SLRT-II); Spelling (DERET) | One minute pseudoword reading (SLRT-II) | NR | Subtest (BISC) | Pseudoword repetition (SETK 3-5), SS and RI (BISC), and PA (BAKO) | NR | Symbol comparison (BISC) | DS (K-ABC) |
| Vandermosten M, et al. [20] | 2015 | Belgium | Dutch | FRD+ FRD− | 36 35 | 13:23 17:18 | 5.1 ± 0.2 5.1 ± 0.2 | The last year of kindergarten | Non-verbal (Raven) | Letter knowledge productive/receptive test | NR | NR | Objects; Colors | End-phoneme and end-rhyme identification task (PA) | NR | NR | NR |
| Van Der Auwera S, et al. [21] | 2021 | Belgium | Dutch | PreR: FRD− FRD+ BR: FRD− FRD+ FR: FRD− FRD+ | 24 16 13 24 10 15 | 14:10 8:8 13:10 12:12 2:8 5:10 | 6 ± 0.1 6 ± 0.1 8 ± 0.1 8 ± 0.1 11 ± 0.1 11 ± 0.2 | Kindergarten1st/ 2nd grades 3rd/4th/5th grades | Non-verbal (Raven and Block Design– WISC-III) | Word Reading List; Letter knowledge; and Spelling | Pseudoword Reading Test | NR | NR | Phoneme Deletion and Spoonerism; PA | NR | NR | NR |
| Wang Y, et al. [22] | 2017 | USA | English | PreR: FHD− FHD+ BR: FHD− FHD+ FR: FHD− FHD+ | 16 24 23 24 10 15 | 8:8 14:10 12:12 13:10 2:8 5:10 | 5.3 ± 0.8 5.4 ± 1.1 7.0 ± 2.2 7.3 ± 2.2 10.0 ± 2.5 10.2 ± 1.8 | Nine single words 1st/2nd grades 3rd/4th/5th grades | Non-verbal (KBIT-2) | WID (WRMT-R) (FR); SWE (TOWRE) (BR and FR); and TOSWRF (FR) | PDE (TOWRE) | Gray Oral Reading Test(GORT-5), Reading Fluency WJ-III-TA | Objects (PreR); Colors | CTOPP (FR); PC (WRMT-R, BR) | CELF-4 (BR) | NR | TOMAL2 (FR) |
| Vanderauwera J, et al. [23] | 2017 | Belgium | Dutch | DYX TR FRD+ FRD− | 15 46 34 27 | 7:8 15:31 13:21 9:18 | 7.9 ± 0.1 7.9 ± 0.1 7.9 ± 0.1 7.9 ± 0.1 | PreR—prior 1st grade/ BR—2nd/3rd grade | Non-verbal (WISC) | Word reading, one-minute test (BR); Spelling (BR); and Productive/Receptive Letter Knowledge (PreR) | Pseudoword reading two minute test (BR) | NR | Objects (PreR); Colors | PA (PreR); End-phoneme and end-rhyme identification task | NA | NA | NR |
| Zhao J, et al. [24] | 2022 | France | French | Control DYX | 31 26 | 13:18 13:13 | 12 ± 1:11 ± 2 11 ± 1:12 ± 1 | NR | Verbal (WISC); Non-verbal (WISC) | Word Reading Ability (Odedys); Word Spelling-to- Dictation Test | Nonword Reading Ability (Odedys) | Alouette Test | Digits; Objects | Phoneme deletion and Spoonerism | NR | VAS (Global and Partial Letter Report Task) | Verbal WM (DS- WISC) |
| Meisler SL, [25] | 2022 | USA | English | Control DYX | 582 104 | 195:387 44:60 | 10.8 ± 3.2 10.2 ± 2.5 | NR | Non-verbal (KBIT-2) | SWE (TOWRE-2) | PDE (TOWRE-2) | NR | NR | NR | NR | NR | NR |
| Liu T, et al. [26] | 2022 | France | French | Control DYX | 31 16 | 13:18 13:13 | 11 ± 1 12 ± 1 | NR | Verbal and Non- verbal (WISC-IV) | NR; Global and Partial Letter Report Task (VAS) | NR | NR | Digits; Objects | Phoneme deletion and Spoonerism | NR | NR | Verbal WM (DS- WISC) |
| Farah R, et al. [27] | 2022 | USA | English | Control RD | 24 22 | 12:12 10:12 | 8-12 | NR | Non-verbal (TONI); Verbal (PPVT) | SWE (TOWRE); LWID (WJ-III) | PDE (TOWRE); WA (WJ-III) | NR | NR | Elision (CTOPP) | NR | Conners questionnaires and VSA (TEA-Ch) | DS (WISC); Switching/Inhibition (DKEFS, Color-Word Condition); and Overall EF (BRIEF) |
| Partanen M, et al. [28] | 2020 | Canada | English | Control DYX | 22 13 | 11:11 5:8 | Pre-test 8.5 ± 0.4 8.6 ± 0.4 Post-test 8.9 ± 0.4 8.9 ± 0.4 | 3rd Grade | Non-verbal (TONI-4) | Word Recognition Task (KTEA-II) | Decoding Task (KTEA-II) | Reading Comprehension (KTEA-II) | NR | NR | NR | NR | NR |
| Lou C, et al. [29] | 2020 | Canada | English | RD random group | 64 | 33:31:00 | 10.9 ± 1.3 | NR | NR | SWE (TOWRE) | PDE (TOWRE) | Reading Comprehension (WJ III) | Letters | NR | NR | NR | NR |
| Liu T, et al. [30] | 2021 | France | French | Control DYX | 31 26 | 13:18 13:13 | 11 ± 1 12 ± 1 | NR | Verbal and Non-verbal (WISC) | Word Reading Fluency (Odedys); Word Spelling-to-Dictation Test | Nonword Reading Fluency (Odedys) | Alouette Test | NR | NR | NR | NR | NR |
| Koirala N, et al. [31] | 2021 | USA | English | Random group | 244 | 151:34:00 | 10.2 ± 2.8 | NR | FSIQ(WISC) | SWE (TOWRE-2) | PDE (TOWRE-2) | NR | NR | Elision and Blending Words (CTOPP-2) | NR | NR | NR |
| Huber E, et al. [32] | 2021 | USA | English | Control DYX | 41 32 | 16:25 12:20 | 9.4 9.8 | NR | NR | SWE (TOWRE) | PDE (TOWRE) | WJ-RF | NR | NR | NR | NR | WJ-MFF; WJ-CALC; and WJ-BRS |
| Borghesani V, et al. [33] | 2021 | USA | English | Control DYX | 14 26 | 5:9 14:12 | 10.4 ± 1.6 10.4 ± 2.0 | 1st grade and 4th grade | Non-verbal (WASI) | SWE (TOWRE-2) | PDE (TOWRE-2) | Gray Oral Reading Test (GORT-5) | NR | NR | NR | NR | NA |
| Vander Stappen C, et al. [34] | 2020 | France | French | Control DYX | 13 18 | 5:8 9:9 | 10.5 ± 0.8 10.6 ± 1.0 | NR | Non-verbal (WISC-IV) | SWE - BALE | BALE | BALE | Objects; Colors | Syllable and phoneme deletion task | NR | NR | NR |
| El-Sady S, et al. [35] | 2020 | Egypt | Arabic | DYX | 20 | 05:15 | 8.2 ± 1 | NR | SB4 | 1 min reading DAT | nonsense passage reading DAT | 1 min reading DAT | Objects | Phonemic segmentation subtest of DAT | NR | NR | Bead threading; Postural stability; and DS |
| Wang HLS, et al. [36] | 2019 | Taiwan | Mandarin Chinese | Control DYX | 22 24 | NR | 9 ± 0.9 10 ± 1 | Primary school | Non-verbal (WISC-IV) | Chinese character recognition | NR | NR | NR | NR | NR | NR | Lexical tone awareness; auditory identification of FM test |
| Vanderauwera J, et al. [37] | 2019 | Netherlands | Dutch | TR and RD | 34 | 19:15 | 13.7 ± 0.5 | grade 8 (28), 7 (3) and 9 (3) | WISC-III-NL | One-minute word reading test | Klepel test | NR | NA | NA | NA | NA | NR |
| Lou C, et al. [38] | 2019 | France | French | Control DYX | 31 26 | 13:18 13:13 | 11.5 ± 1.4 11.6 ± 1.3 | NR | Verbal and Non-verbal (WISC) | Word reading test (Odedys) | Nonword reading test (Odedys) | Alouette test | Digits; Objects | Phoneme deletion and spoonerism | Word spelling- to-dictation test | NR | Verbal WM (DS- WISC) |
| Lebel C, et al. [39] | 2019 | USA | English | Dysfluent inaccurate Dysfluent accurate Non-impaired | 20 36 14 | 5:15 13:23 8:6 | 10.0 ± 1.2 9.4 ± 1.3 9.2 ± 1.2 | NR | WASI FSIQ | SWE (TOWRE); LWID (WJ) | PDE (TOWRE); WA (WJ) | Gray Oral ReadingTest (GORT-4) | NR | Phonological Decoding (TOWRE) | NR | NR | NR |
| Banfi C, et al. [40] | 2018 | Austria | German | TR DYX SD | 27 21 21 | 12:15 9:12 6:15 | 9 ± 0.1 9 ± 0.3 10 ± 0.6 | The end of 3rd and 4th grade | Verbal and Non-verbal (WISC) | (SRLT-II); Spelling (DRT-3) | (SRLT-II) | Sentence reading fluency(SLS) | Digits; Objects | PA | Vocabulary standard score (WISC-IV) | Parental questionnaire ADHD | Verbal WM and processing speed (DS, Symbol search WISC-IV) |
| Žarić G, et al. [41] | 2018 | Netherlands | Dutch | TR DYX | 13 15 | 8:5 7:8 | 9 ± 0.8 9 ± 0.6 | 2–3 years of reading instruction | Non-verbal (WISC) | Word reading subtest (3DM) | Pseudoword reading subtest (3DM) | NR | Letters; Digits; Objects | Phoneme deletion; Spelling; and Letter speech sound matching | NR | NR | Memory span (syllables) |
| Su M, et al. [43] | 2018 | China | Mandarin Chinese | Control DYX | 22 18 | 11:11 7:11 | 11 ± 0.8 11 ± 1.0 | Primary school | Non-verbal and Verbal (C-WISC) | Word list reading; Chinese character recognition | NR | NR | Digits | Phoneme deletion | Lexical decision; Morphological production | NR | Verbal WM (Digit recall) |
| Yagle K, et al. [42] | 2017 | USA | English | TR DYG DYX | 10 9 10 | NR | 9–14 | 4–9 grades | Non-Verbal (Wechsler) | Word reading (TOSWRF); word spelling (TOC) | Nonword reading | NR | NR | NR | NR | NR | NR |
| Christodoulou JA, et al. [44] | 2016 | USA | English | TR DYX | 26 26 | NR | 7.8 ± 0.6 7.8 ± 0.6 | NR | Non-verbal (KBIT-2) | WID (WRMT-III); SWE (TOWRE-2) | WA (WRMT-III); PDE (TOWRE-2) | NR | NR | NR | NR | NR | NR |
| Zhao JT, et al. [45] | 2016 | France | French | Control DYX | 31 26 | 13:18 13:13 | 11.5 ± 1.3 11.6 ± 1.3 | NR | Verbal and Non-verbal (WISC) | Word reading fluency (Odedys); Word spelling | Nonword reading fluency (Odedys) | Alouette Test | Digits, Objects | Word spelling- to-dictation test, Spoonerism | NR | NR | DS (WISC) |
| Koerte IK, et al. [46] | 2015 | Germany | German | Control DYX | 24 16 | 0:24 0:16 | 9.9 ± 0.3 9.7 ± 0.4 | 3rd and 4th grades | Non-verbal (CFT-20R) | SLRT-II | SLRT-II | NR | Digits, Letters, Colors, Objects | Phoneme deletion | NR | NR | DS (K-ABC); Verbal WM (Wechsler); Arithmetic test (HRT 1-4); and Number line task (WRT 1-4) |
| Garcia-Zapirain BG, et al. [47] | 2016 | Spain | Spanish | TR DYX MVR | 19 20 18 | 8:11 8:12 8:10 | 10.0 ± 0.9 10.5 ± 1.1 10.4 ± 0.9 | NR | Verbal and Non-verbal (WISC-IV) | Word reading (PROLEC-R) | Pseudoword reading (PROLEC-R) | ELFE 1-6 | NR | NR | NR | NR | WM |
| Fernandez VG, et al. [48] | 2016 | USA | English | TR DYX | 27 29 | 15:12 14:15 | 10.1 ± 2.1 12.1 ± 2.5 | 6–8 grades | Verbal and non-verbal (KBIT-2, SB4) | LWI (WJ-III); WRAT-3; and SWE (TOWRE) | PDE (TOWRE) | PC (WJ-III-TA) | NR | NR | NR | NR | NR |
| De Moura LM, et al. [49] | 2016 | Brazil | Portuguese | TR RD | 23 17 | 12:11 9:8 | 9.7 ± 0.9 9.2 ± 0.9 | NR | Verbal and Non-verbal (WISC-III) | Aloud reading (TDE) | NR | NR | NR | NR | NR | NR | NR |
| Richards TL, et al. [50] | 2015 | USA | English | Control DYX DYG | 9 17 14 | 5:4 7:10 3:11 | mean of 12.25 (from 9 to 15.6) | 4–9 grades | Verbal (WISC-IV) | Spelling dictated words (WIAT III); Sight Spelling (TOC) | NR | NR | NR | NR | NR | NR | Best and Fast writing (DASH) |
| Marino C, et al. [51] | 2014 | Italy | Italian | TR FRD+ TR FRD− DYX FRD+ DYX FRD− | 10 16 11 10 | 5:5 6:10 6:5 4:6 | 19.1 ± 1.9 18.7 ± 2.4 17.5 ± 2.4 16.4 ± 1.0 | 12.8 ± 1.5 12.0 ± 1.1 10.2 ± 1.8 10.8 ± 1.2 | Full-scale IQ (WISC-R) | Word reading(BVDDE); Spelling (BVDDE) | Non-word reading (BVDDE) | Sentences containing homophones | NR | Spoonerism, phonemic blending, and syllable displacement (PA) | NR | NR | ADC, letter and number forward/backward span (TEMA) |
| Fan Q, et al. [52] | 2014 | USA | English | Control DYX | 20 19 | 9:11 8:11 | 12.0 ± 0.7 12.0 ± 0.7 | NR | Verbal and Non-verbal (WISC-IV) | LWID (WJ-III); SWE (TOWRE); and FLI and spelling (WIST) | WA (WJ-III); PDE (TOWRE) | PC and basic reading (WJ-III); TOSCRF | Color Digit Objects (CTOPP) | NR | NR | NR | NR |
| Fan Q, et al. [53] | 2014 | USA | English | TR RD | 16 20 | 8:8 8:12 | 11.7 ± 0.7 12.1 ± 0.7 | NR | Verbal and Non-verbal (WISC-IV) | LWID (WJ-III); SWE (TOWRE); and FLI (WIST) | WA (WJ-III); PDE (TOWRE) | NR | NR | WJ-III-PC | NR | NR | NR |
| Hasan KM, et al. [54] | 2012 | USA | English | TR DYX CFP | 11 24 15 | 3:8 11:1 39:6 | 12.8 ± 1.7 13.7 ± 1.0 13.5 ± 0.8 | NR | Composite IQ (KBIT-2, SB4) | LWID (WJ-III); SWE (TOWRE) | PDE (TOWRE) | PC (WJ-III) | NR | NR | NR | NR | NR |
| Gebauer D, et al. [55] | 2012 | Austria | German | Control SI SRI | 11 11 9 | NR | 12.3 ± 1.9 11.7 ± 1.6 11.3 ± 0.7 | 4th–5th 5–9 graders | Non-verbal (Raven) | SLS 1-4 or 5-8; Spelling (HSP) | SLS 1-4 or 5-8 | ELFE 1-6 | NR | NR | NR | NR | Personality assessment FFQ (Asendropf) |
| Hoeft F, et al. [56] | 2011 | USA | English | Control DYX (rg) DYX (nrg) | 20 13 12 | 14:6 6:7 7:5 | 11.0 ± 2.6 14.5 ± 1.6 13.5 ± 2.2 | NR | Non-verbal (WASI) | (WRMT) *; SWE (TOWRE); and Spelling and writing fluency (WJ) | WA (WRMT); PDE (TOWRE) | Gray Oral ReadingTest(GORT); PC (WRMT) | Colors; Objects; Numbers; and Letters | NR | PPVT | NR | MD (CTOPP) |
| Sihvonen AJ, et al. [57] | 2021 | Finland | Finnish | Control DYX | 21 23 | 11:10 12:11 | 29.9 ± 6.0 31.3 ± 8.6 | 16.1 ± 4.4 15.7 ± 5.2 | Verbal (WAIS-III); PIQ (WAIS-IV) | Word List Reading | Pseudoword List Reading | Text Reading | Test not specified | Pig Latin; PA; phonological short-term memory; and rapid access of information | NR | ASRS v1.1 | ARHQ;Verbal WM (Non-word Span Length, WMS-III) |
| Tschentscher N, et al. [58] | 2019 | Germany | German | Control DYX | 12 12 | 0:12 0:12 | 23.7 ± 2.6 24.2 ± 2.3 | NR | Nonverbal (Raven) | Spelling | NR | Reading speed and comprehension | Letters and Numbers | NR | NR | NR | NR |
| Moreau D, et al. [59] | 2018 | New Zealand | English | Control Dyscalc DYX Comorbid | 11 11 11 12 | 4:7 5:6 5:6 5:7 | 27.7 ± 1.7 32 ± 2.2 29.4 ± 1.9 33.2 ± 1.7 | 15.2 ± 0.60 14.6 ± 0.56 15.6 ± 0.51 14.8 ± 0.61 | FSIQ (WASI) | WID (WJ) | WA (WJ) | NR | NR | NR | WRAT spelling | NR | WRAT mathematics |
| Müller-Axt C, et al. [60] | 2017 | Germany | German | Control DYX | 12 12 | 0:12 0:12 | 23.7 ± 2.6 24.2 ± 2.4 | Undergraduate students ** | Non-verbal (Raven) | Spelling | NR | NR | Numbers; Letters | NR | NR | NR | NR |
| Vandermosten M, et al. [61] | 2013 | Belgium | Dutch | TR DYX | 20 20 | 12:8 13:7 | 21.4 ± 3.0 22.1 ± 3.1 | NR | Non-verbal (WAIS-III) | Word reading; Spelling | Pseudoword reading | NR | NR | NR | NR | NR | NR |
| Lebel C, et al. [62] | 2013 | USA | English | RLD | 136 | 64:13:00 | 20.1 ± 3.1 | NR | FSIQ (WASI) | WID (WJ) | WA (WJ) | Fluency (GORT) | NR | NR | NR | NR | NR |
| Vandermosten M, et al. [63] | 2012 | Belgium | Dutch | TR DYX | 20 20 | 12:8 13:7 | 21.4 ± 3.0 22.1 ± 3.1 | NR | Non-verbal (WAIS) | Word reading; Spelling | Pseudoword reading | NR | NR | PA; Phoneme deletion and Spoonerism | Speech-in- noise perception (Dutch LIST) | NR | NR |
| Frye RE, et al. [64] | 2011 | USA | English | TR DYX/PR | 20 10 | 10:10 5:5 | 23.7 ± 0.7 23.9 ± 1.6 | NR | Non-verbal (CTONI) | LWI (WJ-III); Spelling | WA (WJ-III) | Gray Oral ReadingTest (GORT) | Colors (CTOPP); Digits (CTOPP); Objects (CTOPP); Letters (CTOPP) | PA (CTOPP); APA (CTOPP) | NR | Test of variables of attention: commissions, omissions | NR |
| DTI Acquisition | DTI Processing | DTI Outcomes | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | MRI Field | Sequence | TR/TE (ms) | Slice Number | Slice Thickness (mm) | FOV (mm) | b-Value (s/mm2) | N. of Diffusion Gradients | Time | Software | Corrections | Type of Analyses | DTI Metrics | Atlas | ROIs/ TRACTS | Tracts Difference between Groups | Clinical Correlations |
| Zuk J, et al. [16] | Siemens 3T | DTI | NR | 30 | 2 | 128x128 | 0; 700 | NR | NR | DTIprep, VISTALab | EC, HM (>2 mm or >0.5°) | ROI | FA | NR | AF, SLF | ↑FA in r-SLF of FHD+ TR compared to FHD− TR and FHD+ RD | r-SLF FA, age, gender, parent education, occupation, and phonological awareness significantly predicted decoding skills among children FHD+ |
| Yu X, et al. [17] | Siemens 3T | DTI | NR | NR | 2 | NR | 0; 700; 1000 | NR | NR | DTIprep, VISTALab, AFQ | EC, HM (>2 mm/0.5°), bed vibration, pulsation, venetian blind artifacts, and slice and gradient-wise intensity inconsistencies | Whole brain; ROI | FA | MNI, Native space | Right of SLF, ILF, and AF, sCC, CC2 | ↑FA in r-sCC of FHD+ TR compared to FHD− TR | R-sCC FA had positive correlation with r-IFG activation for FHD−/+ TR |
| Langer N, et al. [18] | Siemens 3T | DTI | 8320/88 | 64 | 2 | 256x256 | 1000 | 30 | 5:59 min | DTIprep, FSL (DTIFIT), Trackvis (Diffusion Toolkit), Trackvis, AFQ | EC and HM (>2 mm and 0.5°) | Whole brain; ROI | FA RD AD | MNI | Bilateral AF and CS | ↓FA in l-AF (central portion) FHD+ compared with FHD−, corrected by age | l-AF FA has positive correlation with age, expressive language |
| Kraft I, et al. [19] | Siemens 3T | DTI | 8000/NR | 66 | 1.9 | NR | 1000 | 60 | 32 min | FSL (Topup tool), FSL (DTIFIT), MRTrix | EC, HM, and susceptibility- induced distortions | ROI | FA | Destrieux Atlas | SMG, ITG (anterior, long, and posterior AF), SOS/TOS, IFoG, IFobG | No group difference | l-aAF was the best predictor of DYX |
| Vanderm- osten M, et al. [20] | Philips 3T | DTI | 7600/65 | 58 | 2.5 | 200x240 | 1300 | 60 | 10 min 32 s | Explore DTI, Trackvis | EC, HM (6 parameters) Reorientation of the b-matrix Motion as covariate | Whole brain; ROI | FA | TrackVis | AF (dorsal FTP, dorsal post TP), ventral IFOF | ↓FA in l-IFOF of FHD+ | Phonological awareness positive correlation with FA of l-AF(TP) and bilateral IFOF/AF-FTP, as also left ventral tracts in FHD+ |
| Van Der Auwera S, et al. [21] | Philips 3T | DTI | 7600/65 | NR | 2.5 | NR | 1300 | 60 | 10:32 min | FSL, VISTALab, AFQ | EC, HM by root mean square | Whole brain; ROI | FA MD | NR | AF | ↓FA in the l-AF in pre-reader RDs | aAF FA was a significant predictor for scores on word reading tests from 2nd grade |
| Wang Y, et al. [22] | Siemens 3T | DTI | 8320/88 | NR | NR | 256x256 | 1000 | 30 | 5:59 min | DTIprep, VISTALab, AFQ | EC, HM (>2 mm and >0.5°) | Whole brain; ROI | FA AD RD | white matter atlas | Left of AF, SLF, ILF | ↓l-AF FA at pre-reader FHD+ versus FHD− and for poor versus good readers all ages; FHD+ good readers had faster WM development in r-SLF compared to poor readers | l-AF and ILF FA positive correlations with word identification skill |
| Vanderau- wera J, et al. [23] | Philips 3T | DTI | 7600/65 | NR | 2.5 | NR | 1300 | 60 | 10:32 min | ExploreDTI, Trackvis | EC, HM | ROI | FA | native space | Long, anterior and posterior dorsal AF, and ventral IFOF | ↑FA in all groups over time. ↓ long AF FA in DYX prior to reading onset, right also kept in early reading. Influence of FHD+ in l-IFOF and long r-AF | FHD+ and rapid naming predicted 80.3% of cases; the l-longAF FA values predicted 84.4% of DYX cases |
| Zhao J. et al. [24] | Siemens 3T | DTI | 14,000/91 | 70 | 1.7 | 218 | 1400 | 60 | 18 min | Explore DTI, Trackvis, FSL | NR | Whole brain | FA | TrackVis MNI-152 | UF, FAT | Males DYX had a ↓HMOA in the UF compared with males TR | HMOA of the UF showed a positive correlation with VAS in DYXs |
| Meisler SL, [25] | Siemens 3T | DKI | 3320/100.2 | NR | 1.8 | NR | 0; 1000; 2000 | 64 | NR | QSIPrep, MRtrix, FSL, and TractSeg | Gibbs unringing, EC, HM, and AP-PA field | Whole brain | FA | FSL and MNI | AF, SLF (I, II, and III), ILF, IFOF, UF, SCP, ICP, MCP, and sCC | No group difference | Age and sex with gFA positive correlation; in older children, FA in r-SLF and l-ICP related to nonword reading skills |
| Liu T, et al. [26] | Siemens 3T | DTI | 14,000/91 | 70 | 1.7 | 218 | 1400 | 60 | 18 min | PANDA, FSL, and Trackvis | EC | Whole brain | FA | MNI and AAL atlas | 90 ROIs of AAL | NR | Positive correlation between node FA for l-SOG and VAS score, l-MTG and l-ORBsupmed and phonological score |
| Farah R, et al. [27] | Philips 3T | DTI | 6652.446/82.6 | 160 | 2 | 224x120x224 | 1000 | 61 | 7 min 25 s | VISTALab, AFQ | EC, HM | Whole brain; ROI | FA | NR | AF, SLF, ILF | ↓ FA in the left of AF, ILF, and SLF in RD | ↓ FA in the l-SLF positive correlated with reading and working memory score in DYX |
| Partanen M, et al. [28] | GE 3T | DTI | 7000/60 | 60 | 2 | 256x256 | 0; 1000 | 60 | 7.5 min | TORTOISE, FDT (FSL), DTIFIT (FSL), and PROBTRACKX (FSL) | EC, HM | Whole brain; ROI | FA MD | MNI305 and Desikan–Killiany atlas | bilateral IFG, Ins, STG, SMG, AnG, and FFG | ↑MD in bilateral Ins; l-IFtG, l-STG, and r-SMG in DYX | SMG, r-IFoG, and l-Ins MD had negative correlation with reading gains and decoding, respectively |
| Lou C, et al. [29] | Siemens 3T | DTI | 3000/50.6 | 64 | 2 | 256x256 | 0; 1000 | 56 | NR | ExploreDTI | EC, HM, EPI distortions | Whole brain; ROI | FA | AAL and MNI152 | 90 ROIs of AAL | NR | IFtG and IFoG, Ins, FFG, IPL, SMG, AnG, HG, STG, MTG, ITG, IOG, PreCG, ROL, and thalamus in the left hemisphere positive correlated with reading efficiency and phonemic decoding, mainly for girls DYX |
| Liu T, et al. [30] | Siemens 3T | DTI | 14,000/91 | 70 | 1.7 | 218x218 | 0; 1400 | 60 | 18 min | PANDA, FSL | EC, HM | Whole brain; ROI | FA | AAL and MNI | 90 ROIs of AAL | NR | Negative correlation between READACC (pseudoword/word reading) and the r-FFG FA in DYX |
| Koirala N, et al. [31] | Siemens 3T | DTI | NR | NR | 1.8 | NR | 0; 1000; 2000 | 64 | NR | FSL (QUAD), FSL (DTIFIT), and FSL (BEDPOSTX), XTRACT | Susceptibility, EC, and HM | Whole brain; ROI | FA MD RD ODI NDI | Native space | 23 tracts (including SLF, which seeds were central sulcus, SFG, ACG, MFG, and AnG) | NR | Positive correlation between phonological processing and the left IFOF, MDLF, SLF2, VOF, CBD and FX FA, and the l- UF MD |
| Huber E, et al. [32] | Phillips 3T | DKI | NR | NR | 2 | NR | 0; 800; 2000 | 32 and 64 | NR | FSL, DIPY, MRTrix, and AFQ | AP-PA, EC, Mean slice-by-slice displacement > 3 mm | Whole brain | FA MD AWF Da MDe | NR | AF, CS, UF, SLF, ILF, ThR, FMj, FMn, and IFOF | l-AF MD difference for Group x time interaction | Positive correlation between MD of l-AF, UF, l-ILF, l-IFOF, FMj, MDe of left of AF, UF, ILF, IFOF, and FMj with word reading and negative correlation between AWF of right ILF, IFOF, and FMn with word reading |
| Borghesani V, et al. [33] | Siemens 3T | DKI | 8200/86 | 60 | 2.2 | 220x220 | 0; 700; 20,000 | 30 and 64 | 15 min | FSL (NODDI model), FS-TRACULA | AP-PA, EC, and HM | Voxel-based; ROI | NDI ODI | FSL, Desikan–Killiany Atlas and MNI | l-VOT | ↑ODI in DYS at the l-VOT | NR |
| Vander Stappen C, et al. [34] | Philips 3T | DTI | 6422/83 | 70 | 2 | 224x224 | 800 | 55 | NR | BrainVoyager | EC, HM | ROI | FA | Talairach space | AF, IFOF, and ILF | NR | RAN Gains negative correlated with FA in the l-long aAF, and the r-pAF, a reduction in naming times was linked to an increase in FA in those tracts at DYX |
| El-Sady S, et al. [35] | Philips 1.5T | DTI | NR | 70 | 2 | 230x230 | NR | 32 | NR | NR | EC, HM | ROI | FA ADC | NR | SLF, AF, CR, PLIC of CS | NR | Negative correlation between r-AF FA and at-risk quotient, l-sCR ADC with writing, and r-SLF ADC with bDS and positive with VF.Positive correlation between l-SLF-aCR FA and RAN, spelling, and VF, as r-PLIC ADC with writhing, and l-aCR ADC with bDS |
| Wang HLS, et al. [36] | Siemens 3T | DTI | 6700/97 | NR | 2.7 | NR | 5000 | 128 | NR | DSI Studio | NR | Whole brain | NR | MNI, AAL atlas | IFOF, CC, cerebellar, and Tha-pontine tracts | NR | l-IFOF, cerebellar, and Tha-pontine tracts had positive correlated with chinese character recognition; pCC association with auditory FM processing in DD |
| Vanderauwera J, et al, [37] | Phillips 3T | DTI | 8872/2.5 | 55 | 2.5 | 240x240x137.5 | 1000 | 60 | 13:52 min | ExploreDTI, Trackvis | HM (>1.5 mm) and EC | ROI | FA | Native Space | AF, IFOF, UF, and ILF | NR | Word reading had positive correlation with l-long-AF FA and negative with l-long-AF RD and UF RD. Paternal educational level had positive correlation with l-long AF FA, and UF FA; after covariate by HM, only the l-UF remained significant |
| Lou C, et al. [38] | Siemens 3T | DTI | 14,000/91 | 70 | 1.7 | 218 | 0; 1400 | 60 | 18 min (3x6 min) | ExploreDTI, FSL (FLIRT) | EC, HM | Whole brain | FA | AAL atlas; MNI; Harvard-Oxford atlas | Left of MTG-MOG, MOG-TPOsup, TPOsup-HG, HG-ROL, Ins-ROL, STG-Ins, and Ins-SMG | ↓mean FA in DYX for all ROIs | Literacy skills had positive correlation with clustering coefficient, local efficiency, transitivity, and global efficiency, in DYX |
| Lebel C, et al. [39] | Siemens 1.5T | SE EPI | 9000/85 | 28 | 5 | 240x240 | 1000 | NR | 7:24 min | FSL | Motion artifacts (signal drop out, venetian blind artifact, and mechanical vibration artifact, >10), EC | ROI | FA MD AD RD | MNI; JHU ICBM- DTI-81 atlas | sCC, ALIC of CS, aCR, pCR, SS (includes the ILF and IFOF), UF, and SLF | ↓MD in r-CR, and l-UF in DYX | Age had a positive correlation with pCR, r-SLF FA, negative with pCR, l-UF MD. Sight words and VF were positively correlated with l-SLF FA and MD, respectively, as well as with l-pCR MD. Phonological decoding had a negative correlation with r-pCR MD and mean MD and positive with mean FA |
| Banfi C, et al. [40] | Siemens 3T | DTI | 3400/105 | 48 | 2.5 | 240 | 0; 2000 | 64 | NR | MRTrix, FSL, and AFQ | AP-PA, EC, HM, and susceptibility- induced distortion | Whole brain | FA | NR | ThR, FMj, FMn, IFOF, ILF, SLF, and AF, UF, CS, and CG | ↑FA in ILF, r-SLF, and r-CG in DYX | Negative correlation between r-ILF FA and reading measures, controlling for spelling. |
| Žarić G, et al. [41] | Siemens 3T | DTI | 10,800/84(protocol1) 11,000/85(protocol2) | 85 | 1.8 | NR | 0; 1000 | 72 | 15 min | VISTALab (mrDiffusion), SPM, AFQ | EC, HM and phase-encoding direction corrections | Whole brain; ROI | FA | NR | AF, SLF, ILF, IFOF, UF, lCS, antThR, FMn, and FMj | ↑FA in the AF, r-SLF, and aThR in DYX | r-SLF showed age effects that differed between groups. Age effect in ILF FA, and CC (FMj and FMn). L-aThR positive correlation with age appropriate reading accuracy scores |
| Su M, Zhao J, et al. [43] | Siemens 3T | DTI | 8000/89 | NR | 2.2 | 282x282 | 0; 1000 | 30 | NR (repeated twice) | ExploreDTI, Trackvis, and FSL | EC and HM | Whole brain; ROI | FA RD AD | MNI152; native space | AF, IFOF, and ILF | ↓FA and AD in the l-AF and I-ILF in DYX | AF and ILF FA positive correlation with character recognition, digit recall, phoneme deletion (only AF), and morphological production (only ILF). ILF FA negative correlation with RAN |
| Yagle K, et al. [42] | NR | DTI | 8593/78 | NR | 2 | 220x220x128 | 0; 1000 | 32 | 9:35 min | FSL | NR | ROI | FA RA AD RD MD | NR | OR, CS, ILF, SLF, and CG | ↓FA in l-OR in DYX | NR |
| Christod- oulou JA, et al. [44] | Siemens 3T | DTI | 9300/84 | 74 | 2 | 256 | 0; 700 | 30 | NR | FS-TRACULA, DTIprep, and FSL (FLIRT) | EC, HM | Tract-based | FA AD RD | MNI152 | SLF, AF | ↓FA in the l-AF in RDs | Positive correlation of l-AF FA and negative DA with real-word reading |
| Zhao JT, et al. [45] | Siemens 3T | DTI | 14,000/91 | 70 | 1.7 | 218 | 0; 1400 | 60 | 18 min | ExploreDTI, FSL, and Trackvis | Motion corrections | Whole brain; ROI | HMOA FA | MNI152 | IFOF, ILF, SLF, and AF | ↓FA of r-IFOF and l-SLF in DYX | r-IFOF FA negative correlation with reading and spelling accuracy |
| Koerte IK, et al. [46] | Siemens 3T | NR | 9600/110 | 65 | 2 | 208 | 0; 1000 | 30 | NR | 3DSlicer, FSL (FLIRT), and FSL (TBSS) | EC, HM | Tract-based | FA AD RD trace | MNI152 | NR | No group difference | Positive correlation arithmetic test with FA and AD and negative with RD (Temporo-parietal) |
| Garcia-Zapirain BG, et al. [47] | Philips 3T | DTI | 6819/81 | 60 | 2 | 224x224 | 800 | 15 | 7min | FSL (BET), FSL (FDT), and FSL (TBSS) | NR | Whole-brain; ROI | FA MD AD RD | MNI; Atlas JHU White- matter | CC, SLF, ILF, lower FOF, l-AF, IFOF | ↓FA in l-AF in DYX | NR |
| Fernandez VG, et al. [48] | Philips 3T | DTI | 6100/84 | 44 | 3 | 240x240 | 0; 1000 | 21 | NR | FSL (DTIFIT) | EC, HM | ROI | FA AD RD | Desikan and Destrieux atlases | LAC/RAC to bilateral TP, OT, and IFG | ↑FA of cerebellar to TP and IFG; ↓RD in TP in DYX | FA of AC-OT had interaction between age and group, younger DYX have ↓FA in this region. |
| De Moura LM, et al. [49] | GE 1.5T | DTI | 11,600/99 | 47 | 3 | 240x240 | 0; 800 | 15 | NR | FSL, FSL(TBSS) | EC correction and non brain voxels removed | Voxel-based | FA RD MD AD | MNI152 | aThR, CG, CS, IFOF, ILF, UF, FMj, FMn, and CGH | ↓FA left of aThR, CG, CS, FMj, FMn, UF, right of IFOF, ILF ↑RD in the left of CG, CS, and SLF in DYX | NR |
| Richards TL, et al. [50] | Philips 3T | DTI | 8593/78 | NR | 2.0 | 220x220x128 | 0; 1000 | 32 | 9 min 35 s | DTIPre (GTRAC), FSL (TBSS), and FSL | NR | ROI | FA AD RD RA MD | FSL white matter atlas (FHU) | aThR, FMn, CS, SLF, ILF, IFOF, UF, and CG | ↓RA in aThR, IFOF, SLF, UF, and l-CG, and FMn; ↓AD in CS, r-ThR, CG, IFOF, SLF, and UF in DYX | NR |
| Marino C, et al. [51] | Philips 3T | DTI | 9775/58 | NR | 2.3 | NR | 0; 1000 | 35 | NR | BrainVoyager (Brainvisa), SPM | EC, smooth 6 mm | Voxel-based | FA | White matter atlases of FSL | ILF, IFOF, AF, SLF, CC, and OR | NR | DYX with DCDC2d gene x without found ↓FA in ILF and l-CC |
| Fan Q, et al. [52] | Philips 3T | DTI | 6237/75 | 60 | 2.2 | 212x212 | 0; 700 | 32 | 3 min 32 s | FSL (FDT), FS-TRACULA | EC, HM | ROI | FA | Desikan–Killiany Atlas | Thalamus to OFC, MPFC, LPFC, SMC, PC, MTC, LTC, OCC, and Ins | ↑FA of LPFC and SMC to ThR in DYX | Th-SMC showed negative correlation with basic reading score |
| Fan Q, et al. [53] | Philips 3T | DTI | 6237/75 | 60 | 2.2 | 212x212 | 700 | 32 | 3 min 38s | FSL | EC, HM | ROI | NR | MNI152 | 5 ROIs of l-OT/F, MTG, ITG, LOCC, PaHipp, and ILF | Left Mid, Inf and sup- TG, lingual, fusiform, Sup and Inf PG in DYX | NR |
| Hasan KM, et al. [54] | Philips 3T | DTI | 6100/84 | 44 | 3 | NR | 1000 | 21 | min | NR | NR | ROI | FA MD AD RD Dav | NR | CC | ↑mFA of CC in DYX | MD and AD correlation with age (CC2); MD positive correlated with Letter- Word ID test in CC5 |
| Gebauer D, et al. [55] | Siemens 3T | DTI | 6700/95 | 35 | 2.5 | 250 | NR | NR | NR | FSL (TBSS, FDT, DTIFIT, and BET) | EC | Voxel-based | FA | JHU ICBM-DTI-81 White-Matter Labels | aCR, CC | ↓FA in the l-aCR and aCC | NR |
| Hoeft F, et al. [56] | GE 3T | DTI | 11,600/64.5 | 23 | 4 | 240 | 800 | 13 | NR | SPM, DTIStudio, and ROQS | EC, HM | Whole-brain | FA | NR | SLF | NR | Positive correlation between r-SLF FA and single-word reading |
| Sihvonen AJ, et al. [57] | Siemens 3T | DTI | 9000/80 | 70 | 2.5 | 240x240 | 0; 1000 | 64 | NR | MRTrix, DSI Studio | Thermal noise with MP-PCA, Gibbs ringing correction | Whole brain | QA | MNI using (QSDR) | NR | ↓QA in VOF, SLF, AF, CC, CSl-UF, and ThR; ↑QA in l-SLF, VOF, and CS in DYX | Reading skill positive association with l-CG and right fornix, and frontal corticopontine tracts and cerebellum |
| Tschentscher N, et al. [58] | Siemens 3T | DTI | 12,900/100 | 88 | 1.7 | 220x220 | 0; 1000 | 60 | 6 min | FSL (FDT), FSL (PROBTRACKX), and FSL (BEDPOSTX) | Head motion corrections | Voxel-based; ROI | FA | MNI; Juelich histological; Harvard-Oxford atlases | A1, l-mPT, and MGB, IC | ↓connectivity between l-mPT-MGB in DYX | Negative correlation of l- mPT-MGB with reading skills in TR |
| Moreau D, et al. [59] | Siemens 1.5T | DTI | 6601/101 | NR | 3 | 230 | 0; 1000 | 30 | NR | FSL (DTIFIT), FSL (FLIRT), and FSL (TBSS) | EC and motion corrections | Whole brain; Voxel-based | FA | MNI152 | Bilateral CR and AF | No group difference | NR |
| Müller-Axt C, et al. [60] | Siemens 3T | DTI | 12,900/100 | 88 | 1.7 | 220x220 | 0; 1000 | 60 | 6 min | FSL | Motion correction | ROI | FA | Talairach; MNI; Juelich Histological atlas | LGN, l-V1, V5/MT | ↓LGN FA and between l-V5/MT-LGA in DYX | DYX showed negative correlation between l- V5/MT-LGN and name letters and numbers aloud time |
| Vanderm- osten M, et al. [61] | Philips 3T | DTI | 11,043/55 | 68 | 2.2 | 220x220 | 0; 800 | 45 | 21 min 8 s | Explore DTI, FSL (CATNAP) | EC and motion- induced artifacts | Whole-brain; ROI | FA | Harvard-Oxford atlas in MNI space | Post STG, AF, sCC | NR | Positive correlation between coherence 20 Hz and FA of the STGp Lat and sCC in DYX and a negative in HC, without outliers |
| Lebel C, et al. [62] | Siemens 1.5T | DTI | 9000/85 | 28 | 5 | 240x240 | 0; 1000 | 6 | 7 min 24 s | SPM | Smooth of 4 mm kernel | Voxel-based | FA MD | ICBM template | ALIC, sCC, ThR, CR, ILF, IFOF, anf aCR | NR | GORT fluency positive correleted with FA of aCC, sCC, right: aLimb, SLF, MCP, aCR, ILF, l-sCC, Th, IFOF; Word attack with FA of aCC, SLF, aLimb; l-Th, SLF, and r-IFOF |
| Vandermosten M, et al. [63] | Philips 3T | DTI | 11,043/55 | 68 | 2.2 | 220x220 | 0; 800 | 45 | 21 min 8 s | Explore DTI, FSL (CATNAP) | EC, motion-induced artifacts correction | ROI | FA RD AD | Native space | AF, IFOF | ↓FA of l-AF in DYX | Direct and l-aAF FA positive correlated with phoneme awareness, and speech perception, respectively, and l-IFOF with orthography |
| Frye RE, et al. [64] | Philips 3T | DTI | 6100/84 | 44 | 3 | 240x240 | 1000 | NR | 7 min | SPM | Distortion correction, masking, and isotropic voxel interpolation | Whole-brain | FA AD RD Dav | ICBM | FTP, SLF, SFOF, IFOF, and CR | No group difference | Negative correlated: FA- word attack in SLF, SFOF, aCR, and pCR; Dav- word attack in SLF; and positive correlation: Dav and AD—word attack in SFOF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, B.; Baba, M.Y.; Dimateo, E.M.; Costa, L.F.; Camara, A.S.; Lukasova, K.; Nucci, M.P. Investigating Dyslexia through Diffusion Tensor Imaging across Ages: A Systematic Review. Brain Sci. 2024, 14, 349. https://doi.org/10.3390/brainsci14040349
Martins B, Baba MY, Dimateo EM, Costa LF, Camara AS, Lukasova K, Nucci MP. Investigating Dyslexia through Diffusion Tensor Imaging across Ages: A Systematic Review. Brain Sciences. 2024; 14(4):349. https://doi.org/10.3390/brainsci14040349
Chicago/Turabian StyleMartins, Bruce, Mariana Yumi Baba, Elisa Monteiro Dimateo, Leticia Fruchi Costa, Aila Silveira Camara, Katerina Lukasova, and Mariana Penteado Nucci. 2024. "Investigating Dyslexia through Diffusion Tensor Imaging across Ages: A Systematic Review" Brain Sciences 14, no. 4: 349. https://doi.org/10.3390/brainsci14040349
APA StyleMartins, B., Baba, M. Y., Dimateo, E. M., Costa, L. F., Camara, A. S., Lukasova, K., & Nucci, M. P. (2024). Investigating Dyslexia through Diffusion Tensor Imaging across Ages: A Systematic Review. Brain Sciences, 14(4), 349. https://doi.org/10.3390/brainsci14040349







