Effects of Cervical Spinal Manipulation on Saccadic Eye Movements
Abstract
1. Introduction
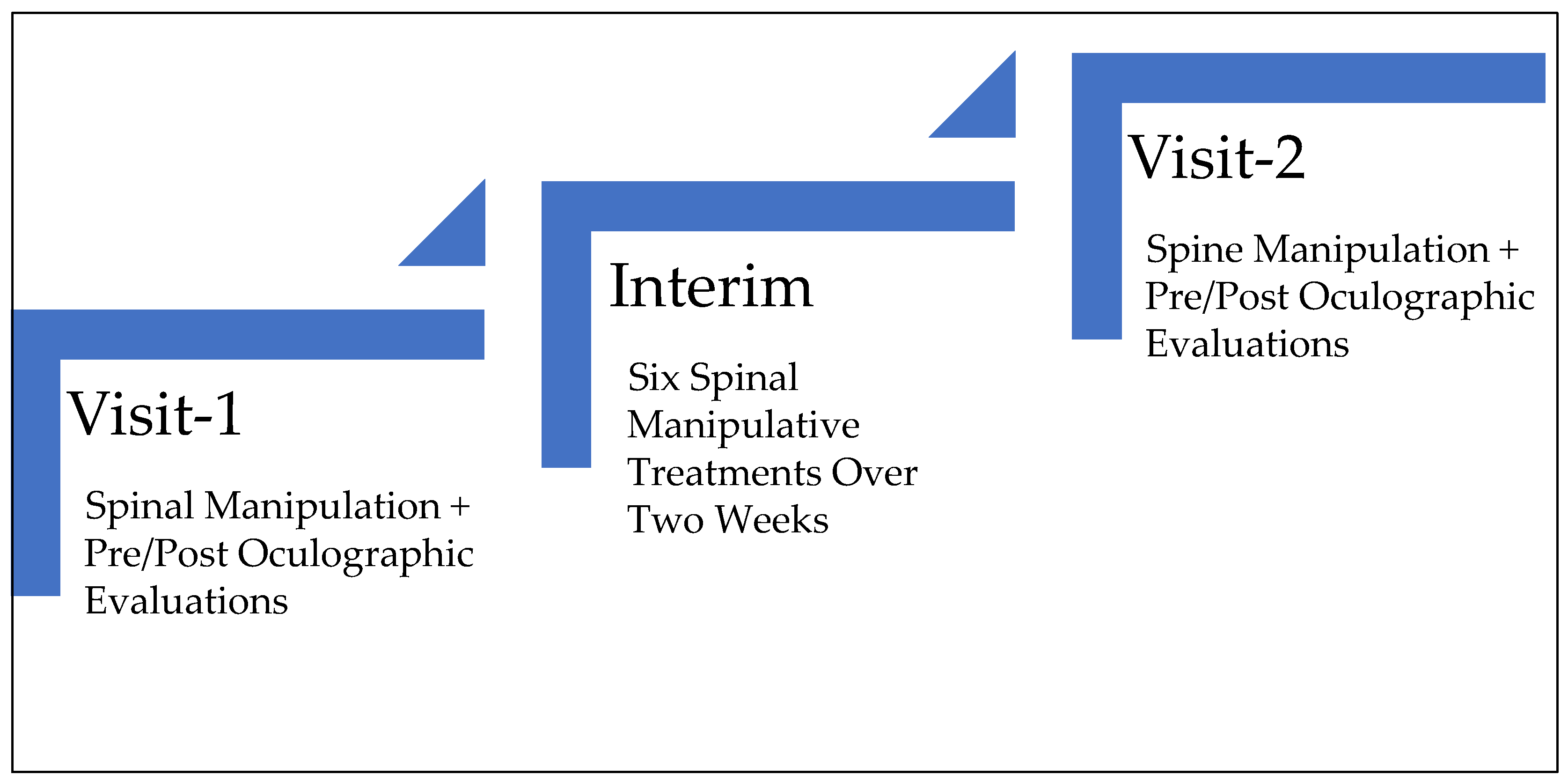
2. Materials and Methods
2.1. Subject
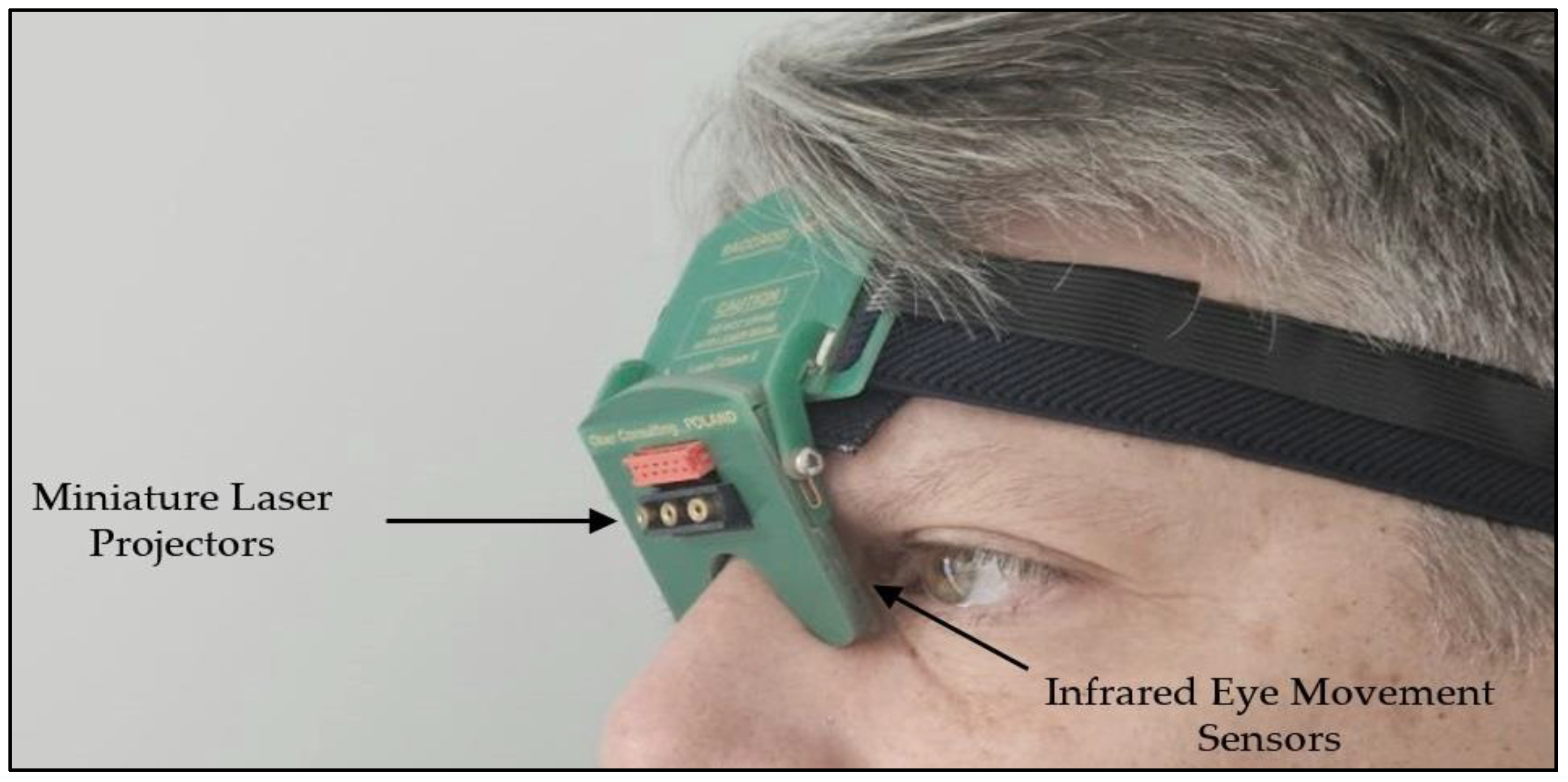
2.2. Saccadic Recording and Quantification
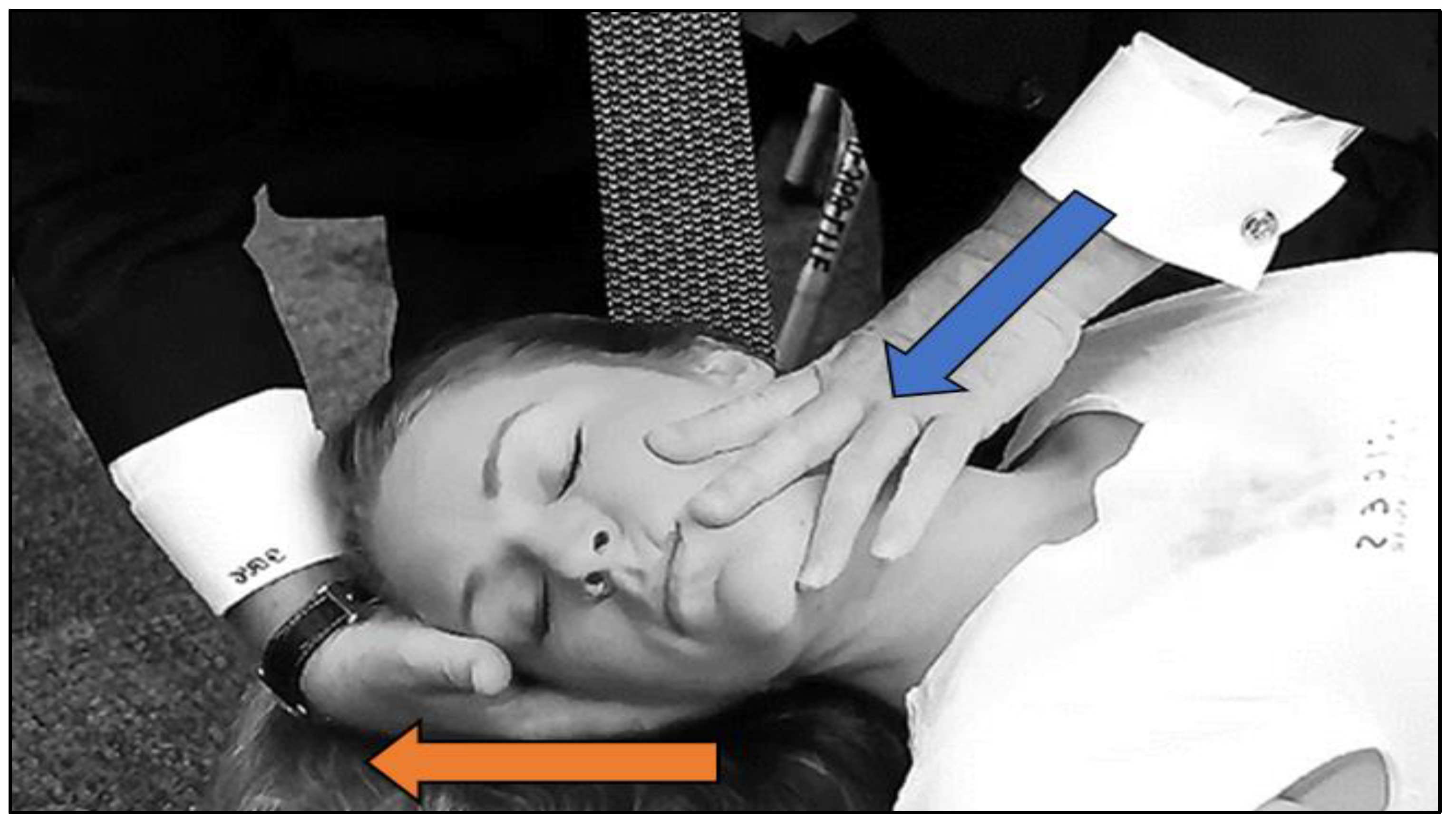
2.3. Treatment (Determining the Cervical Segment to Be Manipulated)
- Immediate Treatment Effect: Separately compares the post to pre-treatment saccadic parameter values in visits 1 and 2.
- Long-Term Treatment Effect: Compares the pre-treatment saccadic parameter values of visit 2 to 1.
- Immediate Treatment Effect Bias: Compares the post-treatment saccadic parameter values in visits 1 and 2.
- Right and Left Asymmetry Effect: Compares the saccadic parameter values between right and leftward saccades.
3. Results
3.1. Visit-1
3.1.1. Visit-1: Immediate Treatment Effect
3.1.2. Visit-1 Saccadic Parameter Profiles: Immediate Treatment Effect
3.2. Visit-2
3.2.1. Visit-2: Immediate Treatment Effect
3.2.2. Visit-2 Saccadic Parameter Profiles: Immediate Treatment Effect
3.3. Long-Term Treatment Effect
Saccadic Parameter Profiles: Long-Term Treatment Effect
3.4. Immediate Treatment Effect Bias between Visit-1 and 2
4. Discussion
4.1. Investigative Purpose
4.2. Primary Outcomes
4.3. Choice of Saccadic Recording Device and Protocol
4.4. Choice of Horizontal Saccade Evaluation over Vertical Saccades
4.5. Saccadic Eye Movements: A Biomarker of Neurological Disease
4.6. Structural and Functional Brain Asymmetry
4.7. Saccadic Generation and Visual System Asymmetry
4.8. Neurophysiological Effects of Spinal Manipulation
4.9. Horizontal Gaze Shifts and Neck Musculature Recruitment
4.10. Case Study’s Perspective
5. Conclusions
6. Limitations and Recommendations
6.1. Limitations
6.2. Recommendations
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierce, J.E.; Clementz, B.A.; McDowell, J.E. Saccades: Fundamentals and Neural Mechanisms. In Eye Movement Research: An Introduction to its Scientific Foundations and Applications; Klein, C., Ettinger, U., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 11–71. [Google Scholar]
- Guidetti, G.; Guidetti, R.; Manfredi, M.; Manfredi, M.; Lucchetta, A.; Livio, S. Saccades and driving. Acta Otorhinolaryngol. Ital. 2019, 39, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.G.; Zee, D.S. Eye Movement Research in the Twenty-First Century—A Window to the Brain, Mind, and More. Cerebellum 2018, 17, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; MacAskill, M.R. Eye movements in patients with neurodegenerative disorders. Nat. Rev. Neurol. 2013, 9, 74–85. [Google Scholar] [CrossRef]
- Karpouzian, T.; Petrovsky, N.; Ettinger, U.; Reilly, J. Eye Movements as Biomarkers to Evaluate Pharmacological Effects on Brain Systems. In Eye Movement Research: An Introduction to Its Scientific Foundations and Applications; Springer International Publishing: Cham, Switzerland, 2019; p. 775. [Google Scholar]
- Baird-Gunning, J.J.; Lueck, C.J. Central control of eye movements. Curr. Opin. Neurol. 2018, 31, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, C.; Legace, E.; Boulay, C.; Macartney, G.; Goulet, K.; Zemek, R.; Sveistrup, H. Balance markers and saccadic eye-movement measures in adolescents with post-concussion syndrome. J. Athl. Train. 2020, 55, 475–481. [Google Scholar] [CrossRef]
- Jain, D.; Arbogast, K.B.; McDonald, C.C.; Podolak, O.E.; Margulies, S.S.; Metzger, K.B.; Howell, D.R.; Scheiman, M.M.O.; Master, C.L. Eye Tracking metrics differences among uninjured adolescents and those with acute or persistent post-concussion symptoms. Optom. Vis. Sci. 2022, 99, 616–625. [Google Scholar] [CrossRef]
- Heitger, M.H.; Jones, R.D.; Macleod, A.D.; Snell, D.L.; Frampton, C.M.; Anderson, T.J. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain 2009, 132, 2850–2870. [Google Scholar] [CrossRef]
- Murray, N.G.; Szekely, B.; Islas, A.; Munkasy, B.; Gore, R.; Berryhill, M.; Reed-Jones, R.J. Smooth Pursuit and Saccades after Sport-Related Concussion. J. Neurotrauma 2020, 37, 340–346. [Google Scholar] [CrossRef]
- Akhand, O.; Balcer, L.J.; Galetta, S.L. Assessment of vision in concussion. Curr. Opin. Neurol. 2019, 32, 68–74. [Google Scholar] [CrossRef]
- Leddy, J.J.; Haider, M.N.; Noble, J.M.; Rieger, B.; Flanagan, S.; McPherson, J.I.; Shubin-Stein, K.; Saleem, G.T.; Corsaro, L.; Willer, B. Clinical Assessment of Concussion and Persistent Post-Concussive Symptoms for Neurologists. Curr. Neurol. Neurosci. Rep. 2021, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Sheeba, S.; Cavaleri, R.; Summers, S.; Browne, C. Effectiveness of non-pharmacological treatments for vestibular and oculomotor dysfunction in patients with persistent post-concussive symptoms: Protocol for a systematic review and meta-analysis. BMJ Open 2023, 13, e066634. [Google Scholar] [CrossRef]
- Marshall, C.M.; Vernon, H.; Leddy, J.J.; Baldwin, B.A. The role of the cervical spine in post-concussion syndrome. Physician Sportsmed. 2015, 43, 274–284. [Google Scholar] [CrossRef]
- Pearson, A.L.; de Haerne, C.M.; Malvankar-Mehta, M.S.; Bursztyn, L.L. Visual Abnormalities in Chronic Post-Concussion Syndrome. Adv. Ophthalmol. Optom. 2020, 5, 171–185. [Google Scholar] [CrossRef]
- Bargary, G.; Bosten, J.M.; Goodbourn, P.T.; Lawrance-Owen, A.J.; Hogg, R.E.; Mollon, J.D. Individual differences in human eye movements: An oculomotor signature? Vis. Res. 2017, 141, 157–169. [Google Scholar] [CrossRef]
- Bijvank, J.A.N.; Petzold, A.; Balk, L.J.; Tan, H.S.; Uitdehaag, B.M.J.; Theodorou, M.; van Rijn, L.J. A standardized protocol for quantification of saccadic eye movements: DEMoNS. PLoS ONE 2018, 13, e0200695. [Google Scholar] [CrossRef]
- Shteyman, A.; DeBroff, B.M. Ophthalmic Manifestations, Evaluation, and Guidelines for Testing of Concussion. Open Ophthalmol. J. 2023, 17, e187436412301050. [Google Scholar] [CrossRef]
- Mosimann, U.P.; Müri, R.M.; Felblinger, J.; Radanov, B.P. Saccadic eye movement disturbances in whiplash patients with persistent complaints. Brain 2000, 123, 828–835. [Google Scholar] [CrossRef][Green Version]
- Hildingsson, C.; Wenngren, B.-I.; Bring, G.; Toolanen, G. Oculomotor problems after cervical spine injury. Acta Orthop. Scand. 1989, 60, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, N.; Lionello, M.; Boscolo-Berto, R.; Giacomelli, L.; Rondinelli, R.; Marioni, G. Video-nystagmographic evidence in more than 700 consecutive cases of road traffic whiplash injury. Am. J. Otolaryngol. 2021, 42, 102909. [Google Scholar] [CrossRef] [PubMed]
- Broglio, S.P.; Surma, T.; Ashton-Miller, J.A. High school and collegiate football athlete concussions: A biomechanical review. Ann. Biomed. Eng. 2012, 40, 37–46. [Google Scholar] [CrossRef]
- Rebbeck, T.; Evans, K.; Elliott, J.M. Concussion in combination with whiplash-associated disorder may be missed in primary care: Key recommendations for assessment and management. J. Orthop. Sports Phys. Ther. 2019, 49, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Treleaven, J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man. Ther. 2008, 13, 2–11. [Google Scholar] [CrossRef]
- Treleaven, J. Dizziness, Unsteadiness, Visual Disturbances, and Sensorimotor Control in Traumatic Neck Pain. J. Orthop. Sports Phys. Ther. 2017, 47, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Treleaven, J.; Takasaki, H. Characteristics of visual disturbances reported by subjects with neck pain. Man. Ther. 2014, 19, 203–207. [Google Scholar] [CrossRef]
- Froment Tilikete, C. How to assess eye movements clinically. Neurol. Sci. 2022, 43, 2969–2981. [Google Scholar] [CrossRef]
- Rubinstein, S.M.; de Zoete, A.; van Middelkoop, M.; Assendelft, W.J.J.; de Boer, M.R.; van Tulder, M.W. Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: Systematic review and meta-analysis of randomized controlled trials. BMJ 2019, 364, l689. [Google Scholar] [CrossRef]
- Gevers-Montoro, C.; Provencher, B.; Descarreaux, M.; de Mues, A.O.; Piché, M. Neurophysiological mechanisms of chiropractic spinal manipulation for spine pain. Eur. J. Pain. 2021, 25, 1429–1448. [Google Scholar] [CrossRef] [PubMed]
- Cade, A.; Jones, K.; Holt, K.; Penkar, A.M.; Haavik, H. The Effects of Spinal Manipulation on Oculomotor Control in Children with Attention Deficit Hyperactivity Disorder: A Pilot and Feasibility Study. Brain Sci. 2021, 11, 1047. [Google Scholar] [CrossRef]
- Gyer, G.; Michael, J.; Inklebarger, J.; Tedla, J.S. Spinal manipulation therapy: Is it all about the brain? A current review of the neurophysiological effects of manipulation. J. Integr. Med. 2019, 17, 328–337. [Google Scholar] [CrossRef]
- Lelic, D.; Niazi, I.K.; Holt, K.; Jochumsen, M.; Dremstrup, K.; Yielder, P.; Murphy, B.; Drewes, A.M.; Haavik, H. Manipulation of dysfunctional spinal joints affects sensorimotor integration in the prefrontal cortex: A brain source localization study. Neural Plast. 2016, 2016, 3704964. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Hodge, C.; Rose, K.; Fraser, C. Persistent visual disturbances after concussion. Aust. J. Gen. Pract. 2019, 48, 531–536. [Google Scholar] [CrossRef]
- Gowrisankaran, S.; Shah, A.S.; Roberts, T.L.; Wiecek, E.; Chinn, R.N.; Hawash, K.K.; O’Brien, M.J.; Howell, D.R.; Meehan, W.P.; Raghuram, A. Association between post-concussion symptoms and oculomotor deficits among adolescents. Brain Inj. 2021, 35, 1218–1228. [Google Scholar] [CrossRef]
- Master, C.L.; Bacal, D.; Grady, M.F.; Hertle, R.; Shah, A.S.; Strominger, M.; Whitecross, S.; Bradford, G.E.; Lum, F.; Donahue, S.P.; et al. Vision and Concussion: Symptoms, Signs, Evaluation, and Treatment. Pediatrics 2022, 150, e2021056047. [Google Scholar] [CrossRef]
- Ober, J.; Dylak, J.; Gryncewicz, W.; Przedpelska-Ober, E. Saccadometry—New possibility for monitoring brain functional status. Kwartalnik NAUKA 2009, 109–135. [Google Scholar]
- Ober, J.K.; Przedpelska-Ober, E.; Gryncewicz, W.; Dylak, J.; Carpenter RH, S.; Ober, J.J. Hand-held system for ambulatory measurement of saccadic durations of neurological patients. Model. Meas. Med. 2003, 187–198. [Google Scholar]
- American Chiropractic Neurology Board. Available online: www.acnb.org (accessed on 24 November 2023).
- Carrick, F.R. Cervical radiculopathy: The diagnosis and treatment of pathomechanics in the cervical spine. J. Manip. Physiol. Ther. 1983, 6, 129–137. [Google Scholar]
- Cook, C.; Hegedus, E.; Showalter, C.; Sizer, P.S. Coupling behavior of the cervical spine: A systematic review of the literature. J. Manip. Physiol. Ther. 2006, 29, 570–575. [Google Scholar] [CrossRef]
- Bogduk, N.; Mercer, S. Biomechanics of the cervical spine. I: Normal kinematics. Clin. Biomech. 2000, 15, 633–648. [Google Scholar]
- Klotzek, A. Cervical Coupled Manipulation in Carrick Institute; Carrick Institute Advanced Chiropractic Technique; Manipulation, F.C.C., Ed.; Carrick Institute: Cape Canaveral, FL, USA, 2023. [Google Scholar]
- Tatara, S.; Toda, H.; Maeda, F.; Handa, T. Development of a New Eye Movement Measurement Device Using Eye-Tracking Analysis Technology. Appl. Sci. 2023, 13, 5968. [Google Scholar] [CrossRef]
- Gibaldi, A.; Sabatini, S.P. The saccade main sequence revised: A fast and repeatable tool for oculomotor analysis. Behav. Res. Methods 2021, 53, 167–187. [Google Scholar] [CrossRef]
- Hessels, R.S.; Niehorster, D.C.; Nyström, M.; Andersson, R.; Hooge, I.T.C. Is the eye-movement field confused about fixations and saccades? A survey among 124 researchers. R. Soc. Open Sci. 2018, 5, 180502. [Google Scholar] [CrossRef]
- Lai, H.-Y.; Saavedra-Pena, G.; Sodini, C.G.; Sze, V.; Heldt, T. Measuring Saccade Latency Using Smartphone Cameras. IEEE J. Biomed. Health Inform. 2020, 24, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Tatler, B.W.; Hansen, D.W.; Pelz, J.B. Eye Movement Recordings in Natural Settings. In Eye Movement Research: An Introduction to Its Scientific Foundations and Applications; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Zhang, J.; Zhang, B.; Ren, Q.; Zhong, Q.; Li, Y.; Liu, G.; Ma, X.; Zhao, C. Eye movement especially vertical oculomotor impairment as an aid to assess Parkinson’s disease. Neurol. Sci. 2021, 42, 2337–2345. [Google Scholar] [CrossRef]
- Hunfalvay, M.; Roberts, C.-M.; Murray, N.; Tyagi, A.; Kelly, H.; Bolte, T. Horizontal and vertical self-paced saccades as a diagnostic marker of traumatic brain injury. Concussion 2019, 4, CNC60. [Google Scholar] [CrossRef]
- Irving, E.L.; Lillakas, L. Difference between vertical and horizontal saccades across the human lifespan. Exp. Eye Res. 2019, 183, 38–45. [Google Scholar] [CrossRef]
- Termsarasab, P.; Thammongkolchai, T.; Rucker, J.C.; Frucht, S.J. The diagnostic value of saccades in movement disorder patients: A practical guide and review. J. Clin. Mov. Disord. 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.J.; Zee, D.S. The Neurology of Eye Movements; Contemporary Neurology; Oxford University Press: Cary, NC, USA, 2015. [Google Scholar]
- Becerra-Garcia, R.A.; Garcia-Bermudez, R.; Joya, G. Differentiation of Saccadic Eye Movement Signals. Sensors 2021, 21, 5021. [Google Scholar] [CrossRef]
- Taghdiri, F.; Chung, J.; Irwin, S.; Multani, N.; Tarazi, A.; Ebraheem, A.; Khodadadi, M.; Goswami, R.; Wennberg, R.; Mikulis, D.; et al. Decreased number of self-paced saccades in post-concussion syndrome is associated with higher symptom burden and reduced white matter integrity. J. Neurotrauma 2018, 35, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Beylergil, S.B.; Shaikh, A.G. Slow saccades in cerebellar disease. Cerebellum Ataxias 2019, 6, 1. [Google Scholar]
- Li, H.; Zhang, X.; Yang, Y.; Xie, A. Abnormal eye movements in Parkinson’s disease: From experimental study to clinical application. Park. Relat. Disord. 2023, 115, 105791. [Google Scholar] [CrossRef]
- Neubauer, S.; Gunz, P.; Scott, N.A.; Hublin, J.-J.; Mitteroecker, P. Evolution of brain lateralization: A shared hominid pattern of endocranial asymmetry is much more variable in humans than in great apes. Sci. Adv. 2020, 6, eaax9935. [Google Scholar] [CrossRef]
- Bisiacchi, P.; Cainelli, E. Structural and functional brain asymmetries in the early phases of life: A scoping review. Brain Struct. Funct. 2022, 227, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, K.; Raichle, M.E.; Mitra, A.; Specht, K. On the existence of a generalized non-specific task-dependent network. Front. Hum. Neurosci. 2015, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Düsing, R.; Tops, M.; Radtke, E.L.; Kuhl, J.; Quirin, M. Relative frontal brain asymmetry and cortisol release after social stress: The role of action orientation. Biol. Psychol. 2016, 115, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. A matter of degree: Strength of brain asymmetry and behaviour. Symmetry 2017, 9, 57. [Google Scholar] [CrossRef]
- Beh, S.C.; Frohman, T.C.; Frohman, E.M. Cerebellar Control of Eye Movements. J. Neuroophthalmol. 2017, 37, 87–98. [Google Scholar] [CrossRef]
- Bender, J.; Tark, K.-J.; Reuter, B.; Kathmann, N.; Curtis, C.E. Differential roles of the frontal and parietal cortices in the control of saccades. Brain Cogn. 2013, 83, 1–9. [Google Scholar] [CrossRef]
- Kheradmand, A.; Zee, D.S. Cerebellum and ocular motor control. Front. Neurol. 2011, 2, 53. [Google Scholar] [CrossRef]
- Tagu, J.; Doré-Mazars, K.; Vergilino-Perez, D. Saccade accuracy as an indicator of the competition between functional asymmetries in vision. Exp. Brain Res. 2020, 238, 411–425. [Google Scholar] [CrossRef]
- Jóhannesson, Ó.I.; Tagu, J.; Kristjánsson, Á. Asymmetries of the visual system and their influence on visual performance and oculomotor dynamics. Eur. J. Neurosci. 2018, 48, 3426–3445. [Google Scholar] [CrossRef]
- Kirenskaya, A.V.; Ryabova, A.M.; Gruden, M.A.; Novototsky-Vlasov, V.Y.; Storozheva, Z.I. Oculomotor Control Asymmetry in Antisaccade Task in Carriers of Val 158 Met Polymorphic Variants of the Cateholamine-O-Methyltransferase Gene. Hum. Physiol. 2021, 47, 260–269. [Google Scholar] [CrossRef]
- Navid, M.S. Effects of Chiropractic Spinal Manipulation on Brain Activity; Aalborg Universitetsforlag: Aalborg, Denmark, 2020. [Google Scholar]
- Pickar, J.G. Neurophysiological effects of spinal manipulation. Spine J. 2002, 2, 357–371. [Google Scholar] [CrossRef]
- Haavik, H.; Murphy, B. The role of spinal manipulation in addressing disordered sensorimotor integration and altered motor control. J. Electromyogr. Kinesiol. 2012, 22, 768–776. [Google Scholar] [CrossRef]
- Hoevemeyer, K.; Teng, K.Y.; Mehner, T.; McMunn, R.; Figueroa, J.; Jie, C. The Effect of Osteopathic Manipulative Treatment on Proprioception in Adults: A Pilot Study. AAO J. 2020, 30, 11–19. [Google Scholar] [CrossRef]
- Balslev, D.; Mitchell, A.G.; Faria, P.J.M.; Priba, L.; Macfarlane, J.A. Proprioceptive contribution to oculomotor control in humans. Hum. Brain Mapp. 2022, 43, 5081–5090. [Google Scholar] [CrossRef]
- Blanc, S. The Influence of Altered Cervical Input from Whiplash Injury on Post-Concussion Ocular/Visual Signs and Symptoms. Optom. Vis. Perform. 2019, 7, 257. [Google Scholar]
- Majcen Rosker, Z.; Vodicar, M.; Kristjansson, E. Oculomotor performance in patients with neck pain: Does it matter which angle of neck torsion is used in smooth pursuit eye movement test and is the agreement between angles dependent on target movement amplitude and velocity? Musculoskelet. Sci. Pract. 2022, 59, 102535. [Google Scholar] [CrossRef] [PubMed]
- Carrick, F.R.; Hankir, A.; Zaman, R.; Antonucci, M.M.; Pagnacco, G.; Azzolino, S.; Oggero, E. Improvement of Saccadic Eye Movements after Head-Eye Vestibular Motion (HEVM) Therapy and Neuro-Psychiatric Considerations. Psychiatr. Danub. 2019, 31 (Suppl. 3), 318–323. [Google Scholar] [PubMed]
- Carrick, F.R.; Pagnacco, G.; Wright, C.H.G.; Oggerd, E. Changes in Saccadic Eye Movements produced by Novel Brain and Vestibular rehabilitation Therapy. Biomed. Sci. Instrum. 2015, 51, 9–16. [Google Scholar] [PubMed]
- Gong, W. Effects of cervical joint manipulation on joint position sense of normal adults. J. Phys. Ther. Sci. 2013, 25, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Haavik, H.; Murphy, B. Subclinical neck pain and the effects of cervical manipulation on elbow joint position sense. J. Manip. Physiol. Ther. 2011, 34, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Gercek, H.; Unuvar, B.S.; Yemisci, O.U.; Aytar, A. Acute effects of instrument assisted soft tissue mobilization technique on pain and joint position error in individuals with chronic neck pain: A double-blind, randomized controlled trial. Somat. Mot. Res. 2023, 40, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Cerritelli, F.; Piras, F.; Spanò, B.; Tamburella, F.; Piras, F.; Caltagirone, C.; Gili, T. Brain Connectivity Changes after Osteopathic Manipulative Treatment: A Randomized Manual Placebo-Controlled Trial. Brain Sci. 2020, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Zeng, K.; Wang, W.; Gong, Z.-G.; Chen, Y.-L.; Cheng, J.-M.; Zhang, M.; Huang, Y.-W.; Men, X.-B.; Wang, J.-W.; et al. The Changes of Brain Function After Spinal Manipulation Therapy in Patients with Chronic Low Back Pain: A Rest BOLD fMRI Study. Neuropsychiatr. Dis. Treat. 2022, 18, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, W.; Yang, Y.; Chen, Y.; Kang, Y.; Huang, Y.; Gong, Z.; Zhan, S.; Ke, Z.; Wang, J.; et al. Spinal manipulative therapy alters brain activity in patients with chronic low back pain: A longitudinal brain fMRI study. Front. Integr. Neurosci. 2020, 14, 534595. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, X.-M.; Jin, X.; Ling, D.-Y.; Chen, S.; Huang, Q.; Kong, N.; Chai, J.-E.; Wang, Q.; Xu, M.-S.; et al. A spinal manipulative therapy altered brain activity in patients with lumbar disc herniation: A resting-state functional magnetic resonance imaging study. Front. Neurosci. 2022, 16, 974792. [Google Scholar] [CrossRef]
- Carrick, F.R. Changes in brain function after manipulation of the cervical spine. J. Manip. Physiol. Ther. 1997, 20, 529–545. [Google Scholar]
- Malaya, C.A.; Haworth, J.; Pohlman, K.A.; Powell, C.; Smith, D.L. Impact of extremity manipulation on postural sway characteristics: A preliminary, randomized crossover study. J. Manip. Physiol. Ther. 2020, 43, 457–468. [Google Scholar] [CrossRef]
- Woo, Y.-M.; Shin, B.-C.; Nam, Y. The study of brain function changes after contralateral and ipsilateral application of electroacupuncture. J. Acupunct. Res. 2003, 20, 22–34. [Google Scholar]
- Lima, C.R.; Sozio, R.S.; Law, A.C.; Nelson, A.J.; Singh, H.; Hurt, C.P.; Li, P.; Reed, W.R. Effects of Thrust Magnitude and Duration on Immediate Postspinal Manipulation Trunk Muscle Spindle Responses. J. Manip. Physiol. Ther. 2021, 44, 363–371. [Google Scholar] [CrossRef]
- Cao, D.-Y.; Reed, W.R.; Long, C.R.; Kawchuk, G.N.; Pickar, J.G. Effects of thrust amplitude and duration of high-velocity, low-amplitude spinal manipulation on lumbar muscle spindle responses to vertebral position and movement. J. Manip. Physiol. Ther. 2013, 36, 68–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reed, W.R.; Pickar, J.G.; Sozio, R.S.; Liebschner, M.A.; Little, J.W.; Gudavalli, M.R. Characteristics of Paraspinal Muscle Spindle Response to Mechanically Assisted Spinal Manipulation: A Preliminary Report. J. Manip. Physiol. Ther. 2017, 40, 371–380. [Google Scholar] [CrossRef] [PubMed]
- DeVocht, J.W.; Pickar, J.G.; Wilder, D.G. Spinal manipulation alters electromyographic activity of paraspinal muscles: A descriptive study. J. Manip. Physiol. Ther. 2005, 28, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Currie, S.J.; Myers, C.A.; Enebo, B.A.; Davidson, B.S. Treatment and Response Factors in Muscle Activation during Spinal Manipulation. J. Clin. Med. 2023, 12, 6377. [Google Scholar] [CrossRef] [PubMed]
- Goonetilleke, S.C.; Gribble, P.L.; Mirsattari, S.M.; Doherty, T.J.; Corneil, B.D. Neck muscle responses evoked by transcranial magnetic stimulation of the human frontal eye fields. Eur. J. Neurosci. 2011, 33, 2155–2167. [Google Scholar] [CrossRef]
- Corneil, B.D.; Olivier, E.; Munoz, D.P. Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J. Neurophysiol. 2002, 88, 1980–1999. [Google Scholar] [CrossRef][Green Version]
- Kuo, F.; Massoud, T.F. Structural asymmetries in normal brain anatomy: A brief overview. Ann. Anat. 2022, 241, 151894. [Google Scholar] [CrossRef]
- Ratnarajah, N.; Rifkin-Graboi, A.; Fortier, M.V.; Chong, Y.S.; Kwek, K.; Saw, S.-M.; Godfrey, K.M.; Gluckman, P.D.; Meaney, M.J.; Qiu, A. Structural connectivity asymmetry in the neonatal brain. Neuroimage 2013, 75, 187–194. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, B.; Suárez, L.E.; Markello, R.D.; Shafiei, G.; Paquola, C.; Hagmann, P.; Heuvel, M.P.v.D.; Bernhardt, B.C.; Spreng, R.N.; Misic, B. Gradients of structure-function tethering across neocortex. Proc. Natl. Acad. Sci. USA 2019, 116, 21219–21227. [Google Scholar] [CrossRef]
- Li, H.H.; Pan, J.; Carrasco, M. Presaccadic attention improves or impairs performance by enhancing sensitivity to higher spatial frequencies. Sci. Rep. 2019, 9, 2659. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef] [PubMed]
- Jemni, M.; Zaman, R.; Carrick, F.R.; Clarke, N.D.; Marina, M.; Bottoms, L.; Matharoo, J.S.; Ramsbottom, R.; Hoffman, N.; Groves, S.J.; et al. Exercise improves depression through positive modulation of brain-derived neurotrophic factor (BDNF). A review based on 100 manuscripts over 20 years. Front. Physiol. 2023, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Fukuda, H.; Hikosaka, O. What do eye movements tell us about patients with neurological disorders?—An introduction to saccade recording in the clinical setting. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 772–801. [Google Scholar] [CrossRef] [PubMed]
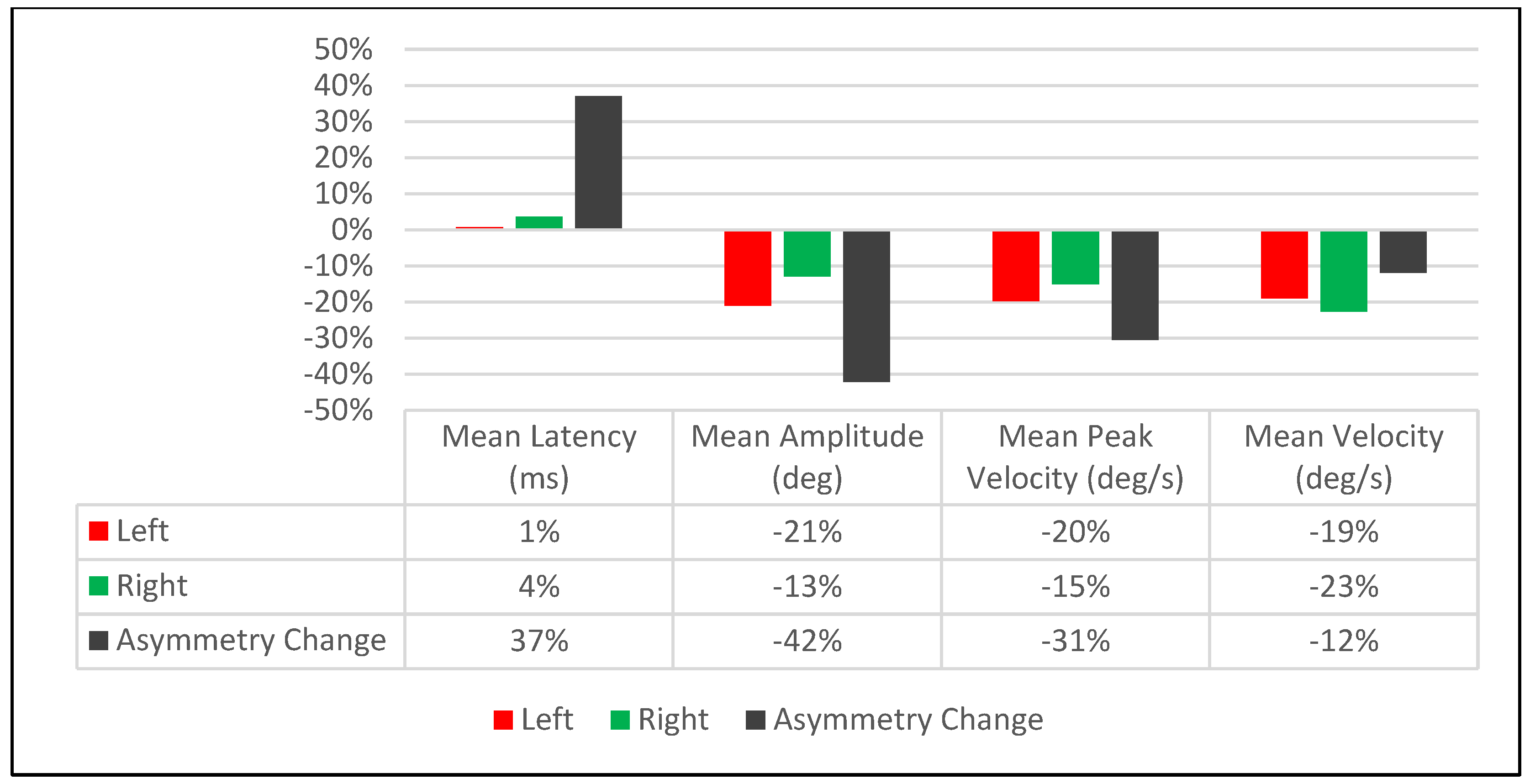
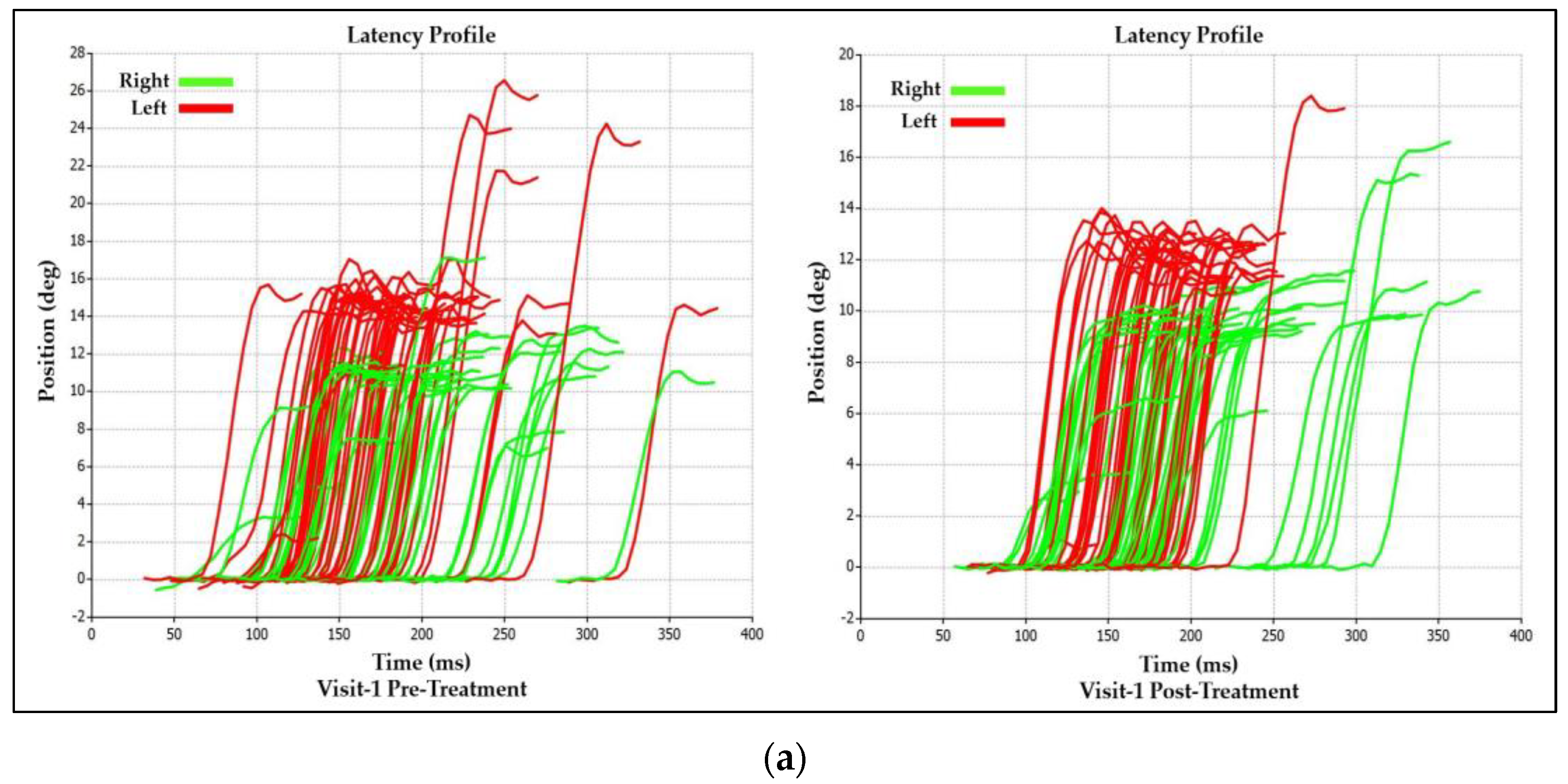
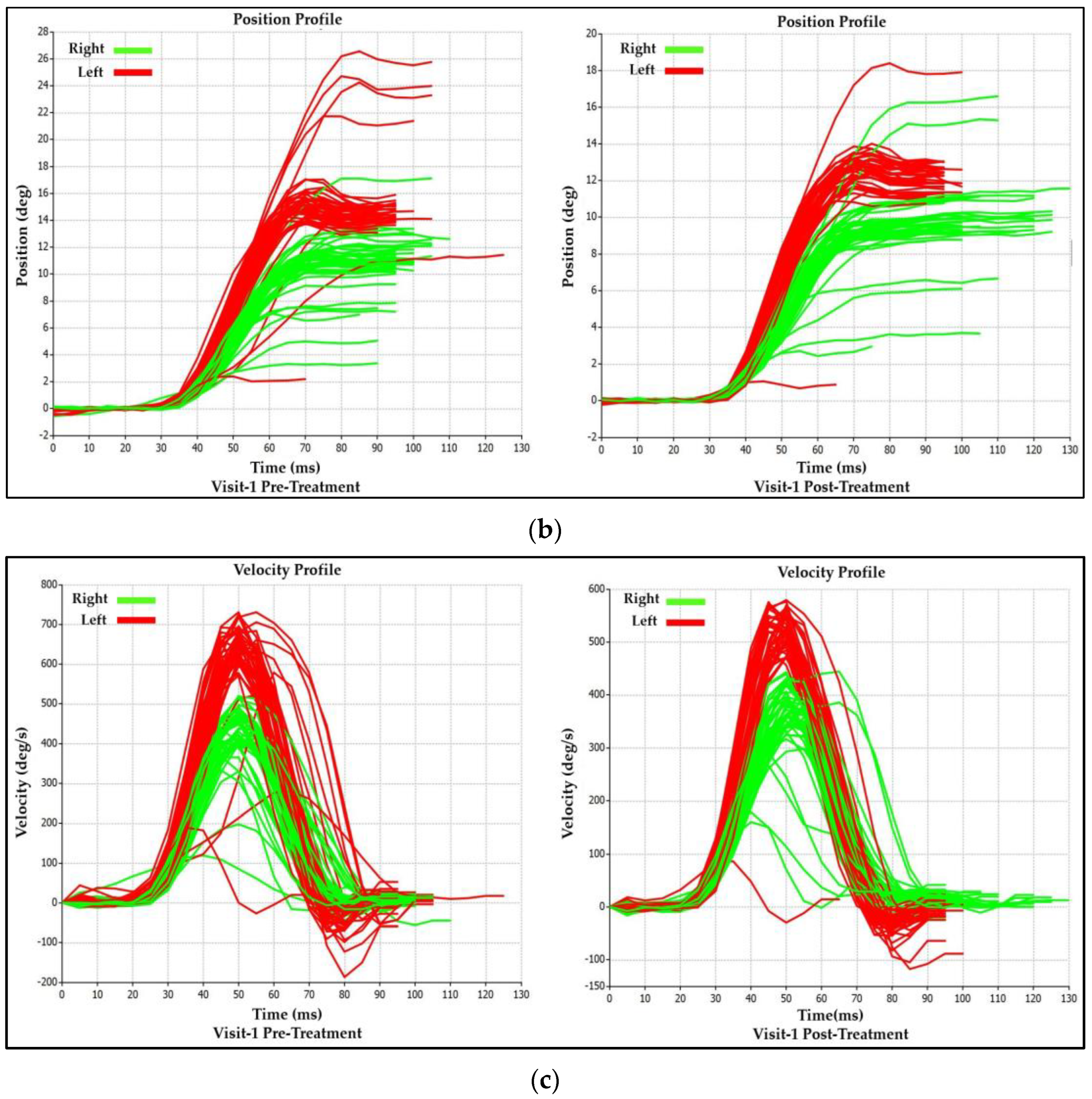
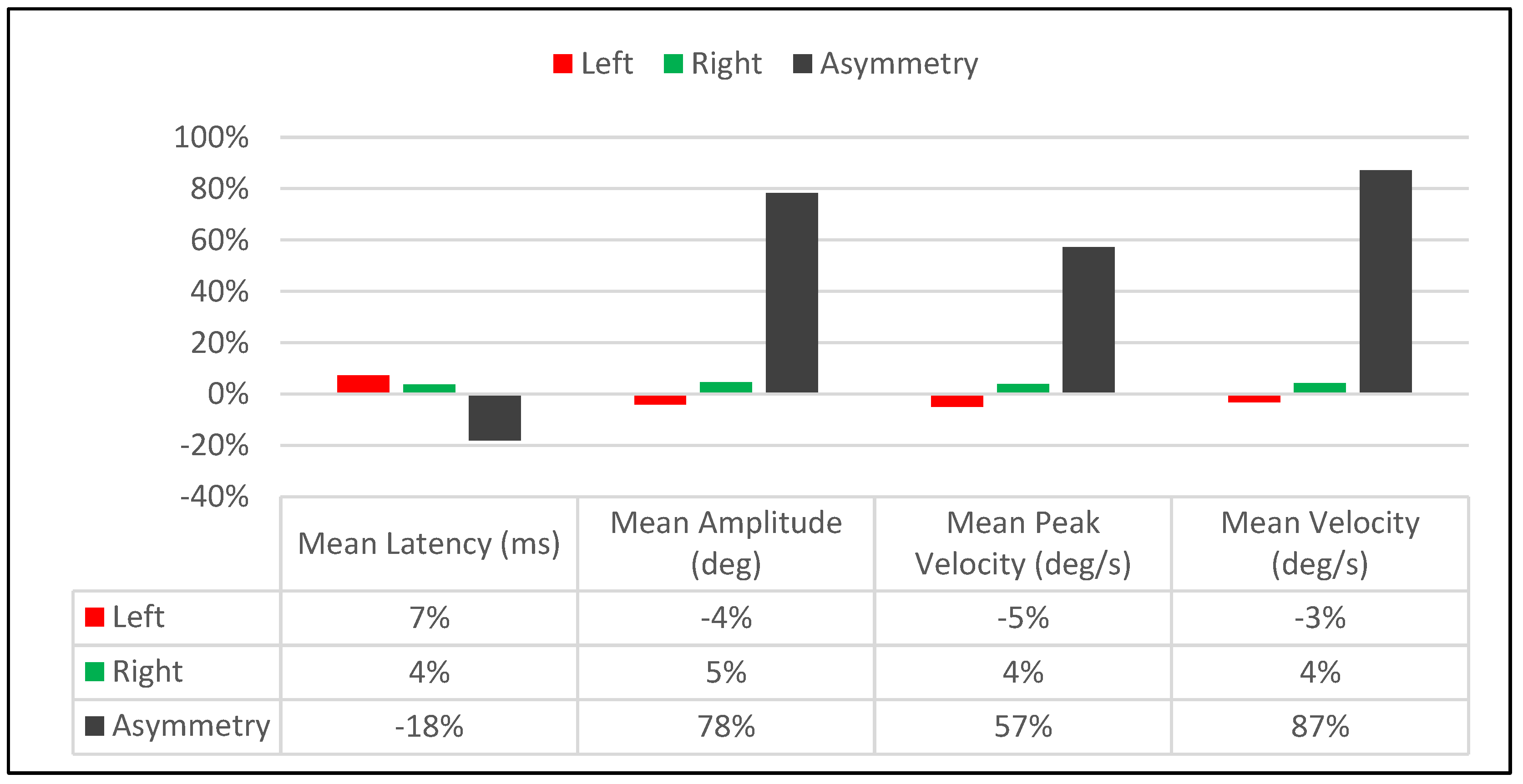
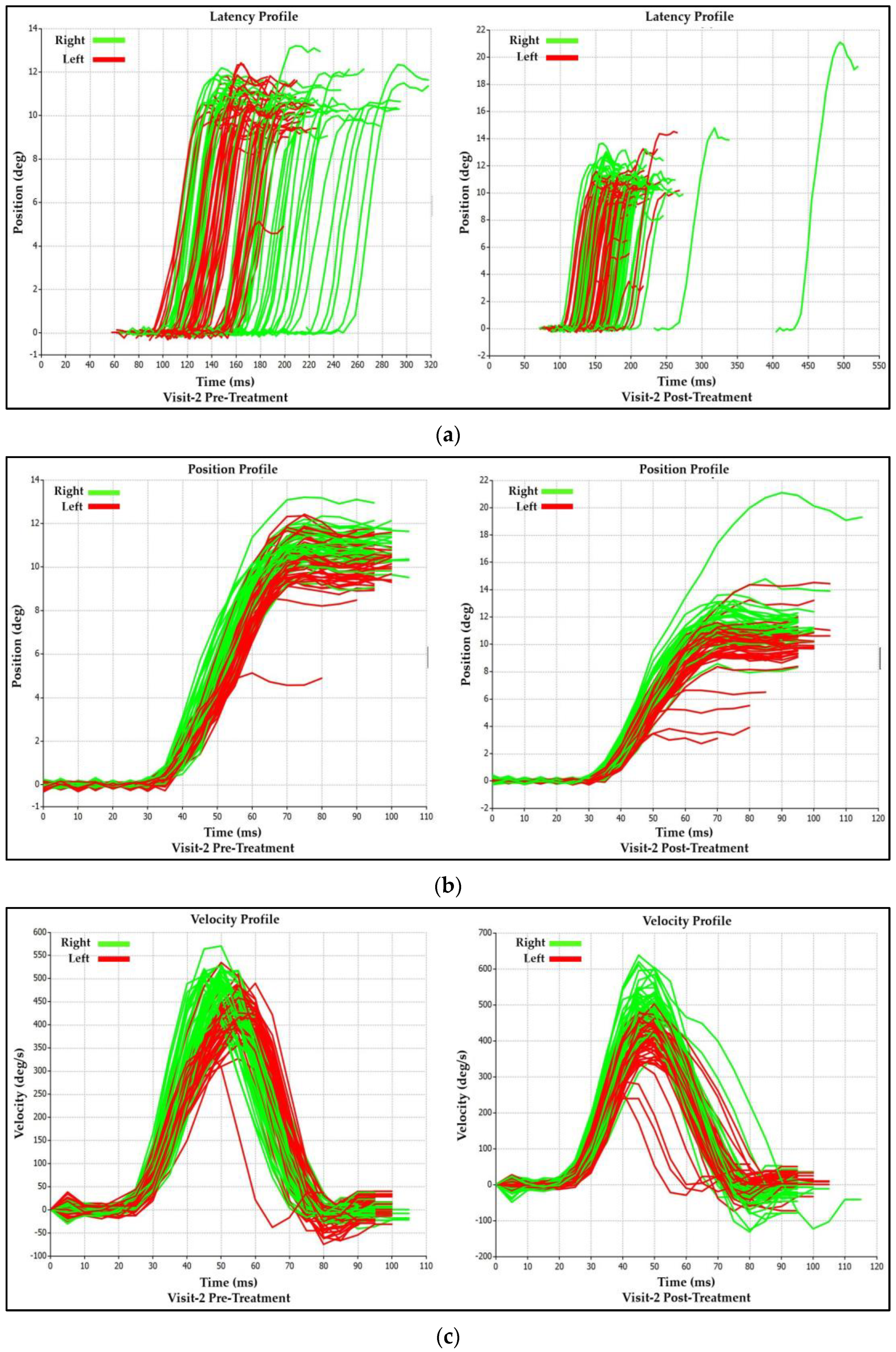
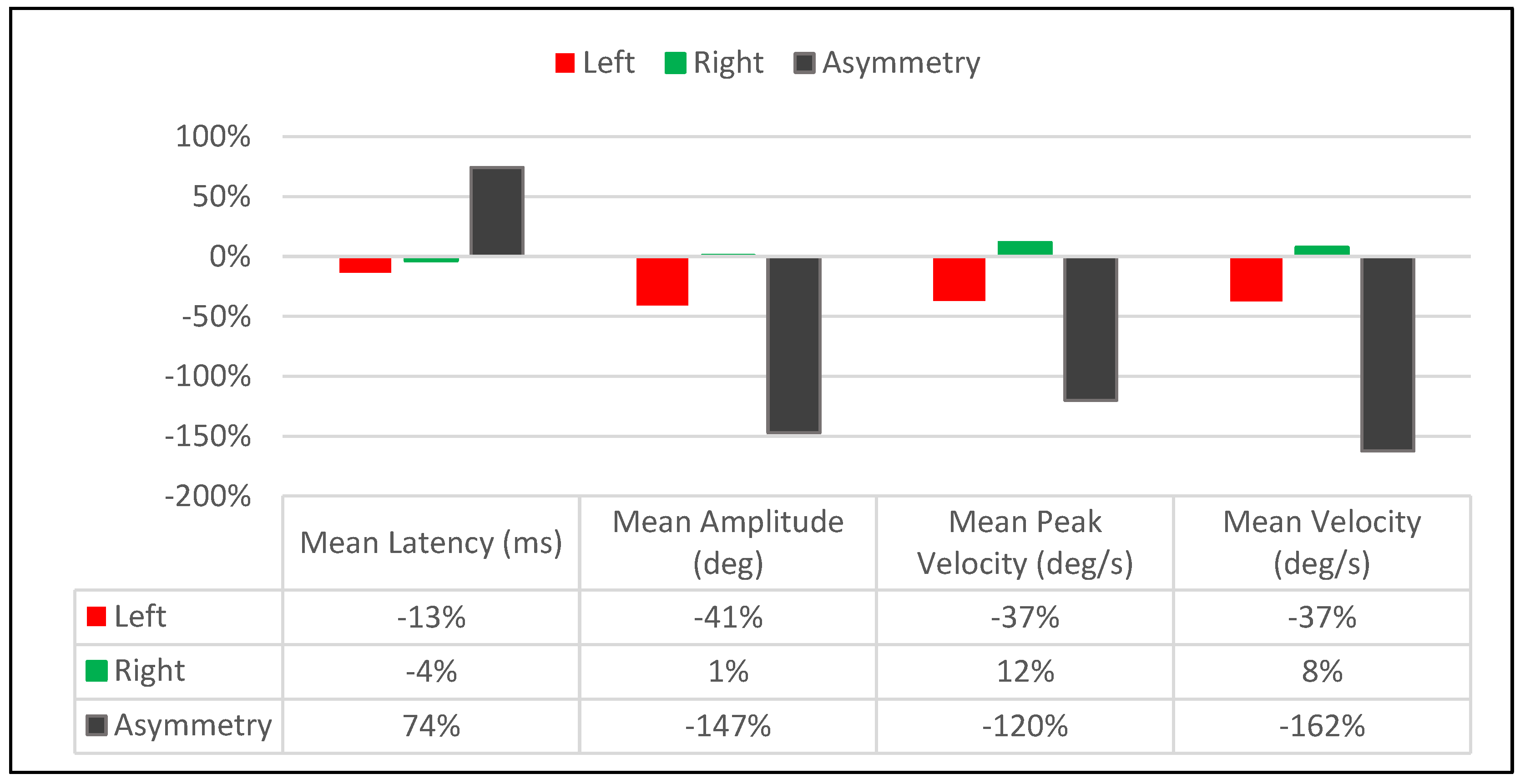
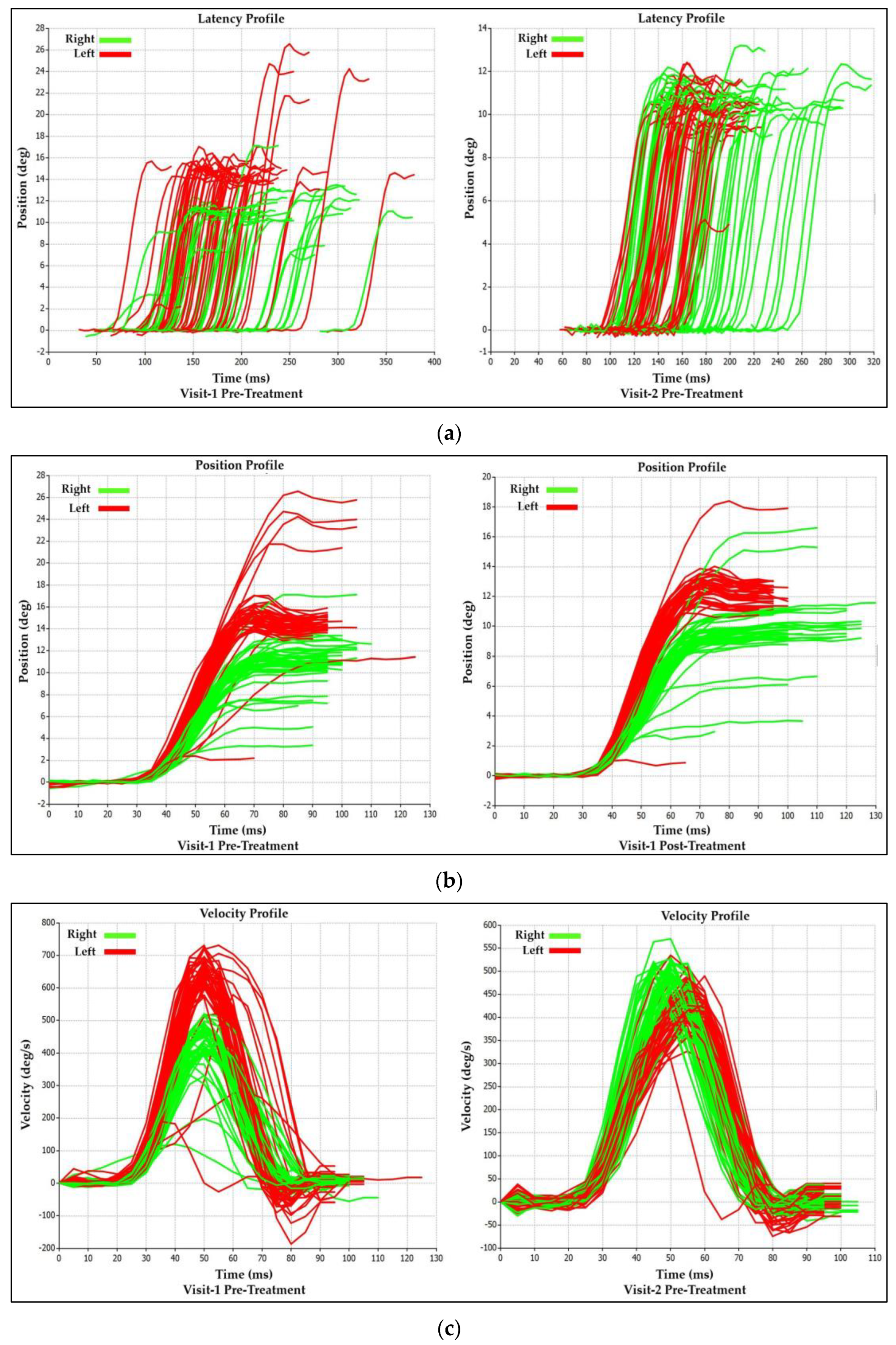
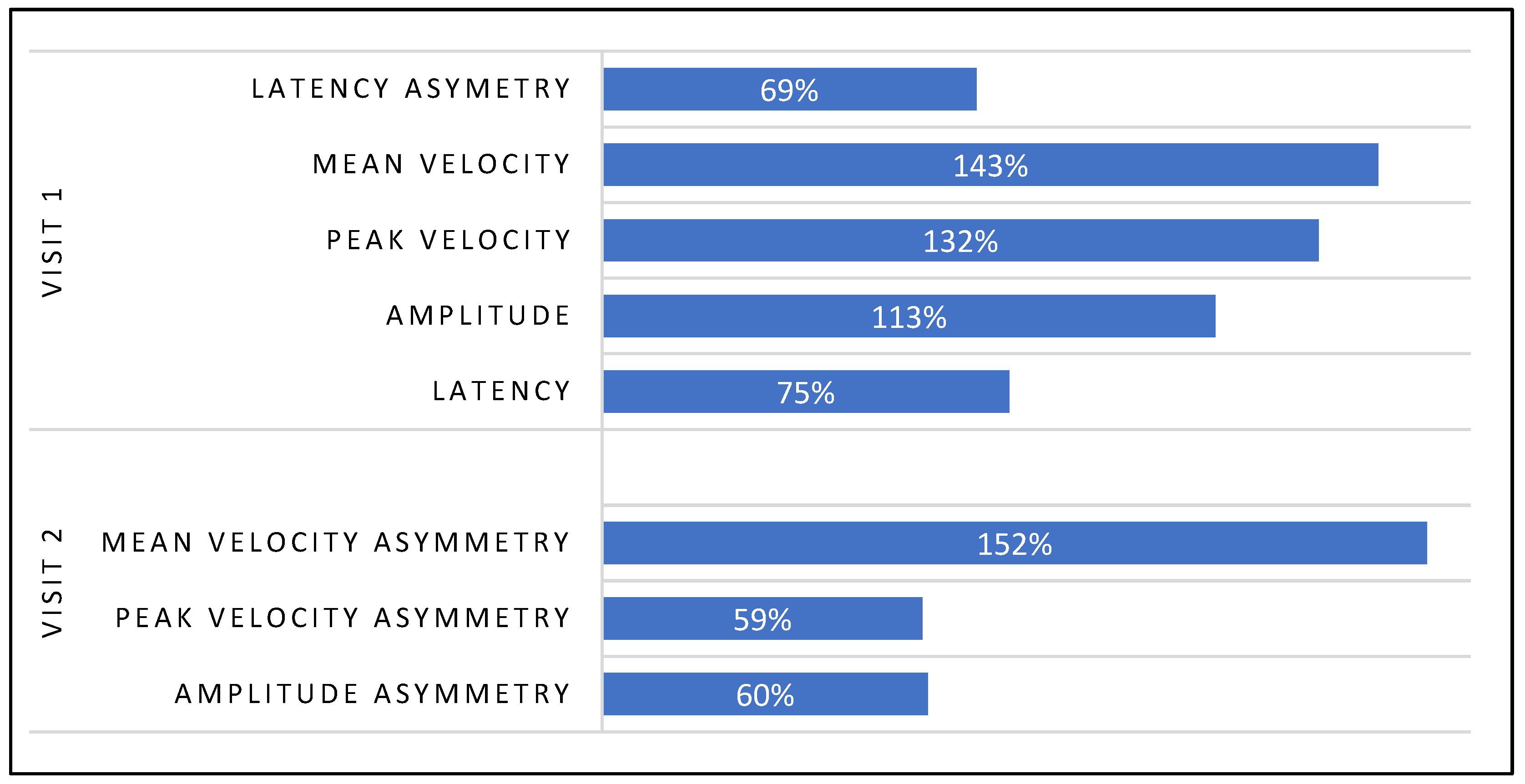
| Saccadic Performance Parameter | Left Saccade | Right Saccade | R/L Asymmetry |
|---|---|---|---|
| Mean Latency (ms) | |||
| Pre-Treatment | 151 | 162 | 11 |
| Post-Treatment | 152 | 168 | 16 |
| Delta | 1 | 6 | 5 |
| % Change | 1% | 4% | 37% |
| Mean Amplitude (deg) | |||
| Pre-Treatment | 15.3 | 10.7 | 4.6 |
| Post-Treatment | 12.4 | 9.4 | 3 |
| Delta | 2.9 | 1.3 | 1.6 |
| % Change | −21% | −13% | −42% |
| Mean Peak Velocity (deg/s) | |||
| Pre-Treatment | 630 | 430 | 200 |
| Post-Treatment | 517 | 370 | 147 |
| Delta | 113 | 60 | 53 |
| % Change | −20% | −15% | −31% |
| Mean Velocity (deg/s) | |||
| Pre-Treatment | 317 | 211 | 106 |
| Post-Treatment | 262 | 168 | 94 |
| Delta | 55 | 43 | 12 |
| % Change | −19% | −23% | −12% |
| Saccadic Performance Parameter | Left Saccade | Right Saccade | R/L Asymmetry |
|---|---|---|---|
| Mean Latency (ms) | |||
| Pre-Treatment | 132 | 156 | 24 |
| Post-Treatment | 142 | 162 | 20 |
| Delta | 10 | 6 | 4 |
| % Change | 7% | 4% | −18% |
| Mean Amplitude (deg) | |||
| Pre-Treatment | 10.1 | 10.8 | 0.7 |
| Post-Treatment | 9.7 | 11.3 | 1.6 |
| Delta | 0.4 | 0.5 | 0.9 |
| % Change | −4% | 5% | 78% |
| Mean Peak Velocity (deg/s) | |||
| Pre-Treatment | 433 | 483 | 50 |
| Post-Treatment | 412 | 502 | 90 |
| Delta | 21 | 19 | 40 |
| % Change | −5% | 4% | 57% |
| Mean Velocity (deg/s) | |||
| Pre-Treatment | 217 | 228 | 11 |
| Post-Treatment | 210 | 238 | 28 |
| Delta | 7 | 10 | 17 |
| % Change | −3% | 4% | 87% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klotzek, A.; Jemni, M.; Groves, S.J.; Carrick, F.R. Effects of Cervical Spinal Manipulation on Saccadic Eye Movements. Brain Sci. 2024, 14, 292. https://doi.org/10.3390/brainsci14030292
Klotzek A, Jemni M, Groves SJ, Carrick FR. Effects of Cervical Spinal Manipulation on Saccadic Eye Movements. Brain Sciences. 2024; 14(3):292. https://doi.org/10.3390/brainsci14030292
Chicago/Turabian StyleKlotzek, Adam, Monem Jemni, Shad James Groves, and Frederick Robert Carrick. 2024. "Effects of Cervical Spinal Manipulation on Saccadic Eye Movements" Brain Sciences 14, no. 3: 292. https://doi.org/10.3390/brainsci14030292
APA StyleKlotzek, A., Jemni, M., Groves, S. J., & Carrick, F. R. (2024). Effects of Cervical Spinal Manipulation on Saccadic Eye Movements. Brain Sciences, 14(3), 292. https://doi.org/10.3390/brainsci14030292








