Selective Alteration of the Left Arcuate Fasciculus in Two Patients Affected by Creatine Transporter Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Tractography
2.2. Patients
2.3. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Entry—#300352—Cerebral Creatine Deficiency Syndrome 1; CCDS1—OMIM. Available online: https://www.omim.org/entry/300352 (accessed on 15 October 2023).
- van de Kamp, J.M.; Mancini, G.M.; Salomons, G.S. X-Linked Creatine Transporter Deficiency: Clinical Aspects and Pathophysiology. J. Inherit. Metab. Dis. 2014, 37, 715–733. Available online: https://onlinelibrary.wiley.com/doi/10.1007/s10545-014-9713-8 (accessed on 14 October 2023). [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and Creatinine Metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A. Chapter 188—Creatine Deficiency Syndromes. In Handbook of Clinical Neurology; Dulac, O., Lassonde, M., Sarnat, H.B., Eds.; Pediatric Neurology Part III; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1837–1843. [Google Scholar]
- Comeaux, M.S.; Wang, J.; Wang, G.; Kleppe, S.; Zhang, V.W.; Schmitt, E.S.; Craigen, W.J.; Renaud, D.; Sun, Q.; Wong, L.-J. Biochemical, Molecular, and Clinical Diagnoses of Patients with Cerebral Creatine Deficiency Syndromes. Mol. Genet. Metab. 2013, 109, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Ghirardini, E.; Calugi, F.; Sagona, G.; Di Vetta, F.; Palma, M.; Battini, R.; Cioni, G.; Pizzorusso, T.; Baroncelli, L. The Role of Preclinical Models in Creatine Transporter Deficiency: Neurobiological Mechanisms, Biomarkers and Therapeutic Development. Genes 2021, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Duran-Trio, L.; Fernandes-Pires, G.; Grosse, J.; Soro-Arnaiz, I.; Roux-Petronelli, C.; Binz, P.-A.; De Bock, K.; Cudalbu, C.; Sandi, C.; Braissant, O. Creatine Transporter-Deficient Rat Model Shows Motor Dysfunction, Cerebellar Alterations, and Muscle Creatine Deficiency without Muscle Atrophy. J. Inherit. Metab. Dis. 2022, 45, 278–291. [Google Scholar] [CrossRef] [PubMed]
- deGrauw, T.J.; Salomons, G.S.; Cecil, K.M.; Chuck, G.; Newmeyer, A.; Schapiro, M.B.; Jakobs, C. Congenital Creatine Transporter Deficiency. Neuropediatrics 2002, 33, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, M.; Jaggumantri, S.; Sargent, M.; Stockler-Ipsiroglu, S.; Van Karnebeek, C.D.M. Treatment of X-Linked Creatine Transporter (SLC6A8) Deficiency: Systematic Review of the Literature and Three New Cases. Mol. Genet. Metab. 2014, 112, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Battini, R. Pre-Symptomatic Treatment of Creatine Biosynthesis Defects. Subcell. Biochem. 2007, 46, 167–181. [Google Scholar] [CrossRef]
- Stockler, S.; Schutz, P.W.; Salomons, G.S. Cerebral Creatine Deficiency Syndromes: Clinical Aspects, Treatment and Pathophysiology. Subcell. Biochem. 2007, 46, 149–166. [Google Scholar] [CrossRef]
- Ducray, A.D.; Schläppi, J.-A.; Qualls, R.; Andres, R.H.; Seiler, R.W.; Schlattner, U.; Wallimann, T.; Widmer, H.R. Creatine Treatment Promotes Differentiation of GABA-Ergic Neuronal Precursors in Cultured Fetal Rat Spinal Cord. J. Neurosci. Res. 2007, 85, 1863–1875. [Google Scholar] [CrossRef]
- Di Santo, S.; Mina, A.; Ducray, A.; Widmer, H.R.; Senn, P. Creatine Supports Propagation and Promotes Neuronal Differentiation of Inner Ear Progenitor Cells. Neuroreport 2014, 25, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Van De Kamp, J.M.; Betsalel, O.T.; Mercimek-Mahmutoglu, S.; Abulhoul, L.; Grünewald, S.; Anselm, I.; Azzouz, H.; Bratkovic, D.; De Brouwer, A.; Hamel, B.; et al. Phenotype and Genotype in 101 Males with X-Linked Creatine Transporter Deficiency. J. Med. Genet. 2013, 50, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Heussinger, N.; Saake, M.; Mennecke, A.; Dörr, H.-G.; Trollmann, R. Variable White Matter Atrophy and Intellectual Development in a Family With X-Linked Creatine Transporter Deficiency Despite Genotypic Homogeneity. Pediatr. Neurol. 2017, 67, 45–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stockler-Ipsiroglu, S.; van Karnebeek, C.D.M. Cerebral Creatine Deficiencies: A Group of Treatable Intellectual Developmental Disorders. Semin. Neurol. 2014, 34, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Parents Find the Cause for Their Son’s Speech Delays with a Genetic Test. Available online: https://www.usatoday.com/story/sponsor-story/association-for-creatine-deficiencies/2020/11/04/parents-find-cause-their-sons-speech-delays-genetic-test/6137248002/ (accessed on 7 February 2024).
- Paldino, M.J.; Hedges, K.; Golriz, F. The Arcuate Fasciculus and Language Development in a Cohort of Pediatric Patients with Malformations of Cortical Development. AJNR Am. J. Neuroradiol. 2016, 37, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, B.; Descoteaux, M.; Mori, S.; Leemans, A. Diffusion MRI Fiber Tractography of the Brain. NMR Biomed. 2019, 32, e3785. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.V.; Zhong, A.; Turken, A.; Baldo, J.V.; Dronkers, N.F. Functional Contributions of the Arcuate Fasciculus to Language Processing. Front. Hum. Neurosci. 2021, 15, 672665. [Google Scholar] [CrossRef] [PubMed]
- Pardini, M.; Yaldizli, Ö.; Sethi, V.; Muhlert, N.; Liu, Z.; Samson, R.S.; Altmann, D.R.; Ron, M.A.; Wheeler-Kingshott, C.A.M.; Miller, D.H.; et al. Motor Network Efficiency and Disability in Multiple Sclerosis. Neurology 2015, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Kumar, S.; Williamson, V.J.; Scholz, J.; Griffiths, T.D.; Stewart, L. Detection of the Arcuate Fasciculus in Congenital Amusia Depends on the Tractography Algorithm. Front. Psychol. 2015, 6, 9. [Google Scholar] [CrossRef]
- Item, C.B.; Stöckler-Ipsiroglu, S.; Stromberger, C.; Mühl, A.; Alessandrì, M.G.; Bianchi, M.C.; Tosetti, M.; Fornai, F.; Cioni, G. Arginine:Glycine Amidinotransferase Deficiency: The Third Inborn Error of Creatine Metabolism in Humans. Am. J. Hum. Genet. 2001, 69, 1127–1133. [Google Scholar] [CrossRef]
- Stöckler, S.; Isbrandt, D.; Hanefeld, F.; Schmidt, B.; von Figura, K. Guanidinoacetate Methyltransferase Deficiency: The First Inborn Error of Creatine Metabolism in Man. Am. J. Hum. Genet. 1996, 58, 914–922. [Google Scholar] [PubMed]
- Greenhaff, P.L. The Creatine-Phosphocreatine System: There’s More than One Song in Its Repertoire. J. Physiol. 2001, 537, 657. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A. Creatine Deficiency Syndromes. Handb. Clin. Neurol. 2013, 113, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.C.; Tosetti, M.; Fornai, F.; Alessandri’, M.G.; Cipriani, P.; De Vito, G.; Canapicchi, R. Reversible Brain Creatine Deficiency in Two Sisters with Normal Blood Creatine Level. Ann. Neurol. 2000, 47, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, K.T.; Sijens, P.E.; Schulze, A.; Lunsing, R.J.; Jakobs, C.; Salomons, G.S.; van Spronsen, F.J. Successful Treatment of a Guanidinoacetate Methyltransferase Deficient Patient: Findings with Relevance to Treatment Strategy and Pathophysiology. Mol. Genet. Metab. 2007, 91, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Battini, R.; Alessandrì, M.G.; Leuzzi, V.; Moro, F.; Tosetti, M.; Bianchi, M.C.; Cioni, G. Arginine:Glycine Amidinotransferase (AGAT) Deficiency in a Newborn: Early Treatment Can Prevent Phenotypic Expression of the Disease. J. Pediatr. 2006, 148, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Sartini, S.; Sestili, P.; Colombo, E.; Martinelli, C.; Bartolini, F.; Ciuffoli, S.; Lattanzi, D.; Sisti, D.; Cuppini, R. Creatine Affects In Vitro Electrophysiological Maturation of Neuroblasts and Protects Them from Oxidative Stress. J. Neurosci. Res. 2012, 90, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.H.; Ducray, A.D.; Huber, A.W.; Pérez-Bouza, A.; Krebs, S.H.; Schlattner, U.; Seiler, R.W.; Wallimann, T.; Widmer, H.R. Effects of Creatine Treatment on Survival and Differentiation of GABA-Ergic Neurons in Cultured Striatal Tissue. J. Neurochem. 2005, 95, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Henry, H.; Villard, A.-M.; Zurich, M.-G.; Loup, M.; Eilers, B.; Parlascino, G.; Matter, E.; Boulat, O.; Honegger, P.; et al. Ammonium-Induced Impairment of Axonal Growth Is Prevented through Glial Creatine. J. Neurosci. 2002, 22, 9810–9820. [Google Scholar] [CrossRef]
- Chamberlain, K.A.; Chapey, K.S.; Nanescu, S.E.; Huang, J.K. Creatine Enhances Mitochondrial-Mediated Oligodendrocyte Survival After Demyelinating Injury. J. Neurosci. 2017, 37, 1479–1492. [Google Scholar] [CrossRef]
- Rosko, L.M.; Gentile, T.; Smith, V.N.; Manavi, Z.; Melchor, G.S.; Hu, J.; Shults, N.V.; Albanese, C.; Lee, Y.; Rodriguez, O.; et al. Cerebral Creatine Deficiency Affects the Timing of Oligodendrocyte Myelination. J. Neurosci. 2023, 43, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Rosko, L.; Gentile, T.; Smith, V.; Huang, J.K. 86583 The Role of Creatine in Developmental Myelination and Remyelination. J. Clin. Transl. Sci. 2021, 5, 99. [Google Scholar] [CrossRef]
- Catani, M.; Piccirilli, M.; Cherubini, A.; Tarducci, R.; Sciarma, T.; Gobbi, G.; Pelliccioli, G.; Petrillo, S.M.; Senin, U.; Mecocci, P. Axonal Injury within Language Network in Primary Progressive Aphasia. Ann. Neurol. 2003, 53, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Anselm, I.M.; Alkuraya, F.S.; Salomons, G.S.; Jakobs, C.; Fulton, A.B.; Mazumdar, M.; Rivkin, M.; Frye, R.; Poussaint, T.Y.; Marsden, D. X-Linked Creatine Transporter Defect: A Report on Two Unrelated Boys with a Severe Clinical Phenotype. J. Inherit. Metab. Dis. 2006, 29, 214–219. [Google Scholar] [CrossRef]
- Morey, K.; Hallinan, B.; Cecil, K.M. Case Report: Clinical and Magnetic Resonance Spectroscopy Presentation of a Female Severely Affected with X-Linked Creatine Transporter Deficiency. Radiol. Case Rep. 2022, 17, 1115–1119. [Google Scholar] [CrossRef]
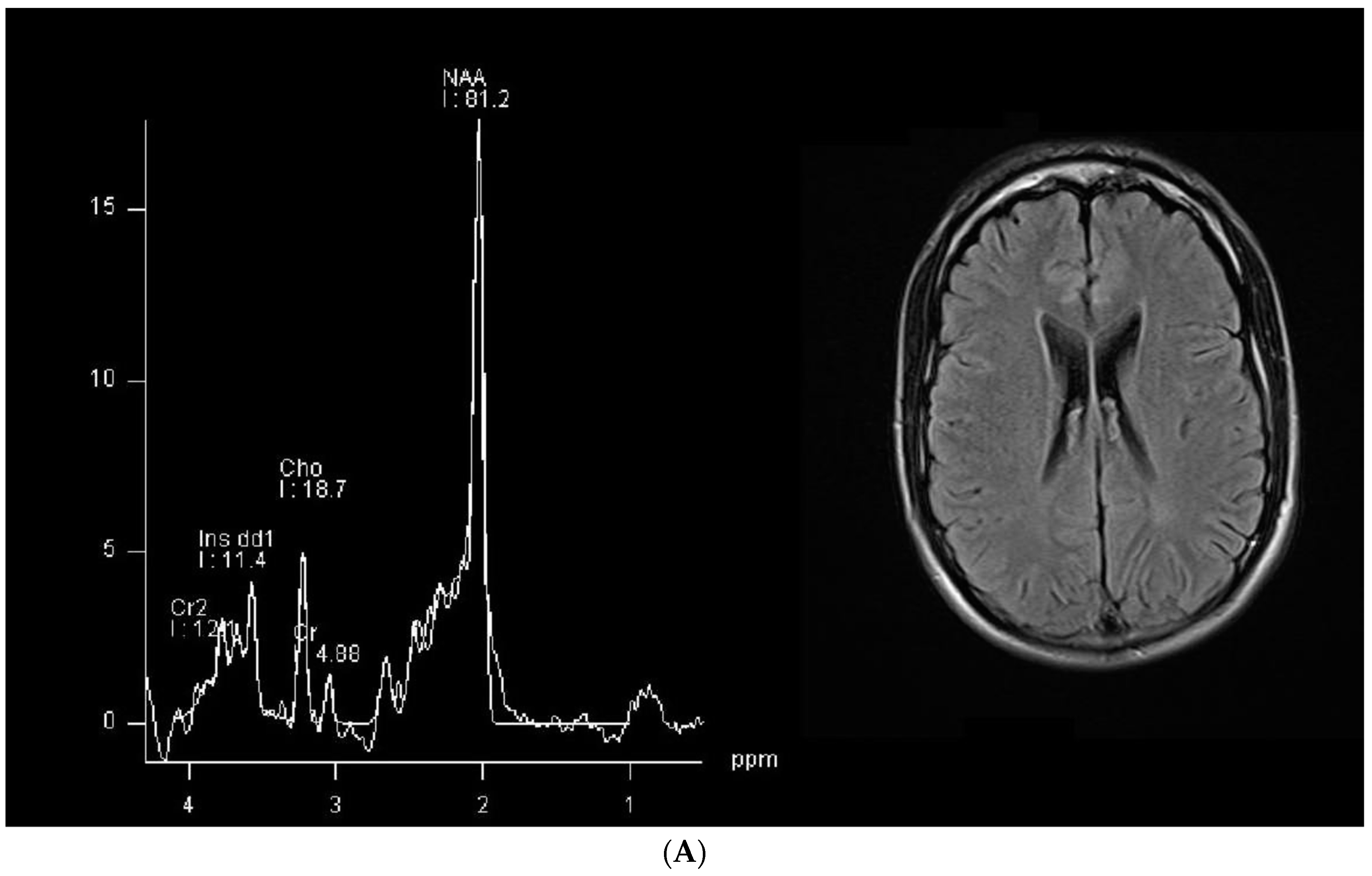
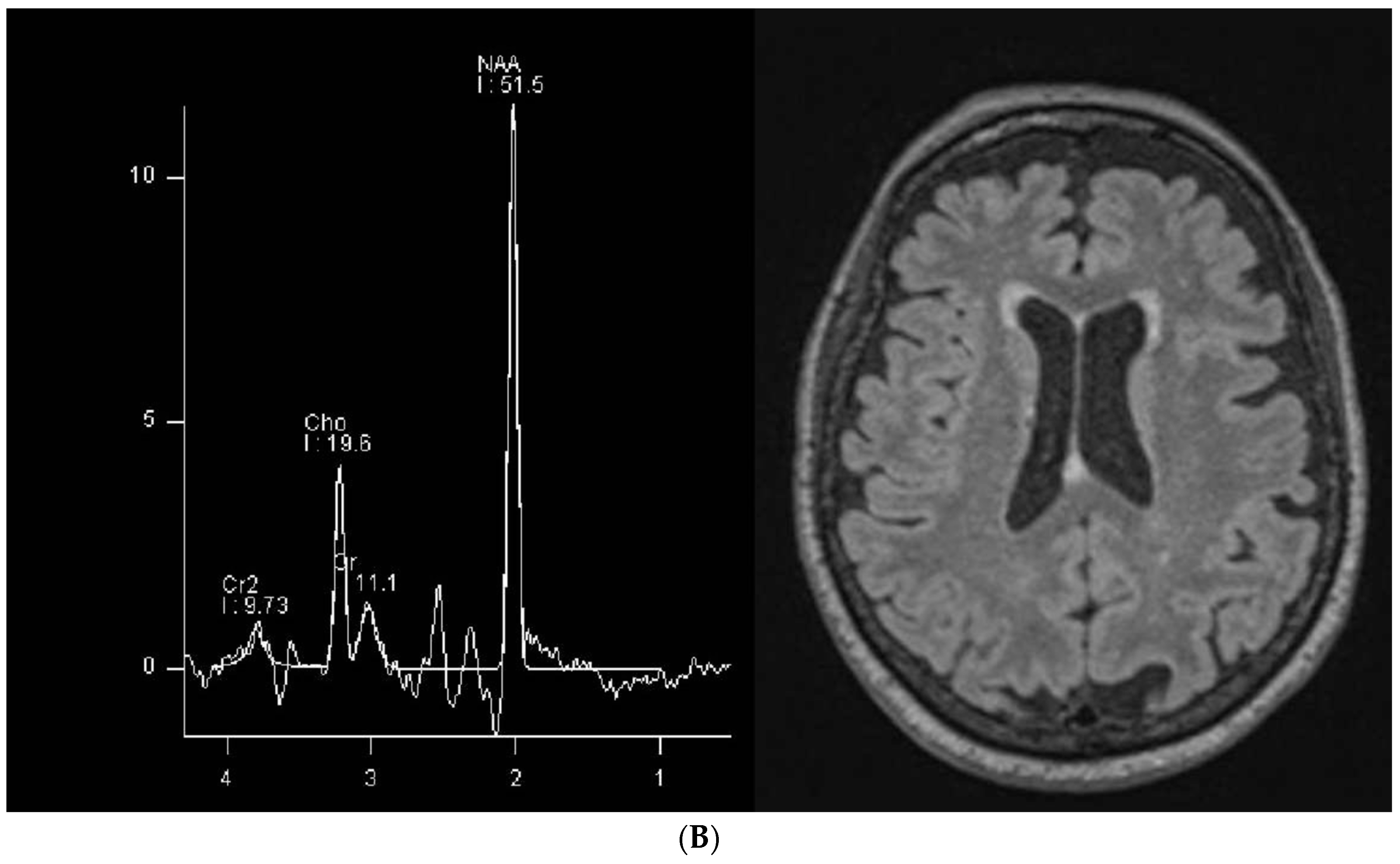


| Patient 1 | Patient 2 | |
|---|---|---|
| Age | 28 years old | 18 years old |
| Social environment | He lived with his family at the time of this investigation; he had to be admitted to institutional care shortly afterwards upon the death of his grandmother who cared for him. | He lived with his family, and he still does so. |
| Speech | He only speaks a few words with semantic meanings; verbal understanding is spared. | Some difficulty in finding words but information is conveyed and his vocabulary has a fair amount of complexity. |
| Cognitive state | Severe cognitive impairment, lack of attention with only occasional collaboration; he is often agitated, especially in unfamiliar situations. | Cognitive impairment but he shows a fair amount of attention and concentration; behavior is not grossly abnormal. |
| Seizures | Seizures in the past, not in the past two years. | Seizures up to the age of 6, but no seizures since then. |
| Neurological examination | No focal lesion signs, global motor impairment, fatuity, hyperkinesia, mild axial hypotonia, internal rotation of the feet. Right hand dominance. | Normal neurological examination except for slight impairment in fine motor movements of the hand fingers, bilaterally. Right hand dominance. |
| Drug therapy |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestrino, M.; Adriano, E.; Alì, P.A.; Pardini, M. Selective Alteration of the Left Arcuate Fasciculus in Two Patients Affected by Creatine Transporter Deficiency. Brain Sci. 2024, 14, 337. https://doi.org/10.3390/brainsci14040337
Balestrino M, Adriano E, Alì PA, Pardini M. Selective Alteration of the Left Arcuate Fasciculus in Two Patients Affected by Creatine Transporter Deficiency. Brain Sciences. 2024; 14(4):337. https://doi.org/10.3390/brainsci14040337
Chicago/Turabian StyleBalestrino, Maurizio, Enrico Adriano, Paolo Alessandro Alì, and Matteo Pardini. 2024. "Selective Alteration of the Left Arcuate Fasciculus in Two Patients Affected by Creatine Transporter Deficiency" Brain Sciences 14, no. 4: 337. https://doi.org/10.3390/brainsci14040337
APA StyleBalestrino, M., Adriano, E., Alì, P. A., & Pardini, M. (2024). Selective Alteration of the Left Arcuate Fasciculus in Two Patients Affected by Creatine Transporter Deficiency. Brain Sciences, 14(4), 337. https://doi.org/10.3390/brainsci14040337






