Akkermansia muciniphila Is Beneficial to a Mouse Model of Parkinson’s Disease, via Alleviated Neuroinflammation and Promoted Neurogenesis, with Involvement of SCFAs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatment and Reagents Preparations
2.1.1. Akk Culture
2.1.2. Antibiotics Preparation
2.1.3. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, M0896, Sigma-Aldrich, St. Louis, MO, USA) Preparation
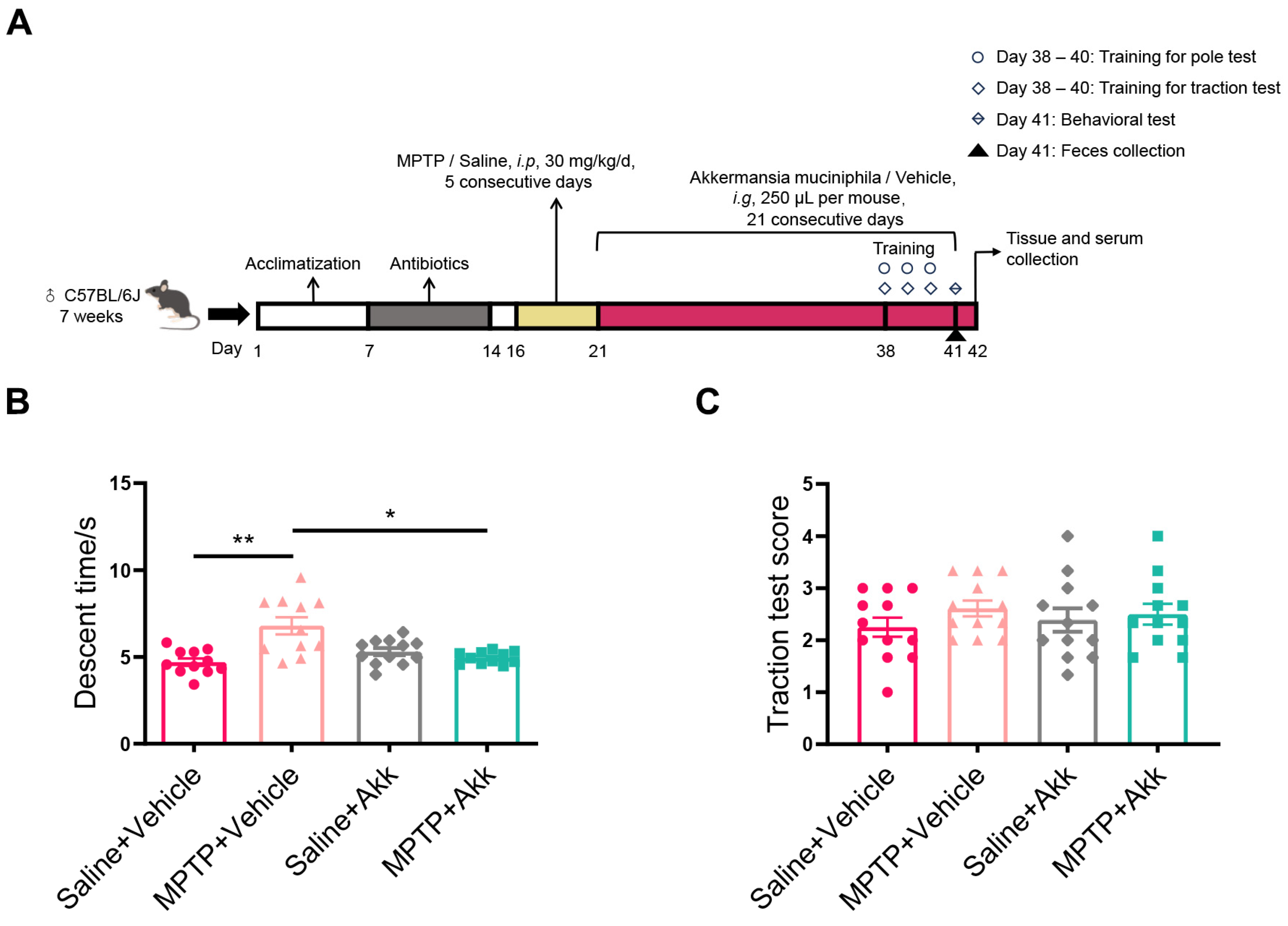
2.2. Mouse Study Design
2.3. Behavioral Tests
2.4. Sample Collection for Feces, Serum, Brain Tissues, and Colonic Tissue
2.5. Immunofluorescence (IF) Staining for SNpc and Hippocampus
2.6. Immunohistochemistry (IHC) Staining for Striatum
2.7. Western Blotting (WB) for Striatum, Hippocampus and Colon
2.8. Gas Chromatography-Mass Spectrometry (GC-MS) for Serum and Feces
2.9. Quantitative Real-Time PCR (qRT-PCR) for Colon
2.10. Statistical Analysis
3. Results
3.1. Akk Treatment Partially Improved Motor Dysfunction in MPTP-Induced PD Mice
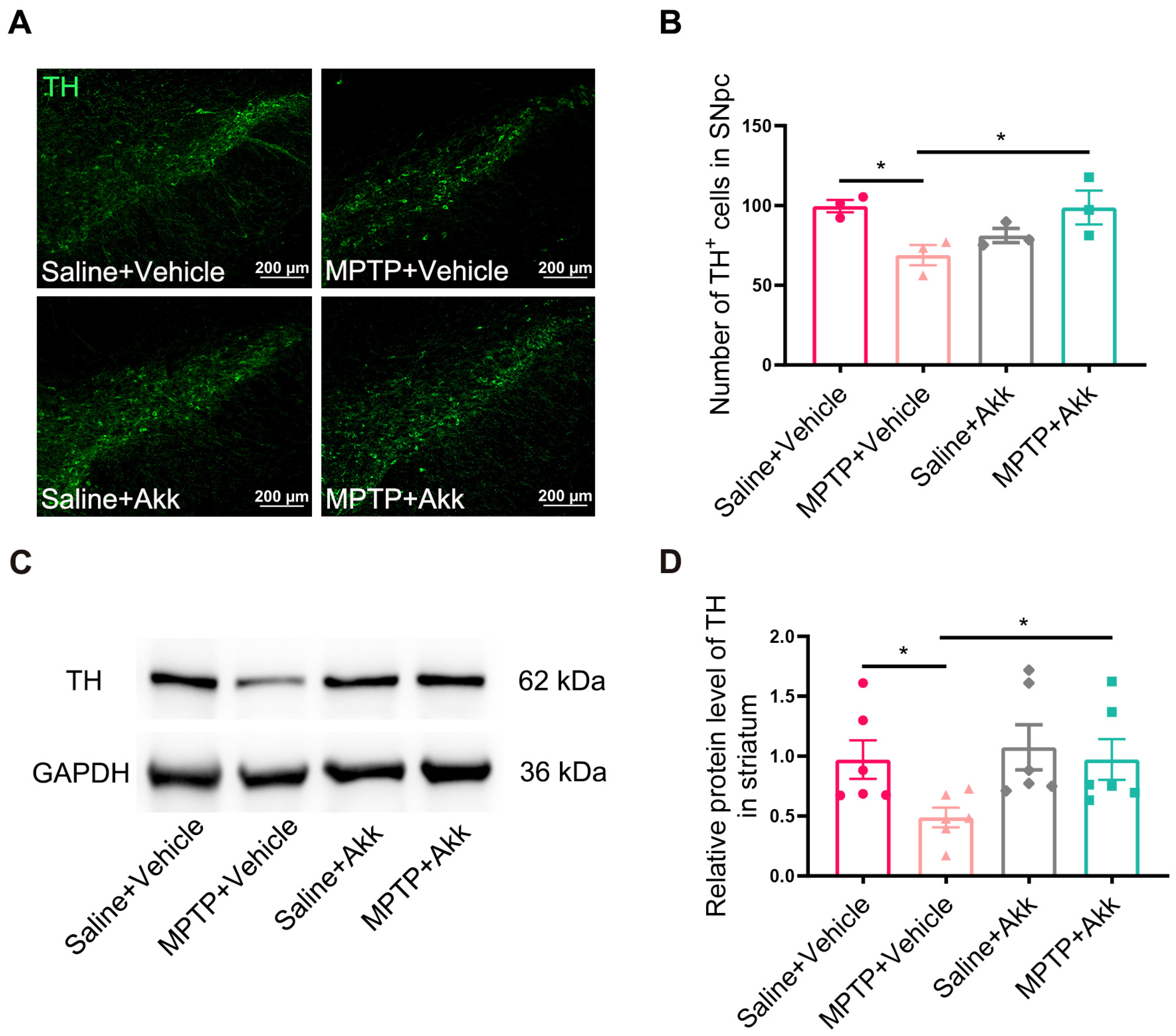
3.2. Akk Suppressed the Loss of Dopaminergic Neurons of SNpc and Reversed Striatal TH Expression in MPTP-Induced PD Mice
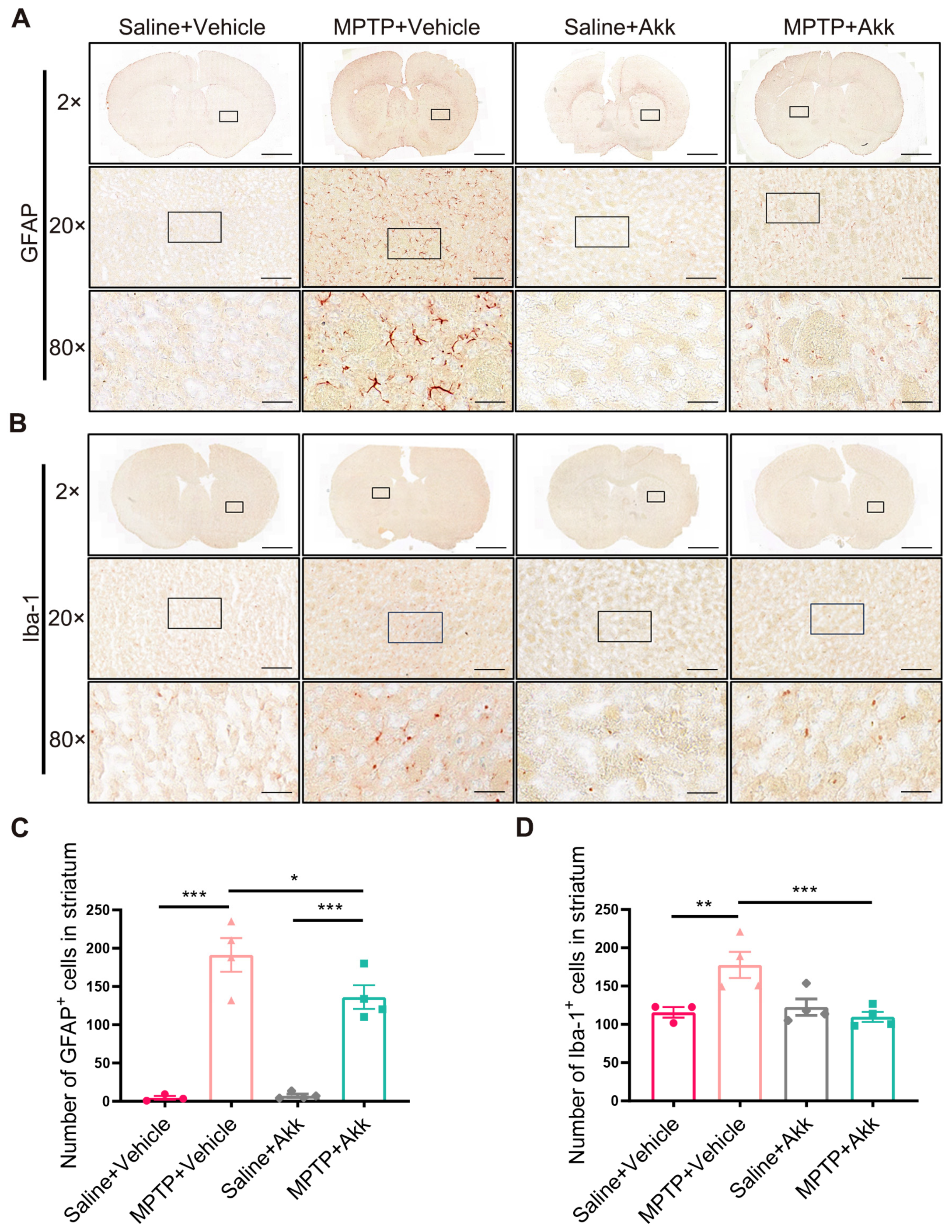
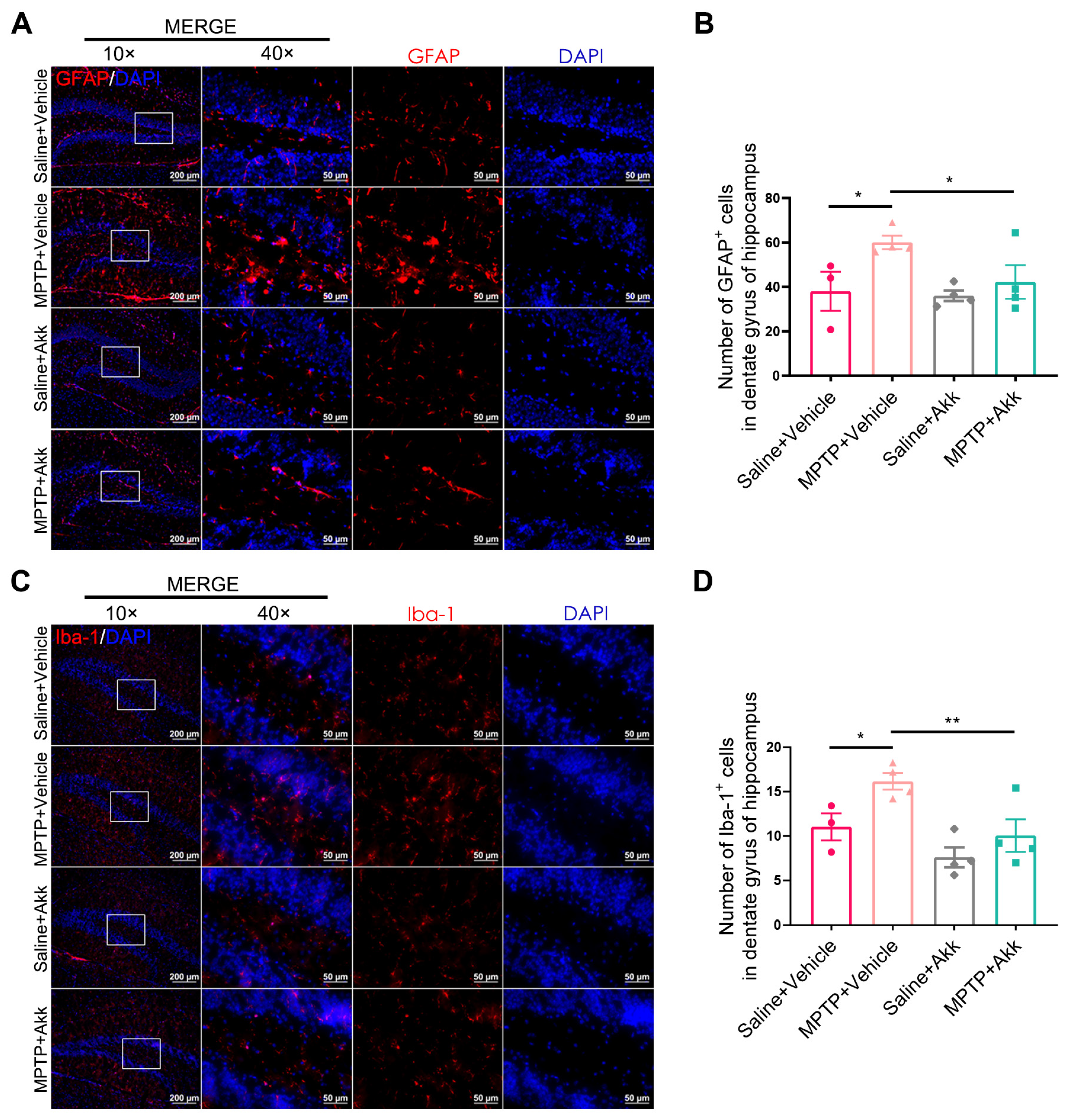
3.3. Akk Treatment Alleviated Neuroinflammation in Striatum and Hippocampus in MPTP-Induced PD Mice
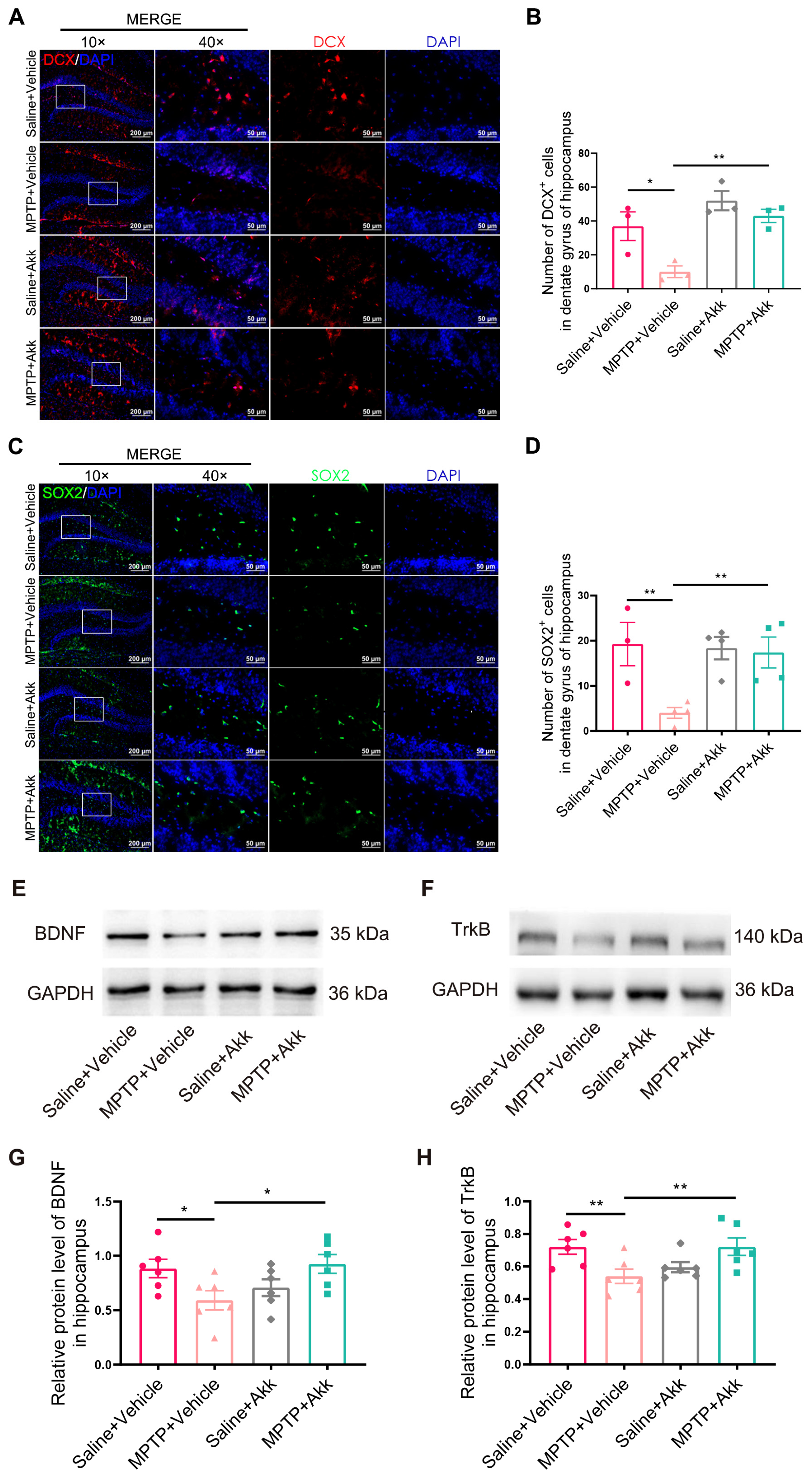
3.4. Akk Treatment Promoted Hippocampal Neurogenesis in MPTP-Induced PD Mice
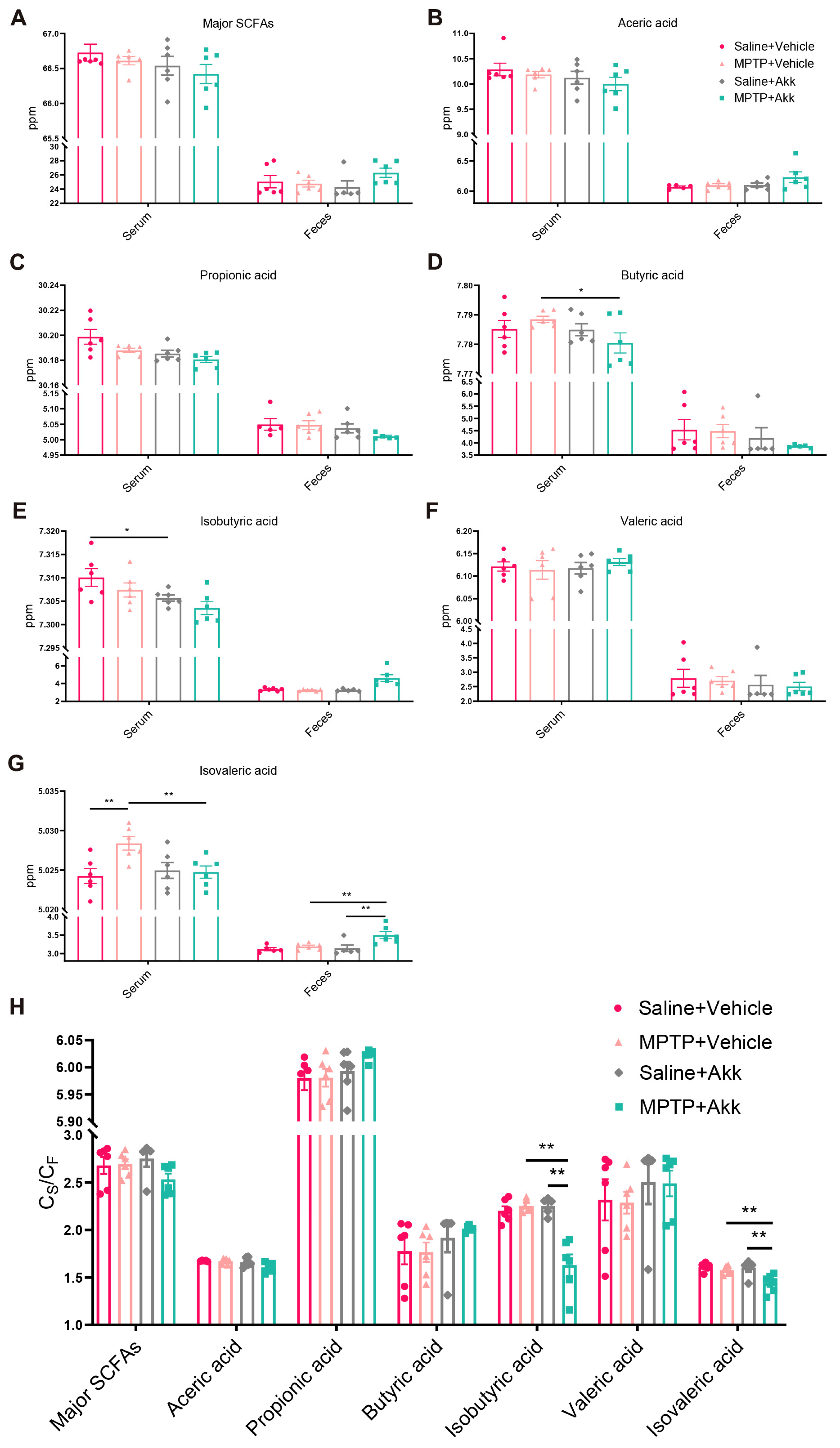
3.5. Akk Treatment Regulated Production of Serum and Fecal SCFAs, Partially Reduced Intestinal Permeability
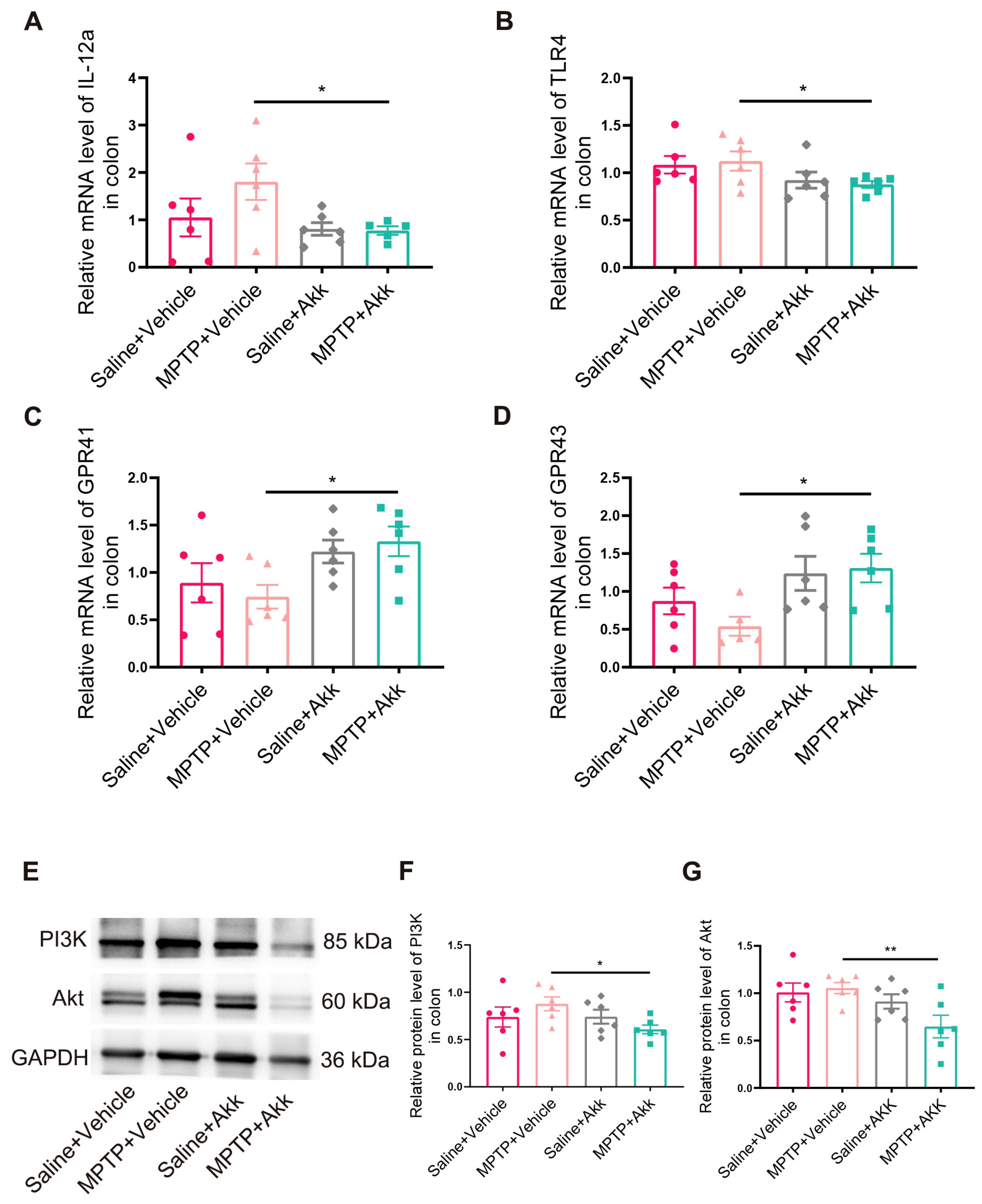
3.6. Effect of Akk Treatment on Intestinal Inflammation in MPTP-Induced PD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic—A Call to Action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Joshi, N.; Singh, S. Neurodegenerative signaling factors and mechanisms in Parkinson’s pathology. Toxicol. Vitro 2017, 43, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Greenamyre, J.T. Gene–environment interactions in Parkinson’s disease: Specific evidence in humans and mammalian models. Neurobiol. Dis. 2013, 57, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, X.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.; Jin, F.; Qin, B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 2017, 60, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-F.; Zhu, Y.-L.; Zhou, Z.-L.; Jia, X.-B.; Xu, Y.-D.; Yang, Q.; Cui, C.; Shen, Y.-Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Qiao, C.-M.; Zhou, Y.; Quan, W.; Ma, X.-Y.; Zhao, L.-P.; Shi, Y.; Hong, H.; Wu, J.; Niu, G.-Y.; Chen, Y.-N.; et al. Fecal Microbiota Transplantation from Aged Mice Render Recipient Mice Resistant to MPTP-Induced Nigrostriatal Degeneration Via a Neurogenesis-Dependent but Inflammation-Independent Manner. Neurotherapeutics 2023, 20, 1405–1426. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Qu, S.; Fan, L.; Qi, Y.; Xu, C.; Hu, Y.; Chen, S.; Liu, W.; Liu, W.; Si, J. Akkermansia muciniphila Alleviates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by NLRP3 Activation. Microbiol. Spectr. 2021, 9, e0073021. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Li, T.; Lin, X.; Shen, B.; Zhang, W.; Liu, Y.; Liu, H.; Wang, Y.; Zheng, L.; Zhi, F. Akkermansia muciniphila suppressing nonalcoholic steatohepatitis associated tumorigenesis through CXCR6+ natural killer T cells. Front. Immunol. 2022, 13, 1047570. [Google Scholar] [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes 2020, 10, 12. [Google Scholar] [CrossRef]
- Liu, S.; Rezende, R.M.; Moreira, T.G.; Tankou, S.K.; Cox, L.M.; Wu, M.; Song, A.; Dhang, F.H.; Wei, Z.; Costamagna, G.; et al. Oral Administration of miR-30d from Feces of MS Patients Suppresses MS-like Symptoms in Mice by Expanding Akkermansia muciniphila. Cell Host Microbe 2019, 26, 779–794.e778. [Google Scholar] [CrossRef]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef]
- Cui, C.; Shi, Y.; Hong, H.; Zhou, Y.; Qiao, C.; Zhao, L.; Jia, X.; Zhao, W.; Shen, Y. 5-HT4 Receptor is Protective for MPTP-induced Parkinson’s Disease Mice Via Altering Gastrointestinal Motility or Gut Microbiota. J. Neuroimmune Pharmacol. 2023, 18, 610–627. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The role of the probiotic Akkermansia muciniphila in brain functions: Insights underpinning therapeutic potential. Crit. Rev. Microbiol. 2023, 49, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhu, F.; Xu, H.; Xu, L.; Li, H.; Yang, X.; Afridi, S.K.; Lai, S.; Qiu, X.; Liu, C.; et al. CHI3L1 signaling impairs hippocampal neurogenesis and cognitive function in autoimmune-mediated neuroinflammation. Sci. Adv. 2023, 9, eadg8148. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Lim, J.; Choi, H.-J.; Kim, S.-H.; Choi, H.J. ERRγ Ligand Regulates Adult Neurogenesis and Depression-like Behavior in a LRRK2-G2019S-associated Young Female Mouse Model of Parkinson’s Disease. Neurotherapeutics 2022, 19, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Lv, L.; Liu, B.; Wang, S.; Zhang, S.; Wu, Z.; Yang, L.; Bian, X.; Wang, Q.; Wang, K.; et al. Akkermansia muciniphila Ameliorates Acetaminophen-Induced Liver Injury by Regulating Gut Microbial Composition and Metabolism. Microbiol. Spectr. 2022, 10, e0159621. [Google Scholar] [CrossRef]
- Shen, J.; Wang, S.; Xia, H.; Han, S.; Wang, Q.; Wu, Z.; Zhuge, A.; Li, S.; Chen, H.; Lv, L.; et al. Akkermansia muciniphila attenuated lipopolysaccharide-induced acute lung injury by modulating the gut microbiota and SCFAs in mice. Food Funct. 2023, 14, 10401–10417. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, C.-C.; Liao, H.-Y.; Lin, Y.-T.; Wu, Y.-W.; Liou, J.-M.; Wu, M.-S.; Kuo, C.-H.; Lin, C.-H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids with Gut Microbiota and Clinical Severity in Patients with Parkinson Disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef]
- Zhou, Z.-L.; Jia, X.-B.; Sun, M.-F.; Zhu, Y.-L.; Qiao, C.-M.; Zhang, B.-P.; Zhao, L.-P.; Yang, Q.; Cui, C.; Chen, X.; et al. Neuroprotection of Fasting Mimicking Diet on MPTP-Induced Parkinson’s Disease Mice via Gut Microbiota and Metabolites. Neurotherapeutics 2019, 16, 741–760. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e1321. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e1713. [Google Scholar] [CrossRef] [PubMed]
- Manocha, G.D.; Floden, A.M.; Puig, K.L.; Nagamoto-Combs, K.; Scherzer, C.R.; Combs, C.K. Defining the contribution of neuroinflammation to Parkinson’s disease in humanized immune system mice. Mol. Neurodegener. 2017, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mishra, A.; Mishra, S.K.; Shukla, S. ALCAR promote adult hippocampal neurogenesis by regulating cell-survival and cell death-related signals in rat model of Parkinson’s disease like-phenotypes. Neurochem. Int. 2017, 108, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Fuchigami, T.; Itokazu, Y.; Morgan, J.C.; Yu, R.K. Restoration of Adult Neurogenesis by Intranasal Administration of Gangliosides GD3 and GM1 in The Olfactory Bulb of A53T Alpha-Synuclein-Expressing Parkinson’s-Disease Model Mice. Mol. Neurobiol. 2023, 60, 3329–3344. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ai, P.; He, X.; Mo, C.; Zhang, Y.; Xu, S.; Lai, Y.; Qian, Y.; Xiao, Q. Parkinson’s Disease Is Associated with Impaired Gut–Blood Barrier for Short-Chain Fatty Acids. Mov. Disord. 2022, 37, 1634–1643. [Google Scholar] [CrossRef]
- Jaworska, K.; Konop, M.; Bielinska, K.; Hutsch, T.; Dziekiewicz, M.; Banaszkiewicz, A.; Ufnal, M. Inflammatory bowel disease is associated with increased gut-to-blood penetration of short-chain fatty acids: A new, non-invasive marker of a functional intestinal lesion. Exp. Physiol. 2019, 104, 1226–1236. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Le, W. Intestinal Permeability, Dysbiosis, Inflammation and Enteric Glia Cells: The Intestinal Etiology of Parkinson’s Disease. Aging Dis. 2022, 13, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zhang, Q.M.; Ni, W.W.; Zhang, X.; Li, Y.; Li, A.L.; Du, P.; Li, C.; Yu, S.S. Modulatory effect of Lactobacillus acidophilus KLDS 1.0738 on intestinal short-chain fatty acids metabolism and GPR41/43 expression in β-lactoglobulin-sensitized mice. Med. Microbiol. Immunol. 2019, 63, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406.e10. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Du, H.; Zhang, M.; Xu, H.; Pu, X.; Chen, Q.; Luo, R.; Hu, Y.; Wang, Y.; Tu, H.; et al. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/mTOR signaling pathway and regulating gut microbiota. Phytother. Res. 2022, 36, 2081–2094. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Cui, C.; Hong, H.; Shi, Y.; Zhou, Y.; Qiao, C.-M.; Zhao, W.-J.; Zhao, L.-P.; Wu, J.; Quan, W.; Niu, G.-Y.; et al. Vancomycin Pretreatment on MPTP-Induced Parkinson’s Disease Mice Exerts Neuroprotection by Suppressing Inflammation Both in Brain and Gut. J. Neuroimmune Pharmacol. 2023, 18, 72–89. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Chaiyasut, C. Role of the Gut–Brain Axis, Gut Microbial Composition, Diet, and Probiotic Intervention in Parkinson’s Disease. Microorganisms 2022, 10, 1544. [Google Scholar] [CrossRef]
- Davey, L.E.; Malkus, P.N.; Villa, M.; Dolat, L.; Holmes, Z.C.; Letourneau, J.; Ansaldo, E.; David, L.A.; Barton, G.M.; Valdivia, R.H. A genetic system for Akkermansia muciniphila reveals a role for mucin foraging in gut colonization and host sterol biosynthesis gene expression. Nat. Microbiol. 2023, 8, 1450–1467. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Lawenius, L.; Scheffler, J.M.; Gustafsson, K.L.; Henning, P.; Nilsson, K.H.; Colldén, H.; Islander, U.; Plovier, H.; Cani, P.D.; de Vos, W.M.; et al. Pasteurized Akkermansia muciniphila protects from fat mass gain but not from bone loss. Am. J. Physiol.-Endocrinol. Metab. 2020, 318, E480–E491. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, J.; Wei, K.; Wei, J.; Tian, P.; Yue, M.; Wang, Y.; Hong, D.; Li, F.; Wang, B.; et al. Neuroprotective Effect of Ceftriaxone on MPTP-Induced Parkinson’s Disease Mouse Model by Regulating Inflammation and Intestinal Microbiota. Oxidative Med. Cell. Longev. 2021, 2021, 9424582. [Google Scholar] [CrossRef]
- Li, T.; Chu, C.; Yu, L.; Zhai, Q.; Wang, S.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients 2022, 14, 4678. [Google Scholar] [CrossRef]
- Yue, M.; Wei, J.; Chen, W.; Hong, D.; Chen, T.; Fang, X. Neurotrophic Role of the Next-Generation Probiotic Strain L. lactis MG1363-pMG36e-GLP-1 on Parkinson’s Disease via Inhibiting Ferroptosis. Nutrients 2022, 14, 4886. [Google Scholar] [CrossRef]
- Nie, S.; Wang, J.; Deng, Y.; Ye, Z.; Ge, Y. Inflammatory microbes and genes as potential biomarkers of Parkinson’s disease. NPJ Biofilms Microbiomes 2022, 8, 101. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Yoshida, T.; Hanada, H.; Takeuchi, I.; et al. Short chain fatty acids-producing and mucin-degrading intestinal bacteria predict the progression of early Parkinson’s disease. NPJ Park. Dis. 2022, 8, 65. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wüllner, U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guo, X.; Zhang, M.; Ou, Z.; Wu, D.; Deng, L.; Lu, Z.; Zhang, J.; Deng, G.; Chen, S.; et al. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe 2020, 61, 102138. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhou, X.; Miao, Y.; Han, Y.; Wei, J.; Chen, T. Therapeutic effect of GLP-1 engineered strain on mice model of Alzheimer’s disease and Parkinson’s disease. AMB Express 2020, 10, 80. [Google Scholar] [CrossRef]
- Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Flor-García, M.; Rodríguez-Moreno, C.B.; Trinchero, M.F.; Cafini, F.; Rábano, A.; Llorens-Martín, M. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science 2021, 374, 1106–1113. [Google Scholar] [CrossRef]
- Wei, Z.-Y.D.; Liang, K.; Shetty, A.K. Role of Microglia, Decreased Neurogenesis and Oligodendrocyte Depletion in Long COVID-Mediated Brain Impairments. Aging Dis. 2023, 14, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Ziv, Y.; Schwartz, A.; Landa, G.; Talpalar, A.E.; Pluchino, S.; Martino, G.; Schwartz, M. Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006, 31, 149–160. [Google Scholar] [CrossRef]
- Du, Y.; Song, L.; Dong, X.; Li, H.; Xie, W.; Wang, Y.; Che, H. Long-Term Krill Oil Administration Alleviated Early Mild Cognitive Impairment in APP/PS1 Mice. Mol. Nutr. Food Res. 2023, e2200652. [Google Scholar] [CrossRef]
- Dehghan, S.; Asadi, S.; Hajikaram, M.; Soleimani, M.; Mowla, S.J.; Fathollahi, Y.; Ahmadiani, A.; Javan, M. Exogenous Oct4 in combination with valproic acid increased neural progenitor markers: An approach for enhancing the repair potential of the brain. Life Sci. 2015, 122, 108–115. [Google Scholar] [CrossRef]
- Lei, E.; Vacy, K.; Boon, W.C. Fatty acids and their therapeutic potential in neurological disorders. Neurochem. Int. 2016, 95, 75–84. [Google Scholar] [CrossRef]
- Liu, M.-J.; Yang, J.-Y.; Yan, Z.-H.; Hu, S.; Li, J.-Q.; Xu, Z.-X.; Jian, Y.-P. Recent findings in Akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin. Nutr. 2022, 41, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-F.; Wang, C.-Y.; Wang, J.-H.; Wang, Q.-N.; Li, S.-J.; Wang, H.-O.; Zhou, F.; Li, J.-M. Short-Chain Fatty Acids Ameliorate Depressive-like Behaviors of High Fructose-Fed Mice by Rescuing Hippocampal Neurogenesis Decline and Blood–Brain Barrier Damage. Nutrients 2022, 14, 1882. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.-J.; Xie, J.; Chen, J.-J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef]
- Ishii, T.; Furuoka, H.; Kaya, M.; Kuhara, T. Oral Administration of Probiotic Bifidobacterium breve Improves Facilitation of Hippocampal Memory Extinction via Restoration of Aberrant Higher Induction of Neuropsin in an MPTP-Induced Mouse Model of Parkinson’s Disease. Biomedicines 2021, 9, 167. [Google Scholar] [CrossRef]
| Genes | Forward and Reverse Sequences |
|---|---|
| Gapdh | Forward: 5′-AGGTCGGTGTGAACGGATTTG-3′ Reverse: 5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
| Gpr41 | Forward: 5′-CTTCTTTCTTGGCAATTACTGGC-3′ Reverse: 5′-CCGAAATGGTCAGGTTTAGCAA-3′ |
| Gpr43 | Forward: 5′-CTTGATCCTCACGGCCTACAT-3′ Reverse: 5′-CCAGGGTCAGATTAAGCAGGAG-3′ |
| Il-12a | Forward: 5′-CTGTGCCTTGGTAGCATCTATG-3′ Reverse: 5′-GCAGAGTCTCGCCATTATGATTC-3′ |
| Tlr4 | Forward: 5′-ATGGCATGGCTTACACCACC-3′ Reverse: 5′-GAGGCCAATTTTGTCTCCACA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, C.-M.; Huang, W.-Y.; Zhou, Y.; Quan, W.; Niu, G.-Y.; Li, T.; Zhang, M.-X.; Wu, J.; Zhao, L.-P.; Zhao, W.-J.; et al. Akkermansia muciniphila Is Beneficial to a Mouse Model of Parkinson’s Disease, via Alleviated Neuroinflammation and Promoted Neurogenesis, with Involvement of SCFAs. Brain Sci. 2024, 14, 238. https://doi.org/10.3390/brainsci14030238
Qiao C-M, Huang W-Y, Zhou Y, Quan W, Niu G-Y, Li T, Zhang M-X, Wu J, Zhao L-P, Zhao W-J, et al. Akkermansia muciniphila Is Beneficial to a Mouse Model of Parkinson’s Disease, via Alleviated Neuroinflammation and Promoted Neurogenesis, with Involvement of SCFAs. Brain Sciences. 2024; 14(3):238. https://doi.org/10.3390/brainsci14030238
Chicago/Turabian StyleQiao, Chen-Meng, Wen-Yan Huang, Yu Zhou, Wei Quan, Gu-Yu Niu, Ting Li, Mei-Xuan Zhang, Jian Wu, Li-Ping Zhao, Wei-Jiang Zhao, and et al. 2024. "Akkermansia muciniphila Is Beneficial to a Mouse Model of Parkinson’s Disease, via Alleviated Neuroinflammation and Promoted Neurogenesis, with Involvement of SCFAs" Brain Sciences 14, no. 3: 238. https://doi.org/10.3390/brainsci14030238
APA StyleQiao, C.-M., Huang, W.-Y., Zhou, Y., Quan, W., Niu, G.-Y., Li, T., Zhang, M.-X., Wu, J., Zhao, L.-P., Zhao, W.-J., Cui, C., & Shen, Y.-Q. (2024). Akkermansia muciniphila Is Beneficial to a Mouse Model of Parkinson’s Disease, via Alleviated Neuroinflammation and Promoted Neurogenesis, with Involvement of SCFAs. Brain Sciences, 14(3), 238. https://doi.org/10.3390/brainsci14030238






