Transcranial Direct Current Stimulation Decreases P3 Amplitude and Inherent Delta Activity during a Waiting Impulsivity Paradigm: Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. General Procedure
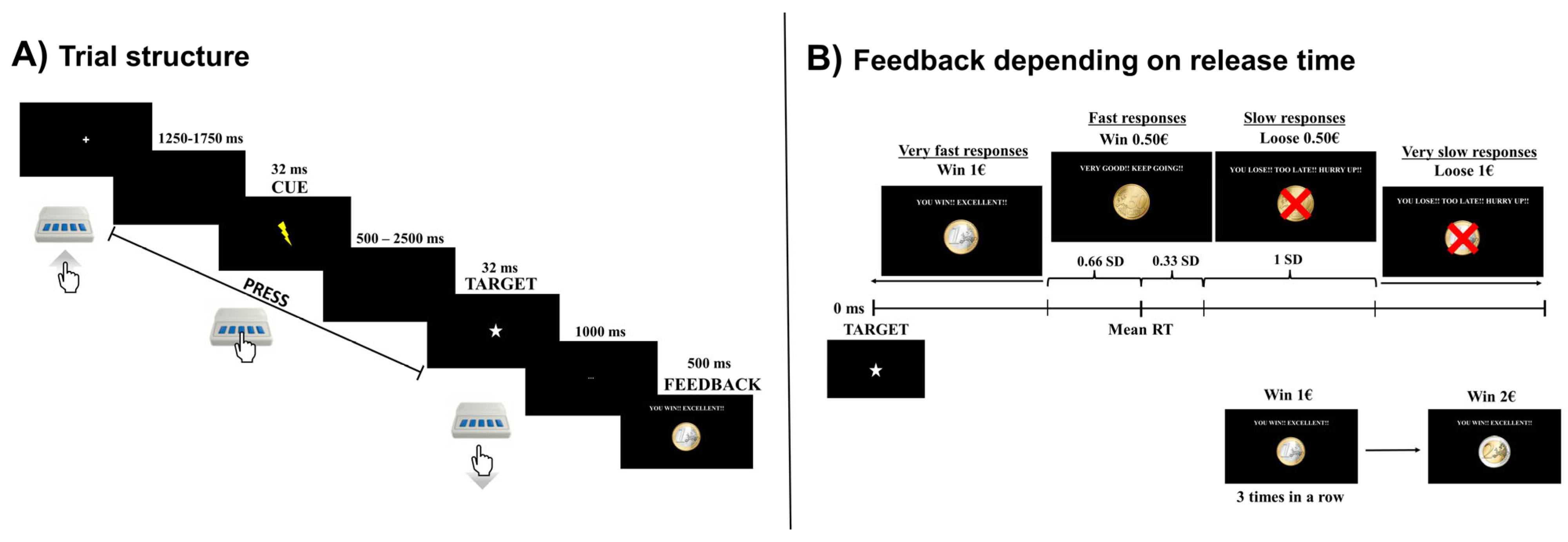
2.3. Cued Premature Response Task
2.4. Control Assignments
2.4.1. Stop-Signal Reaction Time Task
2.4.2. 27-Item Monetary Choice Questionnaires
2.5. Transcranial Direct Current Stimulation
2.6. Electrophysiological Acquisition and Data Analysis
2.6.1. Event-Related Potentials
2.6.2. Event-Related Oscillations
2.7. Statistical Analysis
3. Results
3.1. Behavioral Analysis
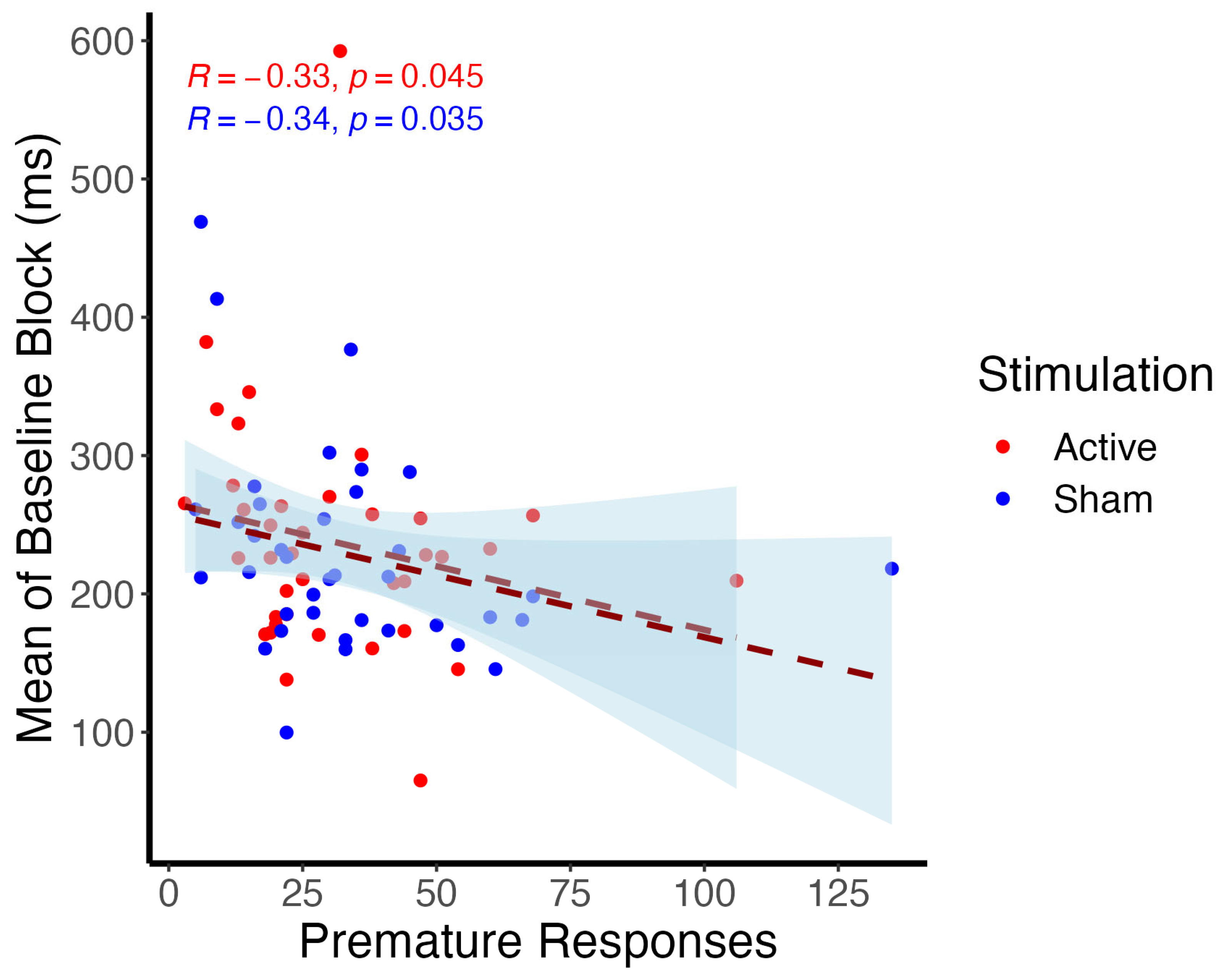
3.1.1. Cued Premature Response Task
3.1.2. Stop Signal Reaction Time Task
3.1.3. 27-Item Monetary Choice Questionnaires
3.2. EEG Analysis
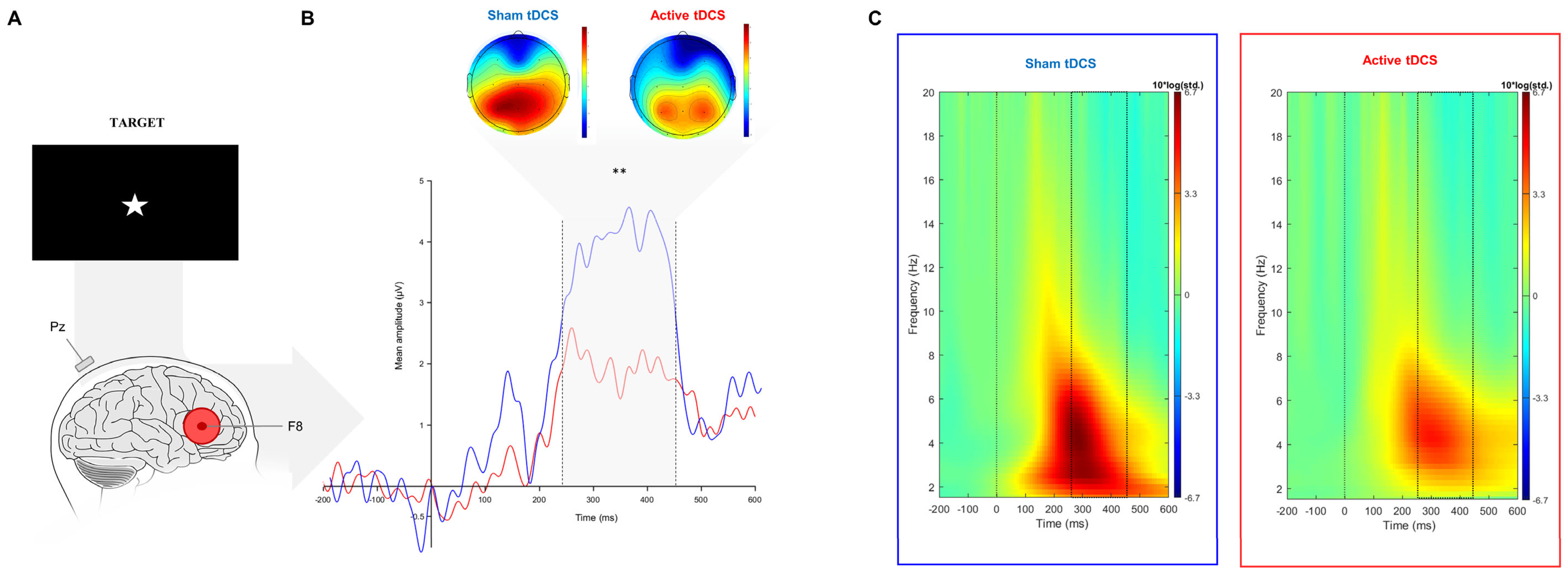
3.2.1. Event-Related Potentials
3.2.2. Event-Related Oscillations
4. Discussion
4.1. Electrophysiological Correlates
4.2. Behavioral Outcomes
4.3. Limitations and Future Directions
4.4. General Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lapenta, O.M.; Marques, L.M.; Rego, G.G.; Comfort, W.E.; Boggio, P.S. TDCS in Addiction and Impulse Control Disorders. J. ECT 2018, 34, 182–192. [Google Scholar] [CrossRef]
- Carvalho, S.; Sampaio, A.; Mendes, A.J.; Lema, A.; Vieira, D.; Gonçalves, Ó.F.; Leite, J. Polarity Specific Effects of Cross-Hemispheric TDCS Coupled with Approach-Avoidance Training on Chocolate Craving. Front. Pharmacol. 2019, 9, 1500. [Google Scholar] [CrossRef] [PubMed]
- Terenzi, D.; Catalan, M.; Polverino, P.; Bertolotti, C.; Manganotti, P.; Rumiati, R.I.; Aiello, M. Effects of TDCS on Reward Responsiveness and Valuation in Parkinson’s Patients with Impulse Control Disorders. J. Neurol. 2021, 269, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.A.; Schwippel, T.; Wolz, I.; Svaldi, J. Meta-Analysis of the Effects of Transcranial Direct Current Stimulation on Inhibitory Control. Brain Stimul. 2020, 13, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.T.; Chopard, G.; Nicolier, M.; Gabriel, D.; Masse, C.; Giustiniani, J.; Vandel, P.; Haffen, E.; Bennabi, D. Can Transcranial Direct Current Stimulation (TDCS) Improve Impulsivity in Healthy and Psychiatric Adult Populations? A Systematic Review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 98, 109814. [Google Scholar] [CrossRef]
- Dalley, J.W.; Everitt, B.J.; Robbins, T.W. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.W.; Robbins, T.W. Fractionating Impulsivity: Neuropsychiatric Implications. Nat. Rev. Neurosci. 2017, 18, 158–171. [Google Scholar] [CrossRef]
- Reynolds, B.; Ortengren, A.; Richards, J.B.; de Wit, H. Dimensions of Impulsive Behavior: Personality and Behavioral Measures. Personal. Individ. Differ. 2006, 40, 305–315. [Google Scholar] [CrossRef]
- Voon, V. Models of Impulsivity with a Focus on Waiting Impulsivity: Translational Potential for Neuropsychiatric Disorders. Curr. Addict. Rep. 2014, 1, 281–288. [Google Scholar] [CrossRef]
- Morris, L.S.; Kundu, P.; Baek, K.; Irvine, M.A.; Mechelmans, D.J.; Wood, J.; Harrison, N.A.; Robbins, T.W.; Bullmore, E.T.; Voon, V. Jumping the Gun: Mapping Neural Correlates of Waiting Impulsivity and Relevance across Alcohol Misuse. Biol. Psychiatry 2016, 79, 499–507. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An Integrative Theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Pfabigan, D.M.; Seidel, E.M.; Sladky, R.; Hahn, A.; Paul, K.; Grahl, A.; Küblböck, M.; Kraus, C.; Hummer, A.; Kranz, G.S.; et al. P300 Amplitude Variation Is Related to Ventral Striatum BOLD Response during Gain and Loss Anticipation: An EEG and FMRI Experiment. NeuroImage 2014, 96, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Broyd, S.J.; Richards, H.J.; Helps, S.K.; Chronaki, G.; Bamford, S.; Sonuga-Barke, E.J.S. An Electrophysiological Monetary Incentive Delay (e-MID) Task: A Way to Decompose the Different Components of Neural Response to Positive and Negative Monetary Reinforcement. J. Neurosci. Methods 2012, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Glazer, J.E.; Kelley, N.J.; Pornpattananangkul, N.; Mittal, V.A.; Nusslock, R. Beyond the FRN: Broadening the Time-Course of EEG and ERP Components Implicated in Reward Processing. Int. J. Psychophysiol. 2018, 132, 184–202. [Google Scholar] [CrossRef]
- Angus, D.J.; Latham, A.J.; Harmon-Jones, E.; Deliano, M.; Balleine, B.; Braddon-Mitchell, D. Electrocortical Components of Anticipation and Consumption in a Monetary Incentive Delay Task. Psychophysiology 2017, 54, 1686–1705. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.D.; Foti, D. Teasing Apart the Anticipatory and Consummatory Processing of Monetary Incentives: An Event-Related Potential Study of Reward Dynamics. Psychophysiology 2015, 52, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Vignapiano, A.; Mucci, A.; Ford, J.; Montefusco, V.; Plescia, G.M.; Bucci, P.; Galderisi, S. Reward Anticipation and Trait Anhedonia: An Electrophysiological Investigation in Subjects with Schizophrenia. Clin. Neurophysiol. 2016, 127, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Rach, S.; Vosskuhl, J.; Strüber, D. Time-Frequency Analysis of Event-Related Potentials: A Brief Tutorial. Brain Topogr. 2014, 27, 438–450. [Google Scholar] [CrossRef]
- Güntekin, B.; Başar, E. Review of Evoked and Event-Related Delta Responses in the Human Brain. Int. J. Psychophysiol. 2016, 103, 43–52. [Google Scholar] [CrossRef]
- Demiralp, T.; Ademoglu, A.; Istefanopulos, Y.; Başar-Eroglu, C.; Başar, E. Wavelet Analysis of Oddball P300. Int. J. Psychophysiol. 2001, 39, 221–227. [Google Scholar] [CrossRef]
- Pornpattananangkul, N.; Nusslock, R. Willing to Wait: Elevated Reward-Processing EEG Activity Associated with a Greater Preference for Larger-but-Delayed Rewards. Neuropsychologia 2016, 91, 141–162. [Google Scholar] [CrossRef]
- Mendes, A.J.; Pacheco-Barrios, K.; Lema, A.; Gonçalves, Ó.F.; Fregni, F.; Leite, J.; Carvalho, S. Modulation of the Cognitive Event-Related Potential P3 by Transcranial Direct Current Stimulation: Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2022, 132, 894–907. [Google Scholar] [CrossRef]
- Lapenta, O.M.; Di Sierve, K.; de Macedo, E.C.; Fregni, F.; Boggio, P.S. Transcranial Direct Current Stimulation Modulates ERP-Indexed Inhibitory Control and Reduces Food Consumption. Appetite 2014, 83, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Cunillera, T.; Brignani, D.; Cucurell, D.; Fuentemilla, L.; Miniussi, C. The Right Inferior Frontal Cortex in Response Inhibition: A TDCS–ERP Co-Registration Study. Neuroimage 2016, 140, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Verveer, I.; Remmerswaal, D.; van der Veen, F.M.; Franken, I.H.A. Long-Term TDCS Effects on Neurophysiological Measures of Cognitive Control in Tobacco Smokers. Biol. Psychol. 2020, 156, 107962. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.; Morales-Quezada, L.; Carvalho, S.; Thibaut, A.; Doruk, D.; Chen, C.-F.; Schachter, S.C.; Rotenberg, A.; Fregni, F. Surface EEG-Transcranial Direct Current Stimulation (TDCS) Closed-Loop System. Int. J. Neural Syst. 2017, 27, 1750026. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the Right Inferior Frontal Cortex: One Decade On. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Steer, R.; Brown, G. Beck Depression Inventory-II. San Antonio 1996, 78, 490–498. [Google Scholar] [CrossRef]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; De La Fente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef]
- Hildebrand, M. The Psychometric Properties of the Drug Use Disorders Identification Test (DUDIT): A Review of Recent Research. J. Subst. Abus. Treat. 2015, 53, 52–59. [Google Scholar] [CrossRef]
- Band, G.P.H.; van Boxtel, G.J.M. Inhibitory Motor Control in Stop Paradigms: Review and Reinterpretation of Neural Mechanisms. Acta Psychol. 1999, 101, 179–211. [Google Scholar] [CrossRef] [PubMed]
- Kirby, K.N.; Petry, N.M.; Bickel, W.K. Heroin Addicts Have Higher Discount Rates for Delayed Rewards than Non-Drug-Using Controls. J. Exp. Psychol. Gen. 1999, 128, 78. [Google Scholar] [CrossRef]
- Voon, V.; Chang-Webb, Y.C.; Morris, L.S.; Cooper, E.; Sethi, A.; Baek, K.; Grant, J.; Robbins, T.W.; Harrison, N.A. Waiting Impulsivity: The Influence of Acute Methylphenidate and Feedback. Int. J. Neuropsychopharmacol. 2016, 19, pyv074. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Aron, A.R.; Band, G.P.H.; Beste, C.; Bissett, P.G.; Brockett, A.T.; Brown, J.W.; Chamberlain, S.R.; Chambers, C.D.; Colonius, H.; et al. A Consensus Guide to Capturing the Ability to Inhibit Actions and Impulsive Behaviors in the Stop-Signal Task. eLife 2019, 8, e46323. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.; Mumford, J.A.; Cohen, J.R.; Galvan, A.; Canli, T.; Poldrack, R.A. Measurement and Reliability of Response Inhibition. Front. Psychol. 2012, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.A.; Amlung, M.; Reed, D.D.; Jarmolowicz, D.P.; McKerchar, T.L.; Lemley, S.M. Automating Scoring of Delay Discounting for the 21- and 27-Item Monetary Choice Questionnaires. Behav. Anal. 2016, 39, 293–304. [Google Scholar] [CrossRef]
- Breitling, C.; Zaehle, T.; Dannhauer, M.; Bonath, B.; Tegelbeckers, J.; Flechtner, H.H.; Krauel, K. Improving Interference Control in ADHD Patients with Transcranial Direct Current Stimulation (TDCS). Front. Cell Neurosci. 2016, 10, 72. [Google Scholar] [CrossRef]
- Leite, J.; Gonçalves, Ó.F.; Pereira, P.; Khadka, N.; Bikson, M.; Fregni, F.; Carvalho, S. The Differential Effects of Unihemispheric and Bihemispheric TDCS over the Inferior Frontal Gyrus on Proactive Control. Neurosci. Res. 2018, 130, 39–45. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Perrin, F.; Pernier, J.; Bertrand, O.; Echallier, J.F. Spherical Splines for Scalp Potential and Current Density Mapping. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 184–187. [Google Scholar] [CrossRef]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. The ICLabel Dataset of Electroencephalographic (EEG) Independent Component (IC) Features. Data Brief. 2019, 25, 104101. [Google Scholar] [CrossRef] [PubMed]
- Grandchamp, R.; Delorme, A. Single-Trial Normalization for Event-Related Spectral Decomposition Reduces Sensitivity to Noisy Trials. Front. Psychol. 2011, 2, 236. [Google Scholar] [CrossRef] [PubMed]
- Gyurkovics, M.; Clements, G.M.; Low, K.A.; Fabiani, M.; Gratton, G. The Impact of 1/f Activity and Baseline Correction on the Results and Interpretation of Time-Frequency Analyses of EEG/MEG Data: A Cautionary Tale. Neuroimage 2021, 237, 118192. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R, version 4.0.3; R Core Team: Vienna, Austria, 2018.
- Harper, J.; Malone, S.M.; Bernat, E.M. Theta and Delta Band Activity Explain N2 and P3 ERP Component Activity in a Go/No-Go Task. Clin. Neurophysiol. 2014, 125, 124–132. [Google Scholar] [CrossRef]
- Dallmer-Zerbe, I.; Popp, F.; Lam, A.P.; Philipsen, A.; Herrmann, C.S. Transcranial Alternating Current Stimulation (TACS) as a Tool to Modulate P300 Amplitude in Attention Deficit Hyperactivity Disorder (ADHD): Preliminary Findings. Brain Topogr. 2020, 33, 191–207. [Google Scholar] [CrossRef]
- Wu, H.; Miller, K.J.; Blumenfeld, Z.; Williams, N.R.; Ravikumar, V.K.; Lee, K.E.; Kakusa, B.; Sacchet, M.D.; Wintermark, M.; Christoffel, D.J.; et al. Closing the Loop on Impulsivity via Nucleus Accumbens Delta-Band Activity in Mice and Man. Proc. Natl. Acad. Sci. USA 2018, 115, 192–197. [Google Scholar] [CrossRef]
- Weafer, J.; Crane, N.A.; Gorka, S.M.; Phan, K.L.; de Wit, H. Neural Correlates of Inhibition and Reward Are Negatively Associated. NeuroImage 2019, 196, 188–194. [Google Scholar] [CrossRef]
- Guleken, Z.; Sutcubasi, B.; Metin, B. The Cognitive Dynamics of Small-Sooner over Large-Later Preferences during Temporal Discounting Task through Event-Related Oscillations (EROs). Neuropsychologia 2021, 162, 108046. [Google Scholar] [CrossRef] [PubMed]
- Jahfari, S.; Waldorp, L.; van den Wildenberg, W.P.M.; Scholte, H.S.; Ridderinkhof, K.R.; Forstmann, B.U. Effective Connectivity Reveals Important Roles for Both the Hyperdirect (Fronto-Subthalamic) and the Indirect (Fronto-Striatal-Pallidal) Fronto-Basal Ganglia Pathways during Response Inhibition. J. Neurosci. 2011, 31, 6891–6899. [Google Scholar] [CrossRef]
- Fertonani, A.; Miniussi, C. Transcranial Electrical Stimulation: What We Know and Do Not Know about Mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Cunillera, T.; Fuentemilla, L.; Brignani, D.; Cucurell, D.; Miniussi, C. A Simultaneous Modulation of Reactive and Proactive Inhibition Processes by Anodal TDCS on the Right Inferior Frontal Cortex. PLoS ONE 2014, 9, e113537. [Google Scholar] [CrossRef] [PubMed]
- Campanella, S.; Schroder, E.; Vanderhasselt, M.A.; Baeken, C.; Kornreich, C.; Verbanck, P.; Burle, B. Short-Term Impact of TDCS Over the Right Inferior Frontal Cortex on Impulsive Responses in a Go/No-Go Task. Clin. EEG Neurosci. 2018, 49, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, S.; Liu, J.; Li, D.; Feng, Z.; Wang, Q.; Chen, C.; Xue, G. The Role of the Frontal and Parietal Cortex in Proactive and Reactive Inhibitory Control: A Transcranial Direct Current Stimulation Study. J. Cogn. Neurosci. 2016, 28, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Voon, V.; Irvine, M.A.; Derbyshire, K.; Worbe, Y.; Lange, I.; Abbott, S.; Morein-Zamir, S.; Dudley, R.; Caprioli, D.; Harrison, N.A.; et al. Measuring “Waiting” Impulsivity in Substance Addictions and Binge Eating Disorder in a Novel Analogue of Rodent Serial Reaction Time Task. Biol. Psychiatry 2014, 75, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.W.; Ersche, K.D. Neural Circuitry and Mechanisms of Waiting Impulsivity: Relevance to Addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180145. [Google Scholar] [CrossRef] [PubMed]
- Ballard, K.; Knutson, B. Dissociable Neural Representations of Future Reward Magnitude and Delay during Temporal Discounting. Neuroimage 2009, 45, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Fonteneau, C.; Redoute, J.; Haesebaert, F.; Le Bars, D.; Costes, N.; Suaud-Chagny, M.-F.; Brunelin, J. Frontal Transcranial Direct Current Stimulation Induces Dopamine Release in the Ventral Striatum in Human. Cereb. Cortex 2018, 28, 2636–2646. [Google Scholar] [CrossRef]
- Fukai, M.; Bunai, T.; Hirosawa, T.; Kikuchi, M.; Ito, S.; Minabe, Y.; Ouchi, Y. Endogenous Dopamine Release under Transcranial Direct-Current Stimulation Governs Enhanced Attention: A Study with Positron Emission Tomography. Transl. Psychiatry 2019, 9, 115. [Google Scholar] [CrossRef]
- Robbins, T.W.; Dalley, J.W. Dissecting Impulsivity: Brain Mechanisms and Neuropsychiatric Implications. In Impulsivity; Nebraska Symposium on Motivation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 201–226. ISBN 978-3-31951-720-9. [Google Scholar]
- Cespón, J.; Rodella, C.; Rossini, P.M.; Miniussi, C.; Pellicciari, M.C. Anodal Transcranial Direct Current Stimulation Promotes Frontal Compensatory Mechanisms in Healthy Elderly Subjects. Front. Aging Neurosci. 2017, 9, 420. [Google Scholar] [CrossRef]
- Breitling, C.; Zaehle, T.; Dannhauer, M.; Tegelbeckers, J.; Flechtner, H.H.; Krauel, K. Comparison between Conventional and HD-TDCS of the Right Inferior Frontal Gyrus in Children and Adolescents with ADHD. Clin. Neurophysiol. 2020, 131, 1146–1154. [Google Scholar] [CrossRef]
- Abellaneda-Pérez, K.; Vaqué-Alcázar, L.; Perellón-Alfonso, R.; Bargalló, N.; Kuo, M.F.; Pascual-Leone, A.; Nitsche, M.A.; Bartrés-Faz, D. Differential TDCS and TACS Effects on Working Memory-Related Neural Activity and Resting-State Connectivity. Front. Neurosci. 2020, 13, 1440. [Google Scholar] [CrossRef]
- Hamidovic, A.; Wang, Y. The P300 in Alcohol Use Disorder: A Meta-Analysis and Meta-Regression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109716. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Aggensteiner, P.M.; Baumeister, S.; Holz, N.E.; Banaschewski, T.; Brandeis, D. Earlier versus Later Cognitive Event-Related Potentials (ERPs) in Attention-Deficit/Hyperactivity Disorder (ADHD): A Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 112, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Brevet-Aeby, C.; Brunelin, J.; Iceta, S.; Padovan, C.; Poulet, E. Prefrontal Cortex and Impulsivity: Interest of Noninvasive Brain Stimulation. Neurosci. Biobehav. Rev. 2016, 71, 112–134. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Zaghlou, K.A.; Jacobs, J.; Williams, R.B.; Sperling, M.R.; Sharan, A.D.; Kahana, M.J. Synchronous and Asynchronous Theta and Gamma Activity during Episodic Memory Formation. J. Neurosci. 2013, 33, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Draguhn, A. Neuronal olscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Miyagishi, Y.; Ikeda, T.; Takahashi, T.; Kudo, K.; Morise, H.; Minabe, Y.; Kikuchi, M. Gamma-band auditory steady-state response after frontal tDCS: A double-blind, randomized, crossover study. PLoS ONE 2018, 13, e0193422. [Google Scholar] [CrossRef]
- Reinhart, R.M.G.; Zhu, J.; Park, S.; Woodman, G.F. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proc. Natl. Acad. Sci. USA 2015, 112, 9448–9453. [Google Scholar] [CrossRef]

| tDCS | df | t/V * | g | p-Value | Adjusted p-Value (BH) | |||
|---|---|---|---|---|---|---|---|---|
| Active | Sham | |||||||
| Cued Premature Response Task | Premature Responses | 27.78 (14.65) | 29.75 (15.77) | 35 | −0.79 | 0.13 | 0.432 | 0.438 |
| Monetary Gain/Loss | 41.82 (66.10) | 31.50 (70.73) | 37 | 0.78 | 0.15 | 0.438 | 0.438 | |
| Release time (ms) | 240.97 (34.19) | 244.89 (42.14) | 37 | −0.87 | 0.1 | 0.388 | 0.438 | |
| Stop-Signal Reaction Time Task | Accuracy Go trials | 97.1 (2) | 96.2 (3) | 30 | 2.11 | 0.35 | 0.043 | 0.215 |
| RT Go trials | 456.49 (70.32) | 443.34 (82.68) | 30 | 1.09 | 0.17 | 0.283 | 0.707 | |
| p(respond|signal) | 44 (10) | 45 (10) | 30 | −0.38 | 0.1 | 0.710 | 0.71 | |
| SSD | 207.71 (55.89) | 212.25 (51.45) | 30 | −0.52 | 0.08 | 0.605 | 0.71 | |
| SSRT | 223.77 (46.38) | 215.85 (61.63) | 30 | 0.73 | 0.15 | 0.471 | 0.71 | |
| Monetary Choice Questionnaire-27 | Overall k | 0.016 (0.02) | 0.015 (0.03) | 39 | 344 * | 0.04 | 0.061 | 0.12 |
| Small k | 0.033 (0.04) | 0.024 (0.04) | 39 | 199 * | 0.23 | 0.004 | 0.016 | |
| Medium k | 0.016 (0.02) | 0.015 (0.03) | 39 | 134 * | 0.04 | 0.120 | 0.12 | |
| Large k | 0.012 (0.02) | 0.010 (0.03) | 39 | 223 * | 0.09 | 0.106 | 0.12 | |
| tDCS | df | t/V * | g | p-Value | Adjusted p-Value (BH) | |||
|---|---|---|---|---|---|---|---|---|
| Active | Sham | |||||||
| Target-P3 (250–450 ms) | ERP (µV) | 0.99 (5.33) | 3.58 (3.14) | 32 | 106 * | 0.59 | 0.001 | 0.003 |
| Delta (dB) | 2.49 (3.18) | 5.01 (6.51) | 32 | −2.29 | 0.49 | 0.015 | 0.03 | |
| Theta (dB) | 3.28 (3.44) | 3.77 (4.72) | 32 | −0.66 | 0.12 | 0.805 | 0.805 | |
| Cue-P3 (300–650 ms) | ERP (µV) | 0.38 (1.97) | 1.39 (1.65) | 32 | 142 * | 0.56 | 0.012 | 0.036 |
| Delta (dB) | −0.17 (1.06) | −0.11 (1.04) | 32 | −0.24 | 0.03 | 0.808 | 0.847 | |
| Theta (dB) | −0.12 (0.91) | −0.07 (1.17) | 32 | −0.19 | 0.05 | 0.847 | 0.847 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, A.J.; Galdo-Álvarez, S.; Lema, A.; Carvalho, S.; Leite, J. Transcranial Direct Current Stimulation Decreases P3 Amplitude and Inherent Delta Activity during a Waiting Impulsivity Paradigm: Crossover Study. Brain Sci. 2024, 14, 168. https://doi.org/10.3390/brainsci14020168
Mendes AJ, Galdo-Álvarez S, Lema A, Carvalho S, Leite J. Transcranial Direct Current Stimulation Decreases P3 Amplitude and Inherent Delta Activity during a Waiting Impulsivity Paradigm: Crossover Study. Brain Sciences. 2024; 14(2):168. https://doi.org/10.3390/brainsci14020168
Chicago/Turabian StyleMendes, Augusto J., Santiago Galdo-Álvarez, Alberto Lema, Sandra Carvalho, and Jorge Leite. 2024. "Transcranial Direct Current Stimulation Decreases P3 Amplitude and Inherent Delta Activity during a Waiting Impulsivity Paradigm: Crossover Study" Brain Sciences 14, no. 2: 168. https://doi.org/10.3390/brainsci14020168
APA StyleMendes, A. J., Galdo-Álvarez, S., Lema, A., Carvalho, S., & Leite, J. (2024). Transcranial Direct Current Stimulation Decreases P3 Amplitude and Inherent Delta Activity during a Waiting Impulsivity Paradigm: Crossover Study. Brain Sciences, 14(2), 168. https://doi.org/10.3390/brainsci14020168









