Social Brain Perspectives on the Social and Evolutionary Neuroscience of Human Language
Abstract
1. Introduction
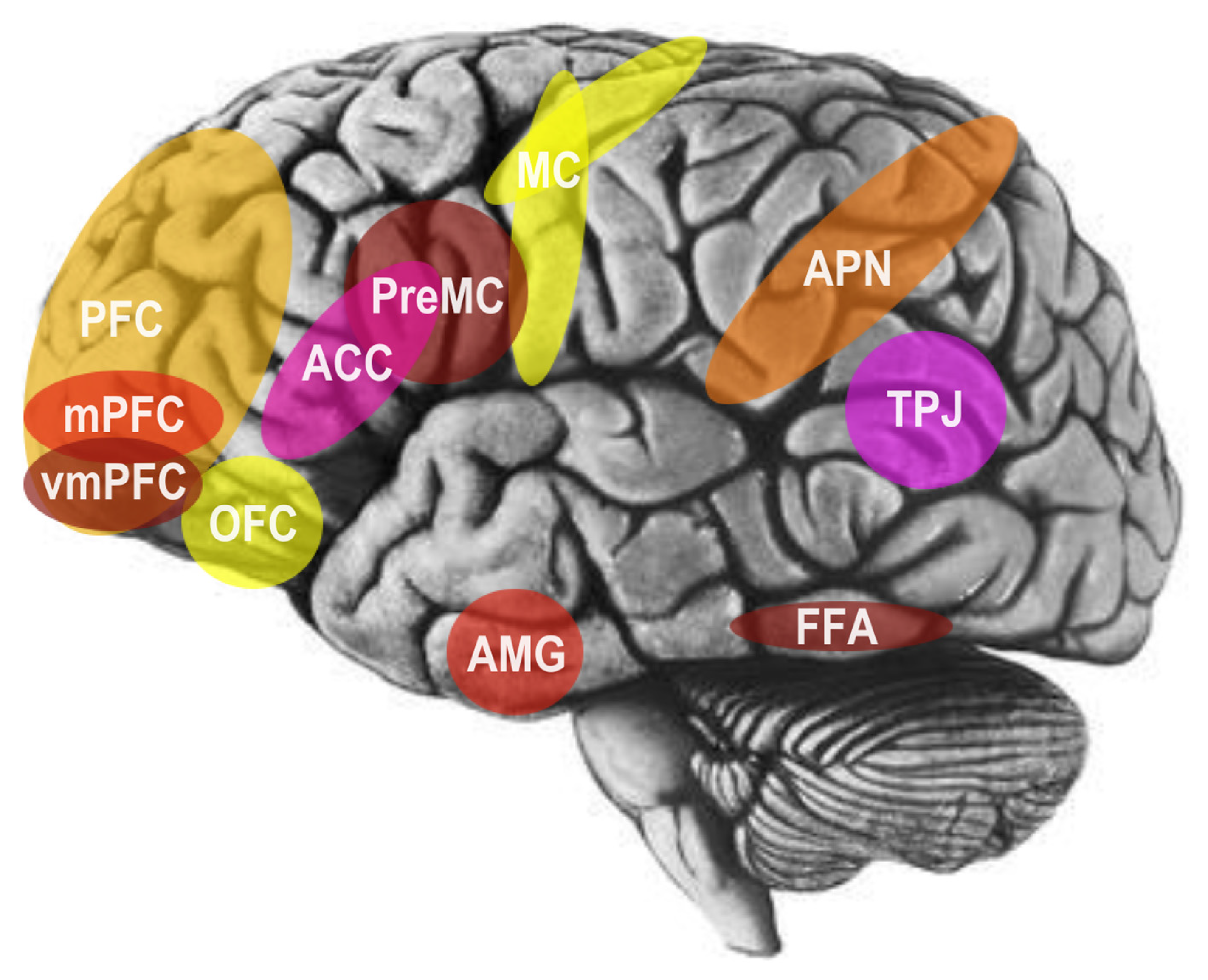
2. The Social Brain and Social Cognitive Neuroscience
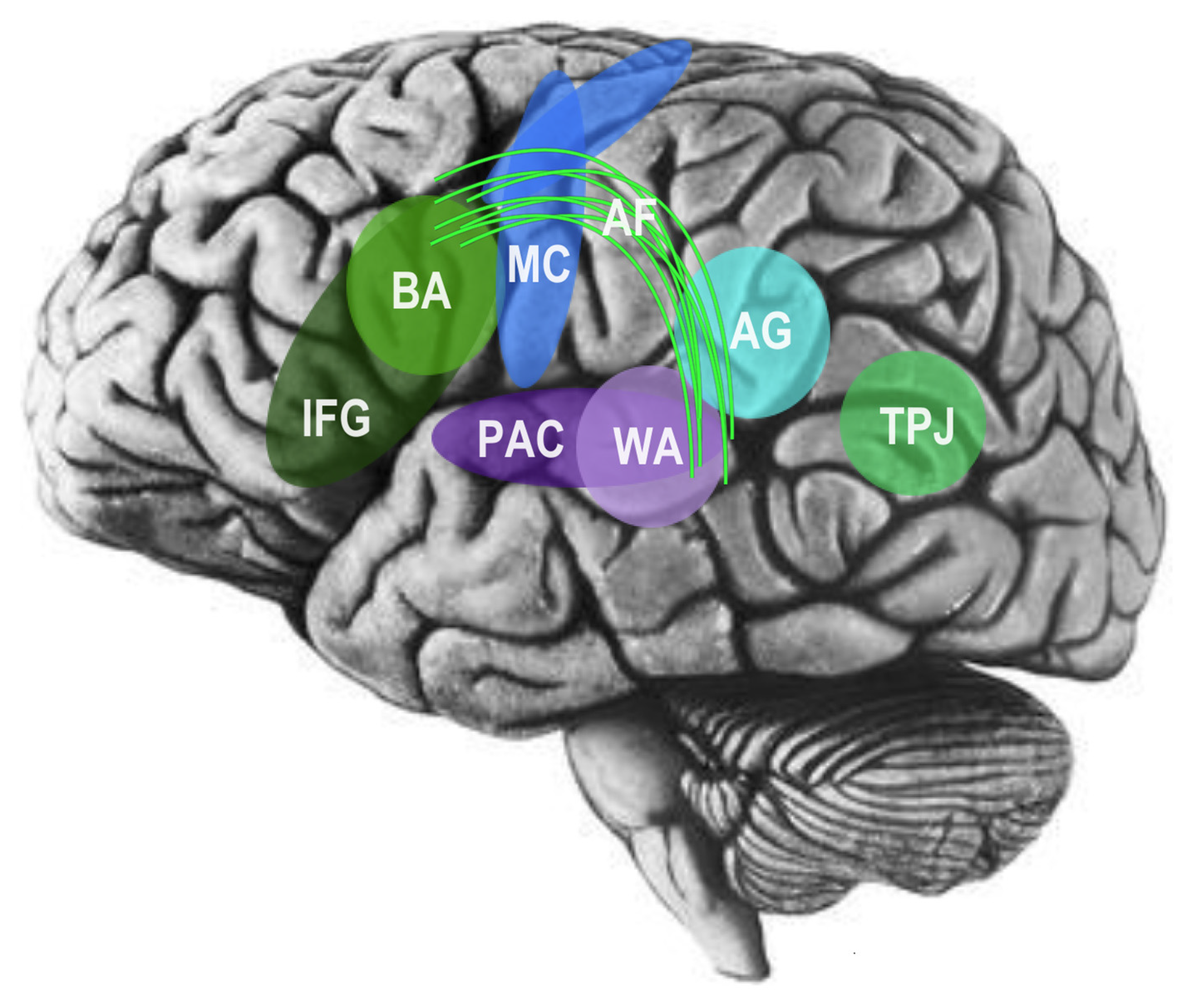
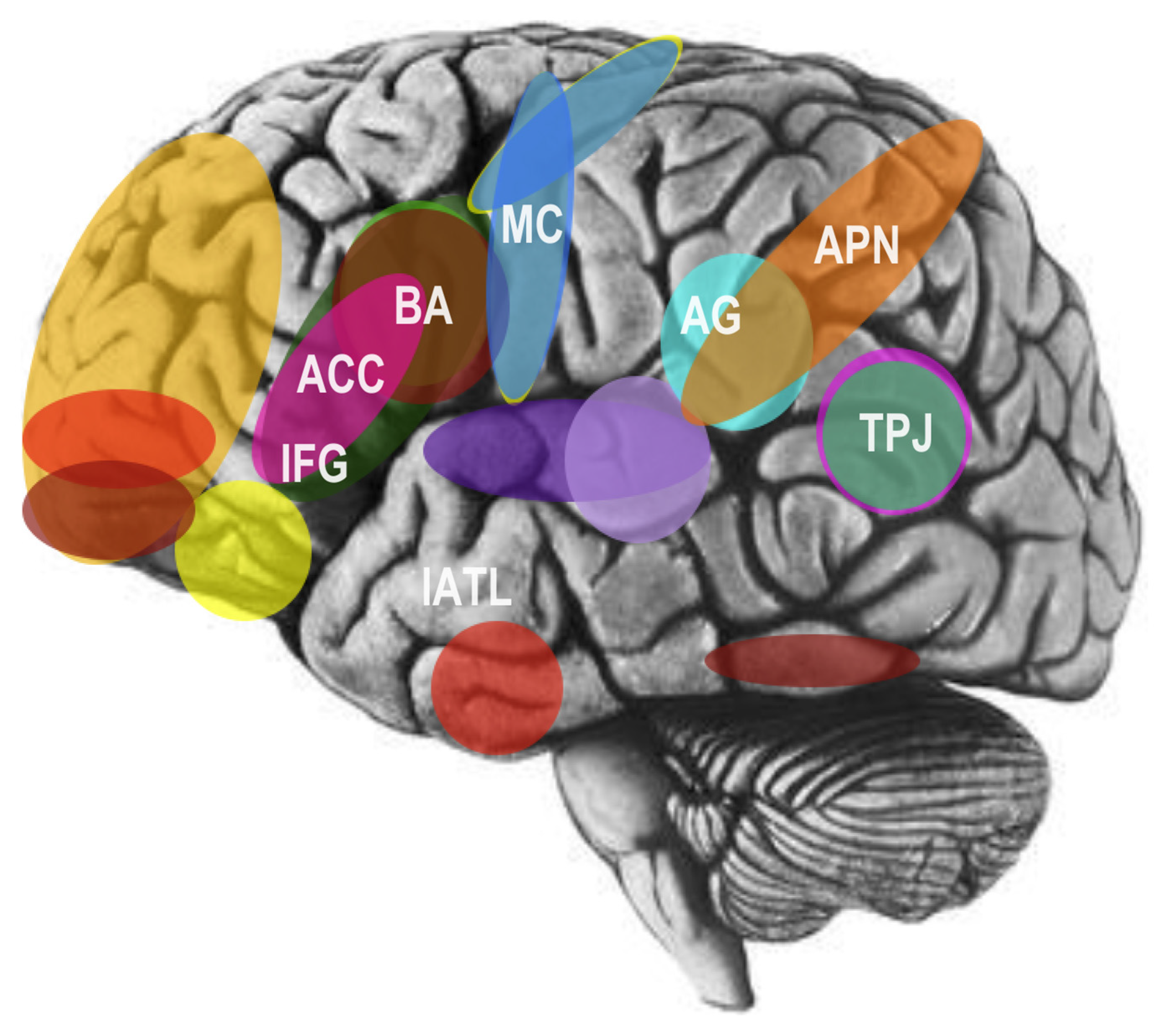
3. The Social Brain and Cognitive Neuroscience of Language
4. The Social Brain and First Language Acquisition
4.1. Social Signals That Facilitate Early Language Acquisition
4.2. Infant-/Child-Directed Speech and Face-to-Face Communication
5. The Social Brain, Cognitive Neuroscience of Language, and First Language Acquisition
6. The Social Brain and Second Language Acquisition
7. The Social Brain, Developmental Dysfunctions, and Psychopathologies
8. The Social Brain and Autism Spectrum Disorder
9. Early Biomarkers of Language-Related Abilities and Relevant Clinical Applications
10. Discussion
10.1. Early Biomarkers of Probable Language Outcomes and Clinical Interventions
10.2. The Social Brain and Cognitive Neuroscience of Language
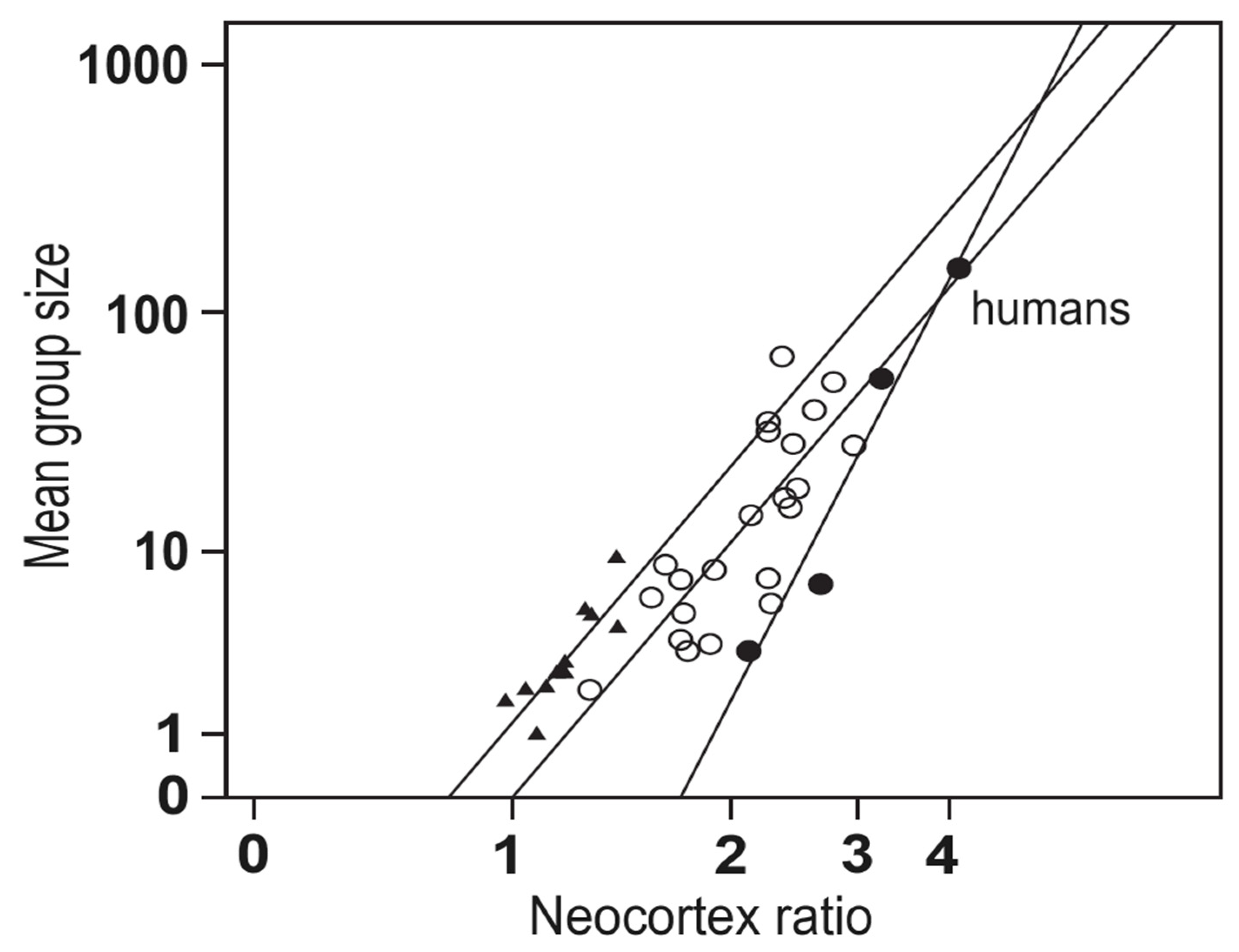
10.3. The Social Brain and Social Complexity Hypotheses as Novel Theoretical Frameworks for Understanding the Evolution and Function of the Human Mind
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunbar, R.I.M.; Shultz, S. Evolution in the social brain. Science 2007, 317, 1344–1347. [Google Scholar] [CrossRef]
- Semendeferi, K.; Armstrong, E.; Schleicher, A.; Zilles, K.; Van Hoesen, G.W. Prefrontal cortex in humans and apes: A comparative study of area. Am. J. Phys. Anthropol. 2001, 114, 224–241. [Google Scholar] [CrossRef]
- Semendeferi, K.; Lu, A.; Schenker, N.; Damasio, H. Humans and great apes share a large frontal cortex. Nat. Neurosci. 2002, 5, 272–276. [Google Scholar] [CrossRef]
- Oesch, N. Social brain hypothesis. In International Encyclopedia of Anthropology; Callan, H., Ed.; John Wiley and Sons: New York, NY, USA, 2018; pp. 1–11. [Google Scholar] [CrossRef]
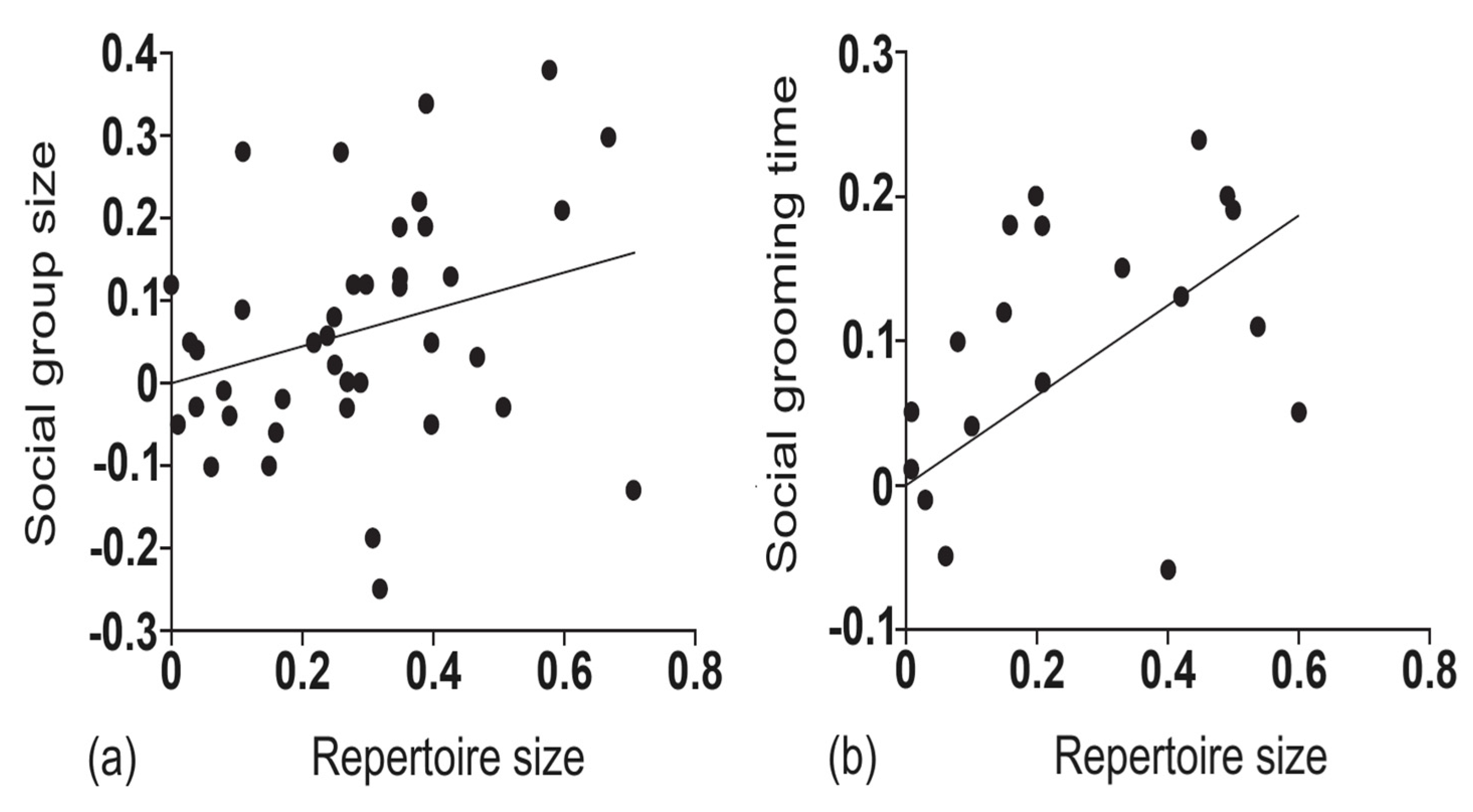
- Freeberg, T.M.; Dunbar, R.I.M.; Ord, T.J. Social complexity as a proximate and ultimate factor in communicative complexity. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1785–1801. [Google Scholar] [CrossRef]
- Oesch, N. Music and language in social interaction: Synchrony, antiphony and functional origins. Front. Psychol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- McComb, K.; Semple, S. Coevolution of vocal communication and sociality in primates. Biol. Lett. 2005, 1, 381–385. [Google Scholar] [CrossRef]
- Gustison, M.L.; le Roux, A.; Bergman, T.J. Derived vocalizations of geladas (Theropithecus gelada) and the evolution of vocal complexity in primates. Philos. Trans. R. Soc. B 2012, 367, 1847–1859. [Google Scholar] [CrossRef]
- King, A.J.; Myatt, J.P.; Fürtbauer, I.; Oesch, N.; Dunbar, R.I.M.; Sumner, S.; Usherwood, J.R.; Hailes, S.; Brown, M.R. Social density processes regulate the functioning and performance of foraging human teams. Sci. Rep. 2015, 5, 18260. [Google Scholar] [CrossRef] [PubMed]
- Oesch, N.; Dunbar, R.I.M. Group size, communication, and familiarity effects in foraging human teams. Ethology 2018, 124, 483–495. [Google Scholar] [CrossRef]
- Dahmardeh, M.; Dunbar, R.I.M. What shall we talk about in Farsi?: Content of everyday conversations in Iran. Hum. Nat. 2017, 28, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, R.I.M.; Marriott, A.; Duncan, N.D. Human conversational behavior. Hum. Nat. 1997, 8, 231–246. [Google Scholar] [CrossRef]
- Feinberg, M.; Willer, R.; Shultz, M. Gossip and ostracism promote cooperation in groups. Psychol. Sci. 2014, 25, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.R.; Bosson, J.K. I feel like I know you: Sharing negative attitudes of others promotes feelings of familiarity. Pers. Soc. Psychol. Bull. 2011, 37, 481–491. [Google Scholar] [CrossRef]
- Laidre, M.E.; Lamb, A.; Shultz, S.; Olsen, M. Making sense of information in noisy networks: Human communication, gossip, and distortion. J. Theor. Biol. 2013, 317, 152–160. [Google Scholar] [CrossRef]
- Adolphs, R. The social brain: Neural basis of social knowledge. Annu. Rev. Psychol. 2009, 60, 693–716. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, R.I.M. The social brain hypothesis and its implications for social evolution. Ann. Hum. Biol. 2009, 36, 562–572. [Google Scholar] [CrossRef]
- Brothers, L. The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990, 1, 27–51. [Google Scholar]
- Johnson, M.H.; Griffin, R.; Csibra, G.; Halit, H.; Farroni, T.; de Haan, M.; Tucker, L.A.; Baron-Cohen, S.; Richards, J. The emergence of the social brain network: Evidence from typical and atypical development. Dev. Psychopathol. 2005, 17, 599–619. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Lewis, P.A.; Birch, A.; Hall, A.; Dunbar, R.I.M. Higher order intentionality tasks are cognitively more demanding. Soc. Cogn. Affect. Neurosci. 2017, 12, 1063–1071. [Google Scholar] [CrossRef]
- Kwak, S.; Joo, W.; Youm, Y.; Chey, J. Social brain volume is associated with in-degree social network size among older adults. Proc. R. Soc. B 2018, 285, 20172708. [Google Scholar] [CrossRef]
- Von Der Heide, R.; Vyas, G.; Olson, I.R. The social network-network: Size is predicted by brain structure and function in the amygdala and paralimbic regions. Soc. Cogn. Affect. Neurosci. 2014, 9, 1962–1972. [Google Scholar] [CrossRef]
- Barbey, A.K.; Colom, R.; Paul, E.J.; Chau, A.; Solomon, J.; Grafman, J.H. Lesion mapping of social problem solving. Brain 2014, 137, 2823–2833. [Google Scholar] [CrossRef]
- Meltzoff, A.N.; Decety, J. What imitation tells us about social cognition: A rapprochement between developmental psychology and cognitive neuroscience. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 491–500. [Google Scholar] [CrossRef]
- Rizzolatti, G. The mirror neuron system and imitation. In Perspectives on Imitation: From Neuroscience to Social Science—I: Mechanisms of Imitation and Imitation in Animals; Hurley, S., Chater, N., Eds.; MIT Press: Cambridge, UK, 2005; pp. 55–76. [Google Scholar]
- Kilner, J.M.; Lemon, R.N. What we know currently about mirror neurons. Curr. Biol. 2013, 23, R1057–R1062. [Google Scholar] [CrossRef] [PubMed]
- Dölen, G.; Darvishzadeh, A.; Huang, K.W.; Malenka, R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013, 501, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, R.I.M. The social brain hypothesis and human evolution. In Oxford Research Encyclopedias: Psychology; Oxford University Press: Oxford, UK, 2016; pp. 1–31. [Google Scholar] [CrossRef]
- Krach, S.; Paulus, F.M.; Bodden, M.; Kircher, T. The rewarding nature of social interactions. Front. Behav. Neurosci. 2010, 4, 1141. [Google Scholar] [CrossRef] [PubMed]
- Machin, A.J.; Dunbar, R.I.M. The brain opioid theory of social attachment: A review of the evidence. Behaviour 2011, 148, 985–1025. [Google Scholar] [CrossRef]
- Watanabe, N.; Yamamoto, M. Neural mechanisms of social dominance. Front. Neurosci. 2015, 9, 154. [Google Scholar] [CrossRef]
- Adolphs, R. Cognitive neurosciences of human social behavior. Nat. Rev. Neurosci. 2003, 4, 165–178. [Google Scholar] [CrossRef]
- Pulvermuller, F. Brain mechanisms linking language to action. Nat. Rev. Neurosci. 2005, 6, 574–582. [Google Scholar] [CrossRef]
- Abrams, D.A.; Chen, T.; Odriozola, P.; Cheng, K.M.; Baker, A.E.; Padmanabhan, A.; Ryali, S.; Kochalkaa, J.; Feinstein, C.; Menon, V. Neural circuits underlying mother’s voice perception predict social communication abilities in children. Proc. Natl. Acad. Sci. USA 2016, 113, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jeong, H. The social brain of language: Grounding second language learning in social interaction. Npj Sci. Learn. 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Rauchbauer, B.; Nazarian, B.; Bourhis, M.; Ochs, M.; Prévot, L.; Chaminade, T. Brain activity during reciprocal social interaction investigated using conversational robots as control condition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180033. [Google Scholar] [CrossRef]
- Tamir, D.I.; Mitchell, J.P. Disclosing information about the self is intrinsically rewarding. Proc. Natl. Acad. Sci. USA 2012, 109, 8038–8043. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Wang, X.; Li, J.; Shi, W.; Bi, Y.; Lin, N. A social-semantic working-memory account for two canonical language areas. Nat. Hum. Behav. 2023, 7, 1980–1997. [Google Scholar] [CrossRef] [PubMed]
- Fancher, R.E. Pioneers of Psychology, 2nd ed.; W. W. Norton and Co.: New York, NY, USA, 1990; pp. 72–93. [Google Scholar]
- Galaburda, A.M.; LeMay, M.; Kemper, T.L.; Geschwind, N. Right-left asymmetrics in the brain. Science 1978, 199, 852–856. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Belin, P. Spectral and temporal processing in human auditory cortex. Cereb. Cortex 2001, 11, 946–953. [Google Scholar] [CrossRef]
- Galuske, R.A.; Schlote, W.; Bratzke, H.; Singer, W. Interhemispheric asymmetries of the modular structure in human in human temporal cortex. Science 2000, 289, 1946–1949. [Google Scholar] [CrossRef]
- Hutsler, J.J. The specialized structure of human language cortex: Pyramidal cell size asymmetries within auditory and language-associated regions of the temporal lobes. Brain Lang. 2003, 86, 226–242. [Google Scholar] [CrossRef]
- Pujol, J.; Deus, J.; Losilla, J.M.; Capdevila, A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology 1999, 52, 1038–1043. [Google Scholar] [CrossRef]
- Springer, J.A.; Binder, J.R.; Hammeke, T.A.; Swanson, S.J.; Frost, J.A.; Bellgowan, P.S.; Mueller, W.M. Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain 1999, 122, 2033–2046. [Google Scholar] [CrossRef]
- Imada, T.; Zhang, Y.; Cheour, M.; Taulu, S.; Ahonen, A.; Kuhl, P.K. Infant speech perception activates Broca’s area: A developmental magnetoencephalography study. Neuroreport 2006, 17, 957–962. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Craighero, L. The mirror-neuron system. Annu. Rev. Neurosci. 2004, 27, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P. Human Language and Our Reptilian Brain: The Subcortical Bases of Speech, Syntax, and Thought; Harvard University Press: Cambridge, UK, 2000. [Google Scholar]
- Oluladea, O.A.; Seydell-Greenwalda, A.; Chambersa, C.E.; Turkeltauba, P.E.; Dromericka, A.W.; Berlb, M.M.; Gaillard, W.D.; Newport, E.L. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. USA 2020, 117, 23477–23483. [Google Scholar] [CrossRef]
- Schenker, N.M.; Hopkins, W.D.; Spocter, M.A.; Garrison, A.R.; Stimpson, C.D.; Erwin, J.M.; Hof, P.R.; Sherwood, C.C. Broca’s area homologue in chimpanzees (Pan troglodytes): Probabilistic mapping, asymmetry, and comparison to humans. Cereb. Cortex 2010, 20, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Striedter, G.F. Principles of Brain Evolution; Sinauer Associates: Sunderland, MA, USA, 2005. [Google Scholar]
- Aboitiz, F. A brain for speech. Evolutionary continuity in primate and human auditory-vocal processing. Front. Neurosci. 2018, 12, 174. [Google Scholar] [CrossRef]
- Ardesch, D.J.; Scholtens, L.H.; Longchuan, L.; Preuss, T.M.; Rilling, J.K.; van den Heuvel, M.P. Evolutionary expansion of connectivity between multimodal association areas in the human brain compared with chimpanzees. Proc. Natl. Acad. Sci. USA 2019, 116, 7101–7106. [Google Scholar] [CrossRef] [PubMed]
- Hage, S.R.; Nieder, A. Dual neural network model for the evolution of speech and language. Trends Neurosci. 2016, 39, 813–829. [Google Scholar] [CrossRef]
- Holstege, G.; Subramanian, H.H. Two different motor systems are needed to generate human speech. J. Comp. Neurol. 2016, 524, 1558–1577. [Google Scholar] [CrossRef]
- Kumar, V.; Croxson, P.L.; Simonyan, K. Structural organization of the laryngeal motor cortical network and its implication for evolution of speech production. J. Neurosci. 2016, 36, 4170–4181. [Google Scholar] [CrossRef]
- Petkov, C.I.; Jarvis, E.D. Birds, primates, and spoken language origins: Behavioral phenotypes and neurobiological substrates. Front. Evol. Neurosci. 2012, 4, 12. [Google Scholar] [CrossRef]
- Schoenemann, P.T. Conceptual complexity and the brain: Understanding language origins. In Language Acquisition, Change and Emergence: Essays in Evolutionary Linguistics; Wang, W.S.-Y., Minett, J.W., Eds.; City University of Hong Kong Press: Hong Kong, China, 2005; pp. 47–94. [Google Scholar]
- Jasmin, K.M.; McGettigan, C.; Agnew, Z.K.; Lavan, N.; Josephs, O.; Cummins, F.; Scott, S.K. Cohesion and joint speech: Right hemisphere contributions to synchronized vocal production. J. Neurosci. 2016, 36, 4669–4680. [Google Scholar] [CrossRef] [PubMed]
- Bruner, J. Child’s Talk: Learning to Use Language; W.W. Norton: New York, NY, USA, 1983. [Google Scholar]
- Vygotsky, L.S. Thought and Language; MIT Press: Cambridge, MA, USA, 1962. [Google Scholar]
- Baldwin, D.A. Understanding the link between joint attention and language. In Joint Attention: Its Origins and Role in Development; Moore, C., Dunham, P.J., Eds.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1995; pp. 131–158. [Google Scholar]
- Brooks, R.; Meltzoff, A.N. The development of gaze following and its relation to language. Dev. Sci. 2005, 8, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, M. Constructing a Language; Harvard University Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Tomasello, M. The key is social cognition. In Language and Thought; Gentner, D., Kuczaj, S., Eds.; MIT Press: Cambridge, MA, USA, 2003; pp. 47–58. [Google Scholar]
- Tomasello, M.; Farrar, M.J. Joint attention and early language. Child Dev. 1986, 57, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, P.K. Is speech learning ‘gated’ by the social brain? Dev. Sci. 2007, 10, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Doupe, A.J.; Kuhl, P.K. Birdsong and human speech: Common themes and mechanisms. Annu. Rev. Neurosci. 1999, 22, 567–631. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.S.; Marler, P. Language and animal communication: Parallels and contrasts. In Comparative Approaches to Cognitive Science: Complex Adaptive Systems; Roitblat, H.L., Meyer, J.-A., Eds.; MIT Press: Cambridge, MA, USA, 1995; pp. 341–382. [Google Scholar]
- Marler, P. The instinct to learn. In The Epigenesis of Mind: Essays on Biology and Cognition; Carey, S., Gelman, R., Eds.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1991; pp. 37–66. [Google Scholar]
- Ferjan Ramírez, N.; Lytle, S.R.; Fish, M.; Kuhl, P.K. Parent coaching at 6 and 10 months improves language outcomes at 14 months: A randomized controlled trial. Dev. Sci. 2019, 22, e12762. [Google Scholar] [CrossRef] [PubMed]
- Heidlage, J.K.; Cunningham, J.E.; Kaiser, A.P.; Trivette, C.M.; Barton, E.E.; Frey, J.R.; Roberts, M.Y. The effects of parent-implemented language interventions on child linguistic outcomes: A meta-analysis. Early Child. Res. Q. 2020, 50, 6–23. [Google Scholar] [CrossRef]
- Leung, C.Y.Y.; Hernandez, M.W.; Suskind, D.L. Enriching home language environment among families from low-SES backgrounds: A randomized controlled trial of a home visiting curriculum. Early Child. Res. Q. 2020, 50, 24–35. [Google Scholar] [CrossRef]
- McGillion, M.; Pine, J.M.; Herbert, J.S.; Matthews, D. A randomised controlled trial to test the effect of promoting caregiver contingent talk on language development in infants from diverse socioeconomic status backgrounds. J. Child Psychol. Psychiatry Allied Discip. 2017, 58, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Dagan, O.; Schuengel, C.; Verhage, M.L.; Madigan, S.; Roisman, G.I.; Bernard, K.; Duschinsky, R.; Bakermans-Kranenburg, M.; Bureau, J.-F.; Sagi-Schwartz, A.; et al. Configurations of mother–child and father–child attachment relationships as predictors of child language competence: An individual participant data meta-analysis. Child Dev. 2024, 95, 50–69. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, P.K. Brain mechanisms in early language acquisition. Neuron 2010, 67, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, P.K. Early language learning and the social brain. Cold Spring Harb. Symp. Quant. Biol. 2014, 79, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Schick, J.; Fryns, C.; Wegdell, F.; Laporte, M.; Zuberbühler, K.; van Schaik, C.P.; Townsend, S.W.; Stoll, S. The function and evolution of child-directed communication. PLoS Biol. 2022, 20, e3001630. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alonso, S.; Aslin, R.N. Towards a model of language neurobiology in early development. Brain Lang. 2022, 224, 105047. [Google Scholar] [CrossRef]
- Kuhl, P.K.; Tsao, F.-M.; Liu, H.-M. Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proc. Natl. Acad. Sci. USA 2003, 100, 9096–9101. [Google Scholar] [CrossRef]
- Bloom, K. Social elicitation of infant vocal behavior. J. Exp. Child Psychol. 1975, 20, 51–58. [Google Scholar] [CrossRef]
- Bloom, K.; Esposito, A. Social conditioning and its proper control procedures. J. Exp. Child Psychol. 1975, 19, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; King, A.; West, M. Social interaction shapes babbling: Testing parallels between birdsong and speech. Proc. Natl. Acad. Sci. USA 2003, 100, 8030–8035. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Tomasello, M. Intersubjectivity in early language learning and use. In Intersubjective Communication and Emotion in Early Ontogeny; Bråten, S., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 316–335. [Google Scholar]
- Meltzoff, A.N. Understanding the intentions of others: Re-enactment of intended acts by 18-month-old children. Dev. Psychol. 1995, 31, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Fernald, A.E.; Marchman, V.A. Causes and consequences of variability in early language learning. In Experience, Variation and Generalization: Learning a First Language; Arnon, I., Clark, E., Eds.; John Benjamins: New York, NY, USA, 2011; pp. 181–202. [Google Scholar] [CrossRef]
- Fernald, A.; Perfors, A.; Marchman, V.A. Picking up speed in understanding: Speech processing efficiency and vocabulary growth across the 2nd year. Dev. Psychol. 2006, 42, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Weisleder, A.; Fernald, A. Talking to children matters: Early language experience strengthens processing and builds vocabulary. Psychol. Sci. 2013, 24, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Fernald, L.C.H.; Weber, A.; Galasso, E.; Ratsifandrihamanana, L. Socioeconomic gradients and child development in a very low income population: Evidence from Madagascar. Dev. Sci. 2011, 14, 832–847. [Google Scholar] [CrossRef]
- Fernald, A.; Marchman, V.A.; Weisleder, A. SES differences in language processing skill and vocabulary are evident at 18 months. Dev. Sci. 2013, 16, 234–248. [Google Scholar] [CrossRef]
- Bergelson, E.; Soderstrom, M.; Schwarz, I.-C.; Rowland, C.F.; Ramírez-Esparza, N.; Hamrick, L.R.; Marklund, E.; Kalashnikova, M.; Guez, A.; Casillas, M.; et al. Everyday language input and production in 1001 children from 6 continents. Proc. Natl. Acad. Sci. USA 2024, 120, e2300671120. [Google Scholar] [CrossRef]
- Holzrichter, A.S.; Meier, R.P. Child-directed signing in American Sign Language. In Language Acquisition by Eye; Chamberlain, C., Morford, J.P., Mayberry, R.I., Eds.; Lawrence Erlbaum: New York, NY, USA, 2000; pp. 25–40. [Google Scholar]
- Lieberman, A.M.; Fitch, A.; Borovsky, A. Flexible fast-mapping: Deaf children dynamically allocate visual attention to learn novel words in American Sign Language. Dev Sci. 2022, 25, e13166. [Google Scholar] [CrossRef]
- Mayberry, R.I.; Squires, B. Sign language: Acquisition. In Encyclopedia of Language & Linguistics, 2nd ed.; Brown, K., Ed.; Elsevier: Oxford, UK, 2006; Volume 11, pp. 291–296. [Google Scholar]
- Lieberman, A.M.; Hatrak, M.; Mayberry, R.I. Learning to look for language: Development of joint attention in young deaf children. Lang. Learn. Dev. 2014, 10, 19–35. [Google Scholar] [CrossRef]
- Carpenter, M.; Nagell, K.; Tomasello, M.; Butterworth, G.; Moore, C. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monogr. Soc. Res. Child Dev. 1998, 63, i+iii+v–vi+1–174. [Google Scholar] [CrossRef]
- Morales, M.; Mundy, P.; Delgado, C.E.F.; Yale, M.; Messinger, D.; Neal, R.; Schwartz, H.K. Responding to joint attention across the 6-through 24-month age period and early language acquisition. J. Appl. Dev. Psychol. 2000, 21, 283–298. [Google Scholar] [CrossRef]
- Çetinçelik, M.; Rowland, C.F.; Snijders, T.M. Do the eyes have it? A systematic review on the role of eye gaze in infant language development. Front. Psychol. 2021, 11, 589096. [Google Scholar] [CrossRef]
- Yu, C.; Smith, L.B. Hand-eye coordination predicts joint attention. Child Dev. 2017, 88, 2060–2078. [Google Scholar] [CrossRef] [PubMed]
- Kannass, K.N.; Oakes, L.M. The development of attention and its relations to language in infancy and toddlerhood. J. Cogn. Dev. 2008, 9, 222–246. [Google Scholar] [CrossRef]
- Lawson, K.R.; Ruff, H.A. Early focused attention predicts outcome for children born prematurely. J. Dev. Behav. Pediatr. 2004, 25, 399–406. [Google Scholar] [CrossRef]
- Ruff, H.A.; Lawson, K.R. Development of sustained, focused attention in young children during free play. Dev. Psychol. 1990, 26, 85–93. [Google Scholar] [CrossRef]
- Macroy-Higgins, M.; Montemanaro, E. Attention and word learning in toddlers who are late talkers. J. Child Lang. 2016, 43, 1020–1037. [Google Scholar] [CrossRef]
- Pereira, A.F.; Smith, L.B.; Yu, C. A bottom-up view of toddler word learning. Psychon. Bull. Rev. 2014, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Salley, B.; Panneton, R.K.; Colombo, J. Separable attentional predictors of language outcome. Infancy 2013, 18, 462–489. [Google Scholar] [CrossRef]
- Yu, C.; Suanda, S.H.; Smith, L.B. Infant sustained attention but not joint attention to objects at 9 months predicts vocabulary at 12 and 15 months. Dev. Sci. 2019, 22, e12735. [Google Scholar] [CrossRef]
- D’Entremont, B.; Hains, S.M.J.; Muir, D.W. A demonstration of gaze following in 3- to 6-month-olds. Infant Behav. Dev. 1997, 20, 569–572. [Google Scholar] [CrossRef]
- Farroni, T.; Csibra, G.; Simion, F.; Johnson, M.H. Eye contact detection in humans from birth. Proc. Natl. Acad. Sci. USA 2002, 99, 9602–9605. [Google Scholar] [CrossRef]
- Gredebäck, G.; Fikke, L.; Melinder, A. The development of joint visual attention: A longitudinal study of gaze following during interactions with mothers and strangers. Dev. Sci. 2010, 13, 839–848. [Google Scholar] [CrossRef]
- Perra, O.; Gattis, M. Attention engagement in early infancy. Infant Behav. Dev. 2012, 35, 635–644. [Google Scholar] [CrossRef]
- Butler, S.C.; Caron, A.J.; Brooks, R. Infant understanding of the referential nature of looking. J. Cogn. Dev. 2000, 1, 359–377. [Google Scholar] [CrossRef]
- Caron, A.J.; Butler, S.; Brooks, R. Gaze following at 12 and 14 months: Do the eyes matter? Br. J. Dev. Psychol. 2002, 20, 225–239. [Google Scholar] [CrossRef]
- Johnson, S.C.; Ok, S.-J.; Luo, Y. The attribution of attention: 9-month-olds? Interpretation of gaze as goal-directed action. Dev. Sci. 2007, 10, 530–537. [Google Scholar] [CrossRef]
- Woodward, A.L. Infants’ developing understanding of the link between looker and object. Dev. Sci. 2003, 6, 297–311. [Google Scholar] [CrossRef]
- Dickstein, S.; Thompson, R.A.; Estes, D.; Malkin, C.; Lamb, M.E. Social referencing and the security of attachment. Infant Behav. Dev. 1984, 7, 507–516. [Google Scholar] [CrossRef]
- Kim, S.; Fonagy, P.; Koos, O.; Dorsett, K.; Strathearn, L. Maternal oxytocin response predicts mother-to-infant gaze. Brain Res. 2014, 580, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.E.; Young, L.J. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009, 30, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.; Atagi, N.; Johnson, S.P. Selective attention to the mouth is associated with expressive language skills in monolingual and bilingual infants. J. Exp. Child Psychol. 2018, 169, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, E.J.; Sobel, D.M.; Sheinkopf, S.J.; Malle, B.F.; Morgan, J.L. Attention to the mouth and gaze following in infancy predict language development. J. Child Lang. 2015, 42, 1173–1190. [Google Scholar] [CrossRef] [PubMed]
- Lewkowicz, D.J.; Hansen-Tift, A.M. Infants deploy selective attention to the mouth of a talking face when learning speech. Proc. Natl. Acad. Sci. USA 2012, 109, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Pons, F.; Bosch, L.; Lewkowicz, D.J. Bilingualism modulates infants’ selective attention to the mouth of a talking face. Psychol. Sci. 2015, 26, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Hillairet de Boisferon, A.; Tift, A.H.; Minar, N.J.; Lewkowicz, D.J. The redeployment of attention to the mouth of a talking face during the second year of life. J. Exp. Child Psychol. 2018, 172, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hoff, E. How social contexts support and shape language development. Dev. Rev. 2006, 26, 55–88. [Google Scholar] [CrossRef]
- Hurtado, N.; Marchman, V.A.; Fernald, A. Does input influence uptake? Links between maternal talk, processing speed and vocabulary size in Spanish-learning children. Dev. Sci. 2008, 11, F31–F39. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, J.; Waterfall, H.; Vasilyeva, M.; Vevea, J.; Hedges, L.V. Sources of variability in children’s language growth. Cogn. Psychol. 2010, 61, 343–365. [Google Scholar] [CrossRef]
- Rowe, M.L. A longitudinal investigation of the role of quantity and quality of child-directed speech in vocabulary development. Child Dev. 2012, 83, 1762–1774. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Schwade, J.A. Social feedback to infants’ babbling facilitates rapid phonological learning. Psychol. Sci. 2008, 19, 515–523. [Google Scholar] [CrossRef]
- Cooper, R.P.; Aslin, R.N. Preference for infant-directed speech in the first month after birth. Child Dev. 1990, 61, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Fernald, A. Four-month-old infants prefer to listen to motherese. Infant Behav. Dev. 1985, 8, 181–195. [Google Scholar] [CrossRef]
- Fisher, C.; Tokura, H. Acoustic cues to grammatical structure in infant-directed speech: Cross-linguistic evidence. Child Dev. 1996, 67, 3192–3218. [Google Scholar] [CrossRef]
- Pegg, J.E.; Werker, J.F.; McLeod, P.J. Preference for infant-directed over adult-directed speech: Evidence from 7-week-old infants. Infant Behav. Dev. 1992, 15, 325–345. [Google Scholar] [CrossRef]
- Nagasawa, M.; Okabe, S.; Mogi, K.; Kikusui, T. Oxytocin and mutual communication in mother-infant bonding. Front. Hum. Neurosci. 2012, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Rilling, J.K.; Young, L.J. The biology of mammalian parenting and its effect on offspring social development. Science 2014, 345, 771–776. [Google Scholar] [CrossRef]
- Hoff, E. The specificity of environmental influence: Socioeconomic status affects early vocabulary development via maternal speech. Child Dev. 2003, 74, 1368–1378. [Google Scholar] [CrossRef]
- Bradley, R.H.; Corwyn, R.F. Socioeconomic status and child development. Annu. Rev. Psychol. 2002, 53, 371–399. [Google Scholar] [CrossRef]
- Halle, T.; Forry, N.; Hair, E.; Perper, K.; Wandner, L.; Wessel, J.; Vick, J. Disparities in Early Learning and Development: Lessons from the Early Childhood Longitudinal Study—Birth Cohort (ECLS-B); Child Trends: Washington, DC, USA, 2009; pp. 1–39. [Google Scholar] [CrossRef]
- Walker, D.; Greenwood, C.; Hart, B.; Carta, J. Prediction of school outcomes based on early language production and socioeconomic factors. Child Dev. 1994, 65, 606–621. [Google Scholar] [CrossRef]
- Fibla, L.; Forbes, S.H.; McCarthy, J.; Mee, K.; Magnotta, V.; Deoni, S.; Cameron, D.; Spencer, J.P. Language exposure and brain myelination in early development. J. Neurosci. 2023, 43, 4279–4290. [Google Scholar] [CrossRef]
- Romeo, R.R.; Leonard, J.A.; Robinson, S.T.; West, M.R.; Mackey, A.P.; Rowe, M.L.; Gabrieli, J.D.E. Beyond the 30-Million-word gap: Children’s conversational exposure is associated with language-related brain function. Psychol. Sci. 2018, 29, 700–710. [Google Scholar] [CrossRef]
- Romeo, R.R.; Segaran, J.; Leonard, J.A.; Robinson, S.T.; West, M.R.; Mackey, A.P.; Yendiki, A.; Rowe, M.L.; Gabrieli, J.D. Language exposure relates to structural neural connectivity in childhood. J. Neurosci. 2018, 38, 7870–7877. [Google Scholar] [CrossRef]
- Romeo, R.R.; Leonard, J.; Grotzinger, H.; Robinson, S.T.; Takada, M.E.; Mackey, A.; Scherer, E.; Rowe, M.; West, M.R.; Gabrieli, J. Neuroplasticity associated with changes in conversational turn-taking following a family-based intervention. Dev. Cogn. Neurosci. 2021, 49, 100967. [Google Scholar] [CrossRef]
- Merz, E.C.; Maskus, E.A.; Melvin, S.A.; He, X.; Noble, K.G. Socioeconomic disparities in language input are associated with children’s language-related brain structure and reading skills. Child Dev. 2020, 91, 846–860. [Google Scholar] [CrossRef]
- Montague, P.R.; Berns, G.S.; Cohen, J.D.; McClure, S.M.; Pagnoni, G.; Dhamala, M.; Fisher, R.E. Hyperscanning: Simultaneous fMRI during linked social interactions. NeuroImage 2002, 16, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Dumas, G.; Lachat, F.; Martinerie, J.; Nadel, J.; George, N. From social behaviour to brain synchronization: Review and perspectives in hyperscanning. IRBM 2011, 32, 48–53. [Google Scholar] [CrossRef]
- Hasson, U.; Ghazanfar, A.A.; Galantucci, B.; Garrod, S.; Keysers, C. Brain-to-brain coupling: A mechanism for creating and sharing a social world. Trends Cogn. Sci. 2012, 16, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Piazza, E.A.; Hasenfratz, L.; Hasson, U.; Lew-Williams, C. Infant and adult brains are coupled to the dynamics of natural communication. Psychol. Sci. 2020, 31, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.; Bellana, B.; Bialystok, E. Perspective-taking ability in bilingual children: Extending advantages in executive control to spatial reasoning. Cogn. Dev. 2013, 28, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.R. Do bilinguals have an advantage in theory of mind? A meta-analysis. Front. Commun. 2018, 3, 36. [Google Scholar] [CrossRef]
- Guiora, A.Z.; Brannon, R.C.L.; Dull, C.Y. Empathy and second language learning. Lang. Learn. 1972, 22, 111–130. [Google Scholar] [CrossRef]
- Kleinmann, H.H. Avoidance behaviour in adult second language learning. Lang. Learn. 1977, 27, 93–101. [Google Scholar] [CrossRef]
- Mishan, F. Designing Authenticity into Language Learning Materials; Intellect Books: New York, NY, USA, 2005. [Google Scholar]
- Brüne, M.; Ribbert, H.; Schiefenhövel, W. The Social Brain: Evolution and Pathology; John Wiley and Sons: West Sussex, UK, 2003. [Google Scholar]
- Groen, W.B.; Zwiers, M.P.; van der Gaag, R.-J.; Buitelaar, J.K. The phenotype and neural correlates of language in autism: An integrative review. Neurosci. Biobehav. Rev. 2008, 32, 1416–1425. [Google Scholar] [CrossRef]
- Klin, A.; Pauls, D.; Schultz, R.; Volkmar, F. Three diagnostic approaches to Asperger syndrome: Implications for research. J. Autism Dev. Disord. 2005, 35, 221–234. [Google Scholar] [CrossRef]
- Lord, C.; Paul, R. Language and communication in autism. In Handbook of Autism and Pervasive Developmental Disorders; Cohen, D., Volkmar, F.R., Eds.; John Wiley: New York, NY, USA, 1997. [Google Scholar]
- Fine, J.; Bartolucci, G.; Szatmari, P.; Ginsberg, G. Cohesive discourse in pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Abell, F.; Krams, M.; Ashburner, J.; Passingham, R.; Friston, K.; Frackowiak, R.; Happe, F.; Frith, C.; Frith, U. The neuroanatomy of autism: A voxel-based whole brain analysis of structural scans. Neuroreport 1999, 10, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- DeFosse, L.; Hodge, S.M.; Makris, N.; Kennedy, D.N.; Caviness, V.S., Jr.; McGrath, L.; Steele, S.; Ziegler, D.A.; Herbert, M.R.; Frazier, J.A.; et al. Language-association cortex asymmetry in autism and specific language impairment. Ann. Neurol. 2004, 56, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.R.; Harris, G.J.; Adrien, K.T.; Ziegler, D.A.; Makris, N.; Kennedy, D.N.; Lange, N.T.; Chabris, C.F.; Bakardjiev, A.; Hodgson, J.; et al. Abnormal asymmetry in language association cortex in autism. Ann. Neurol. 2002, 52, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.G.; Blanton, R.E.; Smalley, S.; Thompson, P.M.; Guthrie, D.; McCracken, J.T.; Sadoun, T.; Heinichen, L.; Toga, A.W. Cortical sulcal maps in autism. Cereb. Cortex 2003, 13, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Boddaert, N.; Chabane, N.; Gervais, H.; Good, C.D.; Bourgeois, M.; Plumet, M.H.; Barthelemy, C.; Mouren, M.C.; Artiges, E.; Samson, Y.; et al. Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel-based morphometry MRI study. Neuroimage 2004, 23, 364–369. [Google Scholar] [CrossRef]
- Colle, L.; Baron-Cohen, S.; Hill, J. Do children with autism have a theory of mind? A non-verbal test of autism vs. specific language impairment. J. Autism Dev. Disord. 2007, 37, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Oesch, N.; Dunbar, R.I.M. The emergence of recursion in human language: Mentalising predicts recursive syntax task performance. J. Neurolinguist. 2017, 43, 95–106. [Google Scholar] [CrossRef]
- Varley, R.; Siegal, M.; Want, S. Severe impairment in grammar does not preclude theory of mind. Neurocase 2001, 7, 489–493. [Google Scholar] [CrossRef]
- Kuhl, P.K.; Coffey-Corina, S.; Padden, D.; Dawson, G. Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Dev. Sci. 2005, 8, F1–F12. [Google Scholar] [CrossRef]
- Fromkin, V.; Krashen, S.; Curtiss, S.; Rigler, D.; Rigler, M. The development of language in Genie: A case of language acquisition beyond the “critical period”. Brain Lang. 1974, 1, 81–107. [Google Scholar] [CrossRef]
- McLaughlin, M.R. Speech and language delay in children. Am. Fam. Physician 2011, 83, 1183–1188. [Google Scholar]
- Catts, H.W.; Fey, M.E.; Tomblin, J.B.; Zhang, X. A longitudinal investigation of reading outcomes in children with language impairments. J. Speech Lang. Hear. Res. 2002, 45, 1142–1157. [Google Scholar] [CrossRef] [PubMed]
- Durand, V.N.; Loe, I.M.; Yeatman, J.D.; Feldman, H.M. Effects of early language, speech, and cognition on later reading: A mediation analysis. Front. Psychol. 2013, 4, 586. [Google Scholar] [CrossRef]
- Snowling, M.J.; Bishop, D.V.M.; Stothard, S.E.; Chipchase, B.; Kaplan, C. Psychosocial outcomes at 15 years of children with a preschool history of speech-language impairment. J. Child Psychol. Psychiatry 2006, 47, 759–765. [Google Scholar] [CrossRef]
- Rosenbaum, S.; Simon, P. Speech and language disorders in children. In Speech and Language Disorders in Children: Implications for the Social Security Administration’s Supplemental Security Income Program; Rosenbaum, S., Simon, P., Eds.; National Academies Press: New York, NY, USA, 2016. [Google Scholar]
- Tomblin, J.B.; Records, N.L.; Buckwalter, P.; Zhang, X.; Smith, E.; O’Brien, M. Prevalence of specific language impairment in kindergarten children. J. Speech Lang. Hear. Res. 1997, 40, 1245–1260. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Schum, R.L. Language screening in the pediatric office setting. Pediatr. Clin. N. Am. 2007, 54, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Rescorla, L. Age 17 language and reading outcomes in late-talking toddlers: Support for a dimensional perspective on language delay. J. Speech Lang. Hear. Res. 2009, 52, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Tager-Flusberg, H.; Paul, R.; Lord, C. Language and communication in autism spectrum disorders. In Handbook of Autism and Pervasive Developmental Disorders: Diagnosis, Development, Neurobiology, and Behavior; Volkmar, F.R., Paul, R., Klin, A., Cohen, J.D., Eds.; John Wiley and Sons Inc.: New York, NY, USA, 2005; pp. 335–364. [Google Scholar]
- Zengin-Akkuş, P.; Çelen-Yoldaş, T.; Kurtipek, G.; Özmert, E.N. Speech delay in toddlers: Are they only ‘late talkers’? Turk. J. Pediatr. 2018, 60, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.M. How young children learn language and speech. Pediatr. Rev. 2019, 40, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Lagercrantz, H.; Kuhl, P.K. Language experienced in utero affects vowel perception after birth: A two-country study. Acta Paediatr. 2013, 102, 156–160. [Google Scholar] [CrossRef]
- Marchman, V.A.; Loi, E.C.; Adams, K.A.; Ashland, M.; Fernald, A.; Feldman, H.M. Speed of language comprehension at 18 Months old predicts school-relevant outcomes at 54 months old in children born preterm. J. Dev. Behav. Pediatr. 2018, 39, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Benasich, A.A.; Tallal, P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav. Brain Res. 2002, 136, 31–49. [Google Scholar] [CrossRef]
- Kuhl, P.K.; Coffey-Corina, S.; Padden, D.; Munson, J.; Estes, A.; Dawson, G. Brain responses to words in 2-year-olds with autism predict developmental outcomes at age. PLoS ONE 2013, 8, e64967. [Google Scholar] [CrossRef]
- Kuhl, P.K.; Rivera-Gaxiola, M. Neural substrates of language acquisition. Annu. Rev. Neurosci. 2008, 31, 511–534. [Google Scholar] [CrossRef]
- Kuhl, P.K. Early language acquisition: Cracking the speech code. Nat. Rev. Neurosci. 2004, 5, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.C.; Boorom, O.; Kuhl, P.K.; Gordon, R. Infants’ neural speech discrimination predicts individual differences in grammar ability at 6 years of age and their risk of developing speech-language disorders. Dev. Cogn. Neurosci. 2021, 48, 100949. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Richardson, D.C. Verbal synchrony and action dynamics in large groups. Front. Psychol. 2016, 7, 2034. [Google Scholar] [CrossRef]
- Collins, N.L.; Miller, L.C. Self-disclosure and liking: A meta-analytic review. Psychol. Bull. 1994, 116, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Cummins, F. Towards an enactive account of action: Speaking and joint speaking as exemplary domains. Adapt. Behav. 2013, 13, 178–186. [Google Scholar] [CrossRef]
- Scott, S. From speech and talkers to the social world: The neural processing of human spoken language. Science 2019, 366, 58–62. [Google Scholar] [CrossRef]
- Kreysa, H.; Kessler, L.; Schweinberger, S.R. Direct speaker gaze promotes trust in truth-ambiguous statements. PLoS ONE 2016, 11, e0162291. [Google Scholar] [CrossRef]
- Poldrack, R.A. Inferring mental states from neuroimaging data: From reverse inference to large-scale decoding. Neuron 2011, 72, 692–697. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oesch, N. Social Brain Perspectives on the Social and Evolutionary Neuroscience of Human Language. Brain Sci. 2024, 14, 166. https://doi.org/10.3390/brainsci14020166
Oesch N. Social Brain Perspectives on the Social and Evolutionary Neuroscience of Human Language. Brain Sciences. 2024; 14(2):166. https://doi.org/10.3390/brainsci14020166
Chicago/Turabian StyleOesch, Nathan. 2024. "Social Brain Perspectives on the Social and Evolutionary Neuroscience of Human Language" Brain Sciences 14, no. 2: 166. https://doi.org/10.3390/brainsci14020166
APA StyleOesch, N. (2024). Social Brain Perspectives on the Social and Evolutionary Neuroscience of Human Language. Brain Sciences, 14(2), 166. https://doi.org/10.3390/brainsci14020166







