Pre-Stimulus Activity of Left and Right TPJ in Linguistic Predictive Processing: A MEG Study
Abstract
1. Introduction
1.1. The Many Facets of the TPJs
1.2. TPJs as Predictive Hubs: Literature’s Findings
1.2.1. Predictive Processing
1.2.2. Linguistic Predictive Processing in Literal and Metaphorical Expressions
1.2.3. Are TPJs Really Involved in Prediction?
1.2.4. The Pre-Stimulus Interval: Alpha, Precision, and Attention
1.2.5. Pre-Stimulus Alpha in Language
1.3. The Present Study
- (a)
- are both TPJs involved in linguistic prediction?
- (b)
- can spontaneous fluctuations of pre-stimulus alpha (associated with predictions’ precision) recorded in the TPJs modulate the subsequent brain responses to target stimuli?
- (c)
- is this modulation local, i.e., limited to the TPJs, or can pre-stimulus TPJ activity influence post-stimulus activations in other task-related (in this case linguistic) areas?
- (d)
- do the left and right TPJ exert different kinds of influence on post-stimulus responses recorded from linguistic ROIs?
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Stimuli and Task
2.4. MEG Data Analyses
2.5. Single-Trial Time–Frequency Analysis
2.6. Statistical Analyses
- the condition (Metaphor vs. Literal) as factor;
- a non-linear effect of time, depending on the condition (this term captures the different changes in the activation over time in the two conditions);
- a non-linear effect of pre-stimulus alpha power from the TPJ itself, capturing the (possibly) non-linear modulation of activation amplitude by different magnitudes of pre-stimulus alpha;
- an interaction term, specifying the non-linear interaction of interest between the continuous variables of time and pre-stimulus alpha power, depending on the condition, was included to capture whether pre-stimulus power modulates, in a possibly non-linear way, the subsequent activations, in either of the two conditions. This term corresponds to the interaction of interest, which, in GAMMs, is modeled by a tensor smooth function, and allows us to answer the question regarding the influence of the pre-stimulus alpha power on the subsequent activation response within the TPJ.
- a non-linear effect of pre-stimulus alpha power from the rTPJ;
- a non-linear effect of pre-stimulus alpha power from the lTPJ;
- an interaction term specifying the non-linear interaction between time and the rTPJ pre-stimulus alpha power, depending on the condition;
- an interaction term specifying the non-linear interaction between time and the lTPJ pre-stimulus alpha power, depending on the condition.
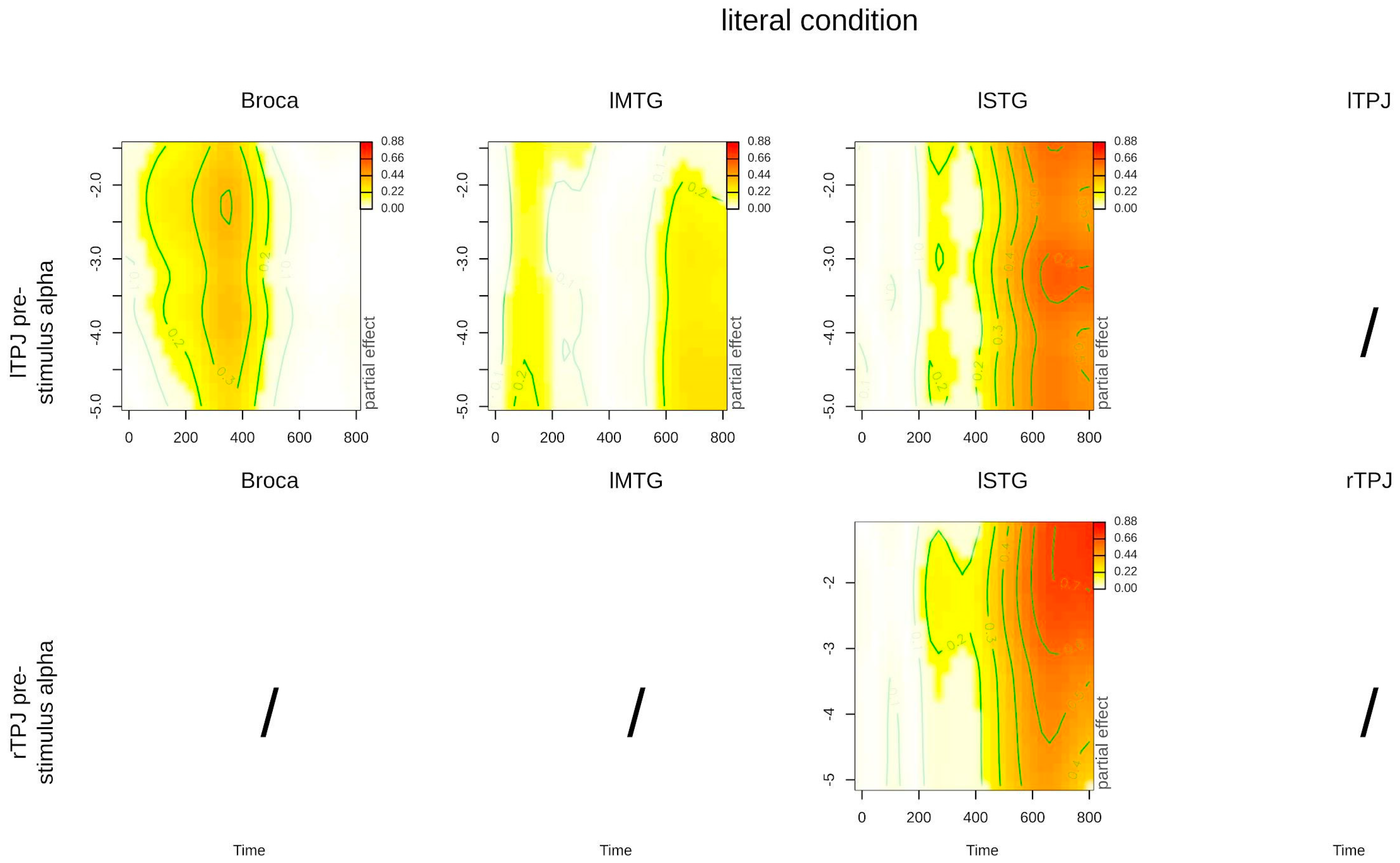
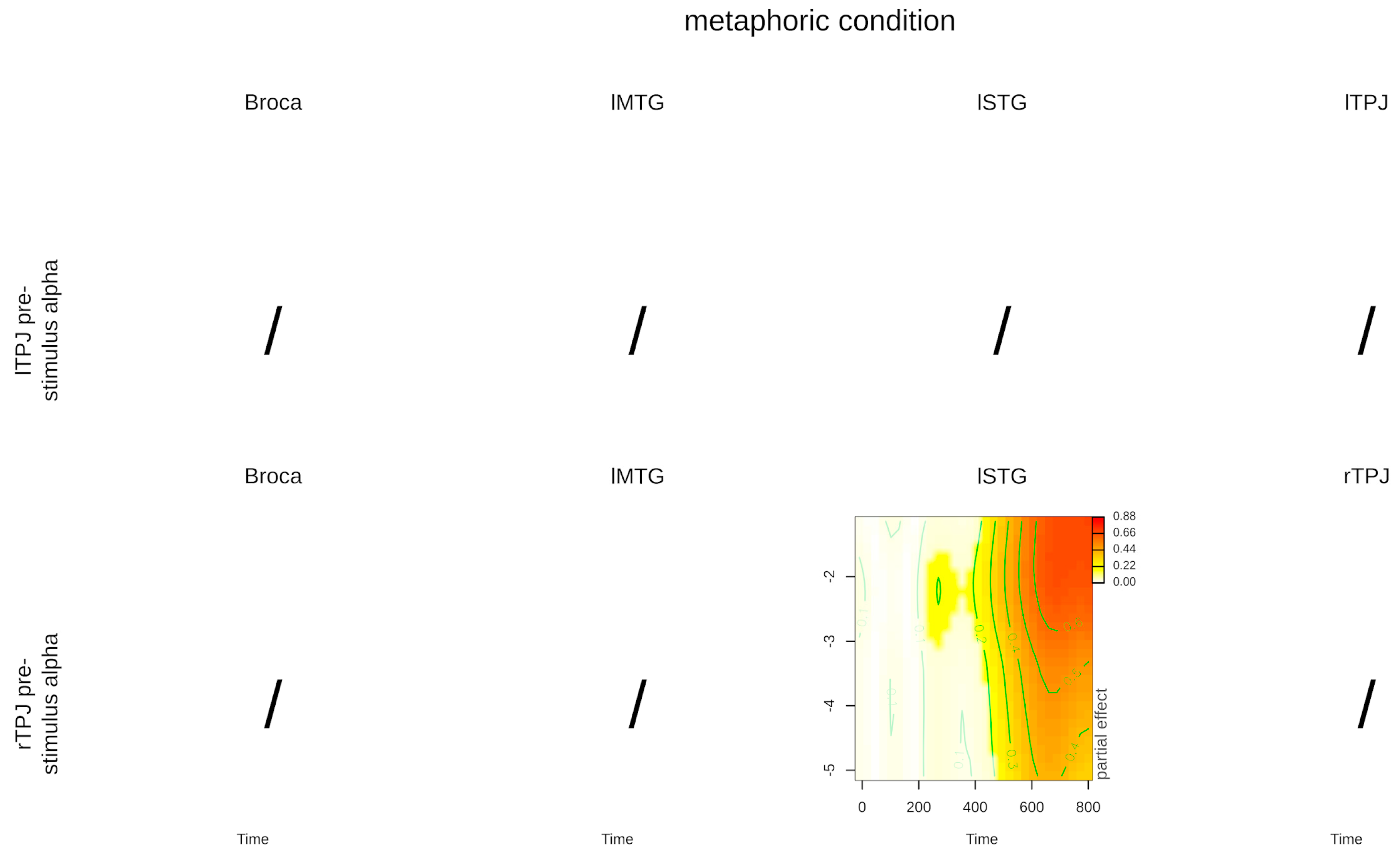
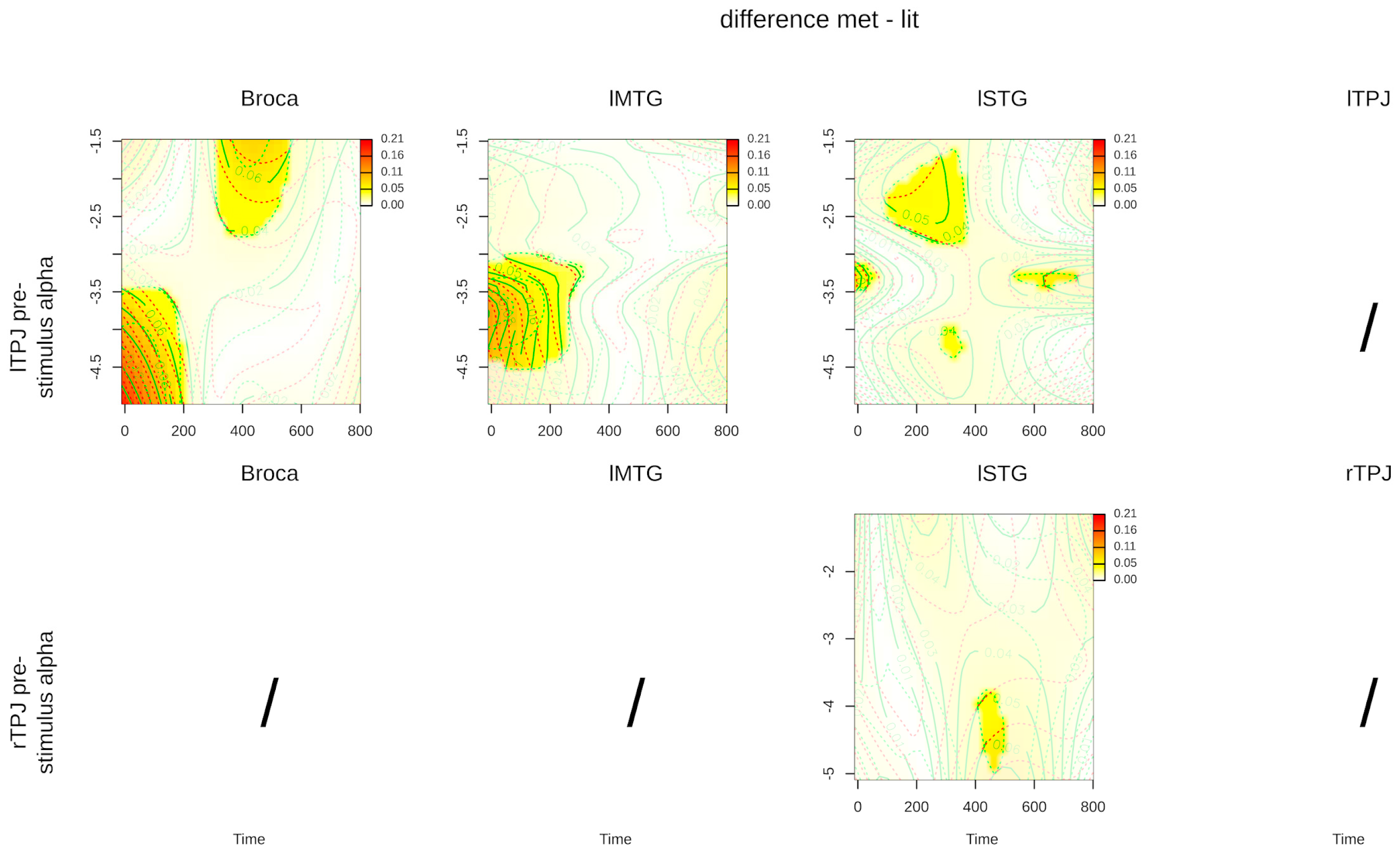
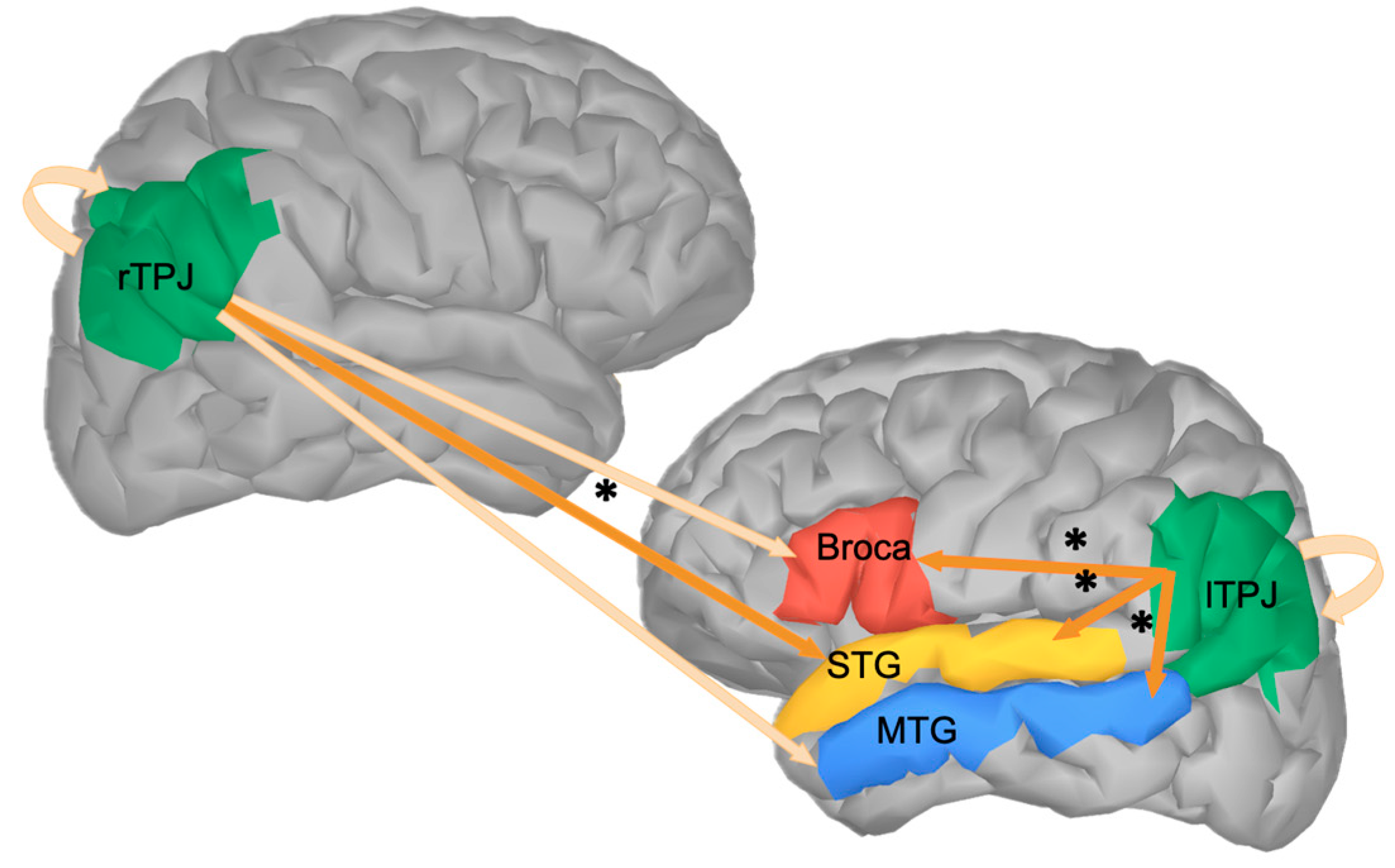
3. Results
4. Discussion
4.1. rTPJ
4.2. lTPJ
4.3. TPJs and Different Aspects of Predictive Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doricchi, F.; Lasaponara, S.; Pazzaglia, M.; Silvetti, M. Left and right temporal-parietal junctions (TPJs) as “match/mismatch” hedonic machines: A unifying account of TPJ function. Phys. Life Rev. 2022, 42, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Masina, F.; Pezzetta, R.; Lago, S.; Mantini, D.; Scarpazza, C.; Arcara, G. Disconnection from prediction: A systematic review on the role of right temporoparietal junction in aberrant predictive processing. Neurosci. Biobehav. Rev. 2022, 138, 104713. [Google Scholar] [CrossRef] [PubMed]
- Doricchi, F.; Macci, E.; Silvetti, M.; Macaluso, E. Neural correlates of the spatial and expectancy components of endogenous and stimulus-driven orienting of attention in the posner task. Cereb. Cortex 2010, 20, 1574–1585. [Google Scholar] [CrossRef]
- Indovina, I.; Macaluso, E. Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cereb. Cortex 2007, 17, 1701–1711. [Google Scholar] [CrossRef]
- Gastaldon, S.; Arcara, G.; Navarrete, E.; Peressotti, F. Commonalities in alpha and beta neural desynchronizations during prediction in language comprehension and production. Cortex 2020, 133, 328–345. [Google Scholar] [CrossRef]
- Blanke, O.; Ortigue, S.; Landis, T.; Seeck, M. Stimulating illusory own-body perceptions. Nature 2002, 419, 269–270. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Van Laere, K.; Dupont, P.; Menovsky, T.; Van de Heyning, P. Visualizing Out-of-Body Experience in the Brain. N. Engl. J. Med. 2007, 357, 1829–1833. [Google Scholar] [CrossRef]
- Daselaar, S.M.; Fleck, M.S.; Cabeza, R. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. J. Neurophysiol. 2006, 96, 1902–1911. [Google Scholar] [CrossRef]
- Yonelinas, A.P.; Otten, L.J.; Shaw, K.N.; Rugg, M.D. Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. 2005, 25, 3002–3008. [Google Scholar] [CrossRef]
- Hughes, C.; Cassidy, B.S.; Faskowitz, J.; Avena-Koenigsberger, A.; Sporns, O.; Krendl, A.C. Age differences in specific neural connections within the Default Mode Network underlie theory of mind. NeuroImage 2019, 191, 269–277. [Google Scholar] [CrossRef]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014, 42, 9–34. [Google Scholar] [CrossRef]
- Corbetta, M.; Patel, G.; Shulman, G.L. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron 2008, 58, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Fiebach, C.J.; Friederici, A.D.; Müller, K.; von Cramon, D.Y. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J. Cogn. Neurosci. 2002, 14, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.R.; Westbury, C.F.; McKiernan, K.A.; Possing, E.T.; Medler, D.A. Distinct brain systems for processing concrete and abstract concepts. J. Cogn. Neurosci. 2005, 17, 905–917. [Google Scholar] [CrossRef]
- Prince, S.E.; Tsukiura, T.; Cabeza, R. Distinguishing the neural correlates of episodic memory encoding and semantic memory retrieval. Psychol. Sci. 2007, 18, 144–151. [Google Scholar] [CrossRef]
- Menenti, L.; Petersson, K.M.; Scheeringa, R.; Hagoort, P. When Elephants Fly: Differential Sensitivity of Right and Left Inferior Frontal Gyri to Discourse and World Knowledge. J. Cogn. Neurosci. 2009, 21, 2358–2368. [Google Scholar] [CrossRef]
- Metusalem, R.; Kutas, M.; Urbach, T.P.; Hare, M.; McRae, K.; Elman, J.L. Generalized event knowledge activation during online sentence comprehension. J. Mem. Lang. 2012, 66, 545–567. [Google Scholar] [CrossRef]
- Poldrack, R.A.; Yarkoni, T. From brain maps to cognitive ontologies: Informatics and the search for mental structure. Annu. Rev. Psychol. 2016, 67, 587–612. [Google Scholar] [CrossRef]
- Corlett, P.R.; Mollick, J.A.; Kober, H. Meta-analysis of human prediction error for incentives, perception, cognition, and action. Neuropsychopharmacology 2022, 47, 1339–1349. [Google Scholar] [CrossRef]
- Carter, R.M.; Huettel, S.A. A nexus model of the temporal-parietal junction. Trends Cogn. Sci. 2013, 17, 328–336. [Google Scholar] [CrossRef]
- Geng, J.J.; Vossel, S. Re-evaluating the role of TPJ in attentional control: Contextual updating? Neurosci. Biobehav. Rev. 2013, 37, 2608–2620. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Verleger, R.; Heide, W.; Butt, C.; Kömpf, D. Reduction of P3b in patients with temporo-parietal lesions. Cogn. Brain Res. 1994, 2, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef]
- Friston, K. Prediction, perception and agency. Int. J. Psychophysiol. 2012, 83, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.; Deane, G.; Miller, M.; Clark, A. Wilding the predictive brain. Wiley Interdiscip. Rev. Cogn. Sci. 2020, 11, e1542. [Google Scholar] [CrossRef]
- Friston, K.; Square, Q.; Kilner, J. Europe PMC Funders Group Action understanding and active inference. Biol. Cybern. 2011, 104, 137–160. [Google Scholar] [CrossRef]
- Siman-Tov, T.; Granot, R.Y.; Shany, O.; Singer, N.; Hendler, T.; Gordon, C.R. Is there a prediction network? Meta-analytic evidence for a cortical-subcortical network likely subserving prediction. Neurosci. Biobehav. Rev. 2019, 105, 262–275. [Google Scholar] [CrossRef]
- Carotenuto, A.; Arcara, G.; Orefice, G.; Cerillo, I.; Giannino, V.; Rasulo, M.; Iodice, R.; Bambini, V. Communication in Multiple Sclerosis: Pragmatic Deficit and its Relation with Cognition and Social Cognition. Arch. Clin. Neuropsychol. 2018, 33, 194–205. [Google Scholar] [CrossRef]
- Brown, M.; Kuperberg, G.R. A Hierarchical Generative Framework of Language Processing: Linking Language Perception, Interpretation, and Production Abnormalities in Schizophrenia. Front. Hum. Neurosci. 2015, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Kuperberg, G.R.; Brothers, T.; Wlotko, E.W. A Tale of Two Positivities and the N400: Distinct neural signatures are evoked by confirmed and violated predictions at different levels of representation. J. Cogn. Neurosci. 2020, 32, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Vespignani, F.; Canal, P.; Molinaro, N.; Fonda, S.; Cacciari, C. Predictive mechanisms in idiom comprehension. J. Cogn. Neurosci. 2010, 22, 1682–1700. [Google Scholar] [CrossRef] [PubMed]
- Weiland, H.; Bambini, V.; Schumacher, P.B. The role of literal meaning in figurative language comprehension: Evidence from masked priming ERP. Front. Hum. Neurosci. 2014, 8, 583. [Google Scholar] [CrossRef]
- Domaneschi, F.; Bambini, V. Pragmatic competence. In The Routledge Handbook of Philosophy of Skill and Expertise; Rutledge: London, UK, 2020; pp. 419–430. [Google Scholar]
- Diaz, M.T.; Eppes, A. Factors Influencing Right Hemisphere Engagement During Metaphor Comprehension. Front. Psychol. 2018, 9, 414. [Google Scholar] [CrossRef]
- Goodman, N.D.; Frank, M.C. Pragmatic Language Interpretation as Probabilistic Inference. Trends Cogn. Sci. 2016, 20, 818–829. [Google Scholar] [CrossRef]
- Mayn, A.; Demberg, V. Pragmatics of Metaphor Revisited: Modeling the Role of Degree and Salience in Metaphor Understanding. In Proceedings of the Annual Meeting of the Cognitive Science Society, Toronto, ON, Canada, 27–30 July 2022; Volume 44. [Google Scholar]
- Carenini, G.; Bischetti, L.; Schaeken, W.; Bambini, V. Towards a Fully Interpretable and More Scalable RSA Model for Metaphor Understanding (Version 1). arXiv 2024. [Google Scholar] [CrossRef]
- Kao, J.; Bergen, L.; Goodman, N. Formalizing the pragmatics of metaphor understanding. In Proceedings of the Annual Meeting of the Cognitive Science Society, Quebec, QC, Canada, 23–26 July 2014; Volume 36. [Google Scholar]
- Friston, K. Does predictive coding have a future? Nat. Neurosci. 2018, 21, 1019–1021. [Google Scholar] [CrossRef]
- Bambini, V.; Gentili, C.; Ricciardi, E.; Bertinetto, P.M.; Pietrini, P. Decomposing metaphor processing at the cognitive and neural level through functional magnetic resonance imaging. Brain Res. Bull. 2011, 86, 203–216. [Google Scholar] [CrossRef]
- Bohrn, I.C.; Altmann, U.; Jacobs, A.M. Looking at the brains behind figurative language—A quantitative meta-analysis of neuroimaging studies on metaphor, idiom, and irony processing. Neuropsychologia 2012, 50, 2669–2683. [Google Scholar] [CrossRef]
- Spotorno, N.; Koun, E.; Prado, J.; Van Der Henst, J.-B.; Noveck, I.A. Neural evidence that utterance-processing entails mentalizing: The case of irony. NeuroImage 2012, 63, 25–39. [Google Scholar] [CrossRef]
- Lago, S.; Pezzetta, R.; Gastaldon, S.; Peressotti, F.; Arcara, G. Trial-by-trial fluctuations of pre-stimulus alpha power predict language ERPs. Psychophysiology 2023, 60, e14388. [Google Scholar] [CrossRef] [PubMed]
- León-Cabrera, P.; Rodríguez-Fornells, A.; Morís, J. Electrophysiological correlates of semantic anticipation during speech comprehension. Neuropsychologia 2017, 99, 326–334. [Google Scholar] [CrossRef]
- Cao, L.; Thut, G.; Gross, J. The role of brain oscillations in predicting self-generated sounds. NeuroImage 2017, 147, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Ergenoglu, T.; Demiralp, T.; Bayraktaroglu, Z.; Ergen, M.; Beydagi, H.; Uresin, Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Cogn. Brain Res. 2004, 20, 376–383. [Google Scholar] [CrossRef]
- Samaha, J.; Boutonnet, B.; Postle, B.R.; Lupyan, G. Effects of meaningfulness on perception: Alpha-band oscillations carry perceptual expectations and influence early visual responses. Sci. Rep. 2018, 8, 6606. [Google Scholar] [CrossRef] [PubMed]
- Grent-’T-Jong, T.; Boehler, C.N.; Kenemans, J.L.; Woldorff, M.G. Differential functional roles of slow-wave and oscillatory-alpha activity in visual sensory cortex during anticipatory visual-spatial attention. Cereb. Cortex 2011, 21, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.v.D.; Appelbaum, L.G.; Clark, K.; Lorist, M.M.; Woldorff, M.G. Visual search performance is predicted by both prestimulus and poststimulus electrical brain activity. Sci. Rep. 2016, 6, 37718. [Google Scholar] [CrossRef]
- Iemi, L.; Busch, N.A.; Laudini, A.; Haegens, S.; Samaha, J.; Villringer, A.; Nikulin, V.V. Multiple mechanisms link prestimulus neural oscillations to sensory responses. eLife 2019, 8, e43620. [Google Scholar] [CrossRef]
- Iemi, L.; Chaumon, M.; Crouzet, S.M.; Busch, N.A. Spontaneous neural oscillations bias perception by modulating baseline excitability. J. Neurosci. 2017, 37, 807–819. [Google Scholar] [CrossRef]
- Min, B.-K.; Herrmann, C.S. Prestimulus EEG alpha activity reflects prestimulus top-down processing. Neurosci. Lett. 2007, 422, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Solís-Vivanco, R.; Jensen, O.; Bonnefond, M. Top–Down Control of Alpha Phase Adjustment in Anticipation of Temporally Predictable Visual Stimuli. J. Cogn. Neurosci. 2018, 30, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Rohenkohl, G.; Nobre, A.C. Alpha Oscillations Related to Anticipatory Attention Follow Temporal Expectations. J. Neurosci. 2011, 31, 14076–14084. [Google Scholar] [CrossRef]
- Van Diepen, R.M.; Foxe, J.J.; Mazaheri, A. The functional role of alpha-band activity in attentional processing: The current zeitgeist and future outlook. Curr. Opin. Psychol. 2019, 29, 229–238. [Google Scholar] [CrossRef]
- Simonet, M.; Meziane, H.B.; Runswick, O.R.; North, J.S.; Williams, A.M.; Barral, J.; Roca, A. The modulation of event-related alpha rhythm during the time course of anticipation. Sci. Rep. 2019, 9, 18226. [Google Scholar] [CrossRef]
- Foxe, J.J.; Snyder, A.C. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Sherman, M.T.; Kanai, R.; Seth, A.K.; VanRullen, R. Rhythmic Influence of Top–Down Perceptual Priors in the Phase of Prestimulus Occipital Alpha Oscillations. J. Cogn. Neurosci. 2016, 28, 1318–1330. [Google Scholar] [CrossRef]
- Bauer, M.; Stenner, M.-P.; Friston, K.J.; Dolan, R.J. Attentional modulation of alpha/beta and gamma oscillations reflect functionally distinct processes. J. Neurosci. 2014, 34, 16117–16125. [Google Scholar] [CrossRef]
- Sedley, W.; Gander, P.E.; Kumar, S.; Kovach, C.K.; Oya, H.; Kawasaki, H.; Howard, M.A.; Griffiths, T.D. Neural signatures of perceptual inference. eLife 2016, 5, e11476. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.; Friston, K.J. Attention, uncertainty, and free-energy. Front. Hum. Neurosci. 2010, 4, 215. [Google Scholar] [CrossRef]
- Hohwy, J. Attention and Conscious Perception in the Hypothesis Testing Brain. Front. Psychol. 2012, 3, 96. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.S.; McGovern, D.P.; Clark, A.; O’Connell, R.G. Evaluating the neurophysiological evidence for predictive processing as a model of perception. Ann. N. Y. Acad. Sci. 2020, 1464, 242–268. [Google Scholar] [CrossRef]
- Becker, R.; Van de Ville, D.; Kleinschmidt, A. Alpha Oscillations Reduce Temporal Long-Range Dependence in Spontaneous Human Brain Activity. J. Neurosci. 2018, 38, 755–764. [Google Scholar] [CrossRef]
- Hindriks, R.; Bijma, F.; van Dijk, B.; van der Werf, Y.; van Someren, E.; van der Vaart, A. Dynamics underlying spontaneous human alpha oscillations: A data-driven approach. NeuroImage 2011, 57, 440–451. [Google Scholar] [CrossRef]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neurosci. 2014, 20, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Gastaldon, S.; Busan, P.; Arcara, G.; Peressotti, F. Inefficient speech-motor control affects predictive speech comprehension: Atypical electrophysiological correlates in stuttering. Cereb. Cortex 2023, 33, 6834–6851. [Google Scholar] [CrossRef] [PubMed]
- Rommers, J.; Dijkstra, T.; Bastiaansen, M. Context-dependent Semantic Processing in the Human Brain: Evidence from Idiom Comprehension. J. Cogn. Neurosci. 2013, 25, 762–776. [Google Scholar] [CrossRef]
- Terporten, R.; Schoffelen, J.-M.; Dai, B.; Hagoort, P.; Kösem, A. The Relation between Alpha/Beta Oscillations and the Encoding of Sentence induced Contextual Information. Sci. Rep. 2019, 9, 20255. [Google Scholar] [CrossRef]
- Wang, L.; Hagoort, P.; Jensen, O. Language Prediction Is Reflected by Coupling between Frontal Gamma and Posterior Alpha Oscillations. J. Cogn. Neurosci. 2018, 30, 432–447. [Google Scholar] [CrossRef]
- Ryskin, R.; Nieuwland, M.S. Prediction during language comprehension: What is next? Trends Cogn. Sci. 2023, 27, 1032. [Google Scholar] [CrossRef]
- Friederici, A.D. Evolution of the neural language network. Psychon. Bull. Rev. 2017, 24, 41–47. [Google Scholar] [CrossRef]
- Rapp, A.M.; Mutschler, D.E.; Erb, M. Where in the brain is nonliteral language? A coordinate-based meta-analysis of functional magnetic resonance imaging studies. NeuroImage 2012, 63, 600–610. [Google Scholar] [CrossRef]
- Sulpizio, S.; Arcara, G.; Lago, S.; Marelli, M.; Amenta, S. Very early and late form-to-meaning computations during visual word recognition as revealed by electrophysiology. Cortex 2022, 157, 167–193. [Google Scholar] [CrossRef]
- Baayen, H.; Linke, M. An introduction to the generalized additive model. In A Practical Handbook of Corpus Linguistics; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Oswald, V.; Zerouali, Y.; Boulet-Craig, A.; Krajinovic, M.; Laverdière, C.; Sinnett, D.; Jolicoeur, P.; Lippé, S.; Jerbi, K.; Robaey, P. Spontaneous brain oscillations as neural fingerprints of working memory capacities: A resting-state MEG study. Cortex 2017, 97, 109–124. [Google Scholar] [CrossRef]
- Alexandrou, A.M.; Saarinen, T.; Kujala, J.; Salmelin, R. Cortical entrainment: What we can learn from studying naturalistic speech perception. Lang. Cogn. Neurosci. 2020, 35, 681–693. [Google Scholar] [CrossRef]
- Pu, Y.; Cheyne, D.; Sun, Y.; Johnson, B.W. Theta oscillations support the interface between language and memory. NeuroImage 2020, 215, 116782. [Google Scholar] [CrossRef]
- Bambini, V.; Bertini, C.; Schaeken, W.; Stella, A.; Di Russo, F. Disentangling Metaphor from Context: An ERP Study. Front. Psychol. 2016, 7, 559. [Google Scholar] [CrossRef]
- Bambini, V.; Lago, S.; Zago, S.; Tonini, E.; Pellegrino, G.; Arcara, G. Brain dynamics during metaphor processing as assessed by magnetoencephalographic imaging. in preparation.
- Peirce, J.W. PsychoPy—Psychophysics software in Python. J. Neurosci. Methods 2007, 162, 8–13. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Destrieux, C.; Fischl, B.; Dale, A.; Halgren, E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 2010, 53, 1–15. [Google Scholar] [CrossRef]
- Lorca-Puls, D.L.; Gajardo-Vidal, A.; Oberhuber, M.; Prejawa, S.; Hope, T.M.H.; Leff, A.P.; Green, D.W.; Price, C.J. Brain regions that support accurate speech production after damage to Broca’s area. Brain Commun. 2021, 3, fcab230. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 15 November 2023).
- Arcara, G.; Petrova, A. erpR: Event-Related Potentials (ERP) Analysis, Graphics and Utility Functions (Version R Package Version 0.2.0) [Computer Software]. 2014. Available online: https://CRAN.R-project.org/package=erpR (accessed on 15 November 2023).
- DeLong, K.A.; Urbach, T.P.; Kutas, M. Probabilistic word pre-activation during language comprehension inferred from electrical brain activity. Nat. Neurosci. 2005, 8, 1117–1121. [Google Scholar] [CrossRef]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- van Rij, J.; Hendriks, P.; van Rijn, H.; Baayen, R.H.; Wood, S.N. Analyzing the Time Course of Pupillometric Data. Trends Hear. 2019, 23, 2331216519832483. [Google Scholar] [CrossRef]
- Wieling, M. Analyzing dynamic phonetic data using generalized additive mixed modeling: A tutorial focusing on articulatory differences between L1 and L2 speakers of English. J. Phon. 2018, 70, 86–116. [Google Scholar] [CrossRef]
- Luck, S.J. Event-related potentials. In APA Handbook of Research Methods in Psychology; Foundations, Planning, Measures, and Psychometrics; Cooper, H., Camic, P.M., Long, D.L., Panter, A.T., Rindskopf, D., Sher, K.J., Eds.; American Psychological Association: Washington, DC, USA, 2012; Volume 1, pp. 523–546. [Google Scholar] [CrossRef]
- van Rij, J.; Wieling, M.; Baayen, R.H.; van Rijn, H. itsadug: Interpreting Time Series and Autocorrelated Data Using GAMMs. (Version R Package Version 2.3) [Computer Software]. 2017. Available online: https://cran.r-project.org/package=itsadug (accessed on 15 November 2023).
- Gillebert, C.R.; Mantini, D.; Peeters, R.; Dupont, P.; Vandenberghe, R. Cytoarchitectonic mapping of attentional selection and reorienting in parietal cortex. NeuroImage 2013, 67, 257–272. [Google Scholar] [CrossRef]
- Schneider, S.; Rapp, A.M.; Haeußinger, F.B.; Ernst, L.H.; Hamm, F.; Fallgatter, A.J.; Ehlis, A.-C. Beyond the N400: Complementary access to early neural correlates of novel metaphor comprehension using combined electrophysiological and haemodynamic measurements. Cortex 2014, 53, 45–59. [Google Scholar] [CrossRef]
- Canal, P.; Bischetti, L.; Bertini, C.; Ricci, I.; Lecce, S.; Bambini, V. N400 differences between physical and mental metaphors: The role of Theories of Mind. Brain Cogn. 2022, 161, 105879. [Google Scholar] [CrossRef]
- Regel, S.; Gunter, T.C.; Friederici, A.D. Isn’t It Ironic? An Electrophysiological Exploration of Figurative Language Processing. J. Cogn. Neurosci. 2011, 23, 277–293. [Google Scholar] [CrossRef]
- Regel, S.; Coulson, S.; Gunter, T.C. The communicative style of a speaker can affect language comprehension? ERP evidence from the comprehension of irony. Brain Res. 2010, 1311, 121–135. [Google Scholar] [CrossRef]
- Federmeier, K.D.; Mai, H.; Kutas, M. Both sides get the point: Hemispheric sensitivities to sentential constraint. Mem. Cogn. 2005, 33, 871–886. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Liu, Y.-N.; Tsai, J.-L. The Time Course of Contextual Effects on Visual Word Recognition. Front. Psychol. 2012, 3, 23036. [Google Scholar] [CrossRef]
- Wlotko, E.W.; Federmeier, K.D. Finding the Right Word: Hemispheric Asymmetries in the Use of Sentence Context Information. Neuropsychologia 2007, 45, 3001–3014. [Google Scholar] [CrossRef]
- Goldstein, A.; Arzouan, Y.; Faust, M. Killing a novel metaphor and reviving a dead one: ERP correlates of metaphor conventionalization. Brain Lang. 2012, 123, 137–142. [Google Scholar] [CrossRef]
- Bastiaansen, M.; Magyari, L.; Hagoort, P. Syntactic Unification Operations Are Reflected in Oscillatory Dynamics during On-line Sentence Comprehension. J. Cogn. Neurosci. 2010, 22, 1333–1347. [Google Scholar] [CrossRef]
- Halgren, E.; Dhond, R.P.; Christensend, N.; Van Petten, C.; Marinkovica, K.; Lewine, J.D.; Dale, A.M. N400-like Magnetoencephalography Responses Modulated by Semantic Context, Word Frequency, and Lexical Class in Sentences. NeuroImage 2002, 17, 1101–1116. [Google Scholar] [CrossRef]
- Kielar, A.; Panamsky, L.; Links, K.A.; Meltzer, J.A. Localization of electrophysiological responses to semantic and syntactic anomalies in language comprehension with MEG. NeuroImage 2015, 105, 507–524. [Google Scholar] [CrossRef]
- Wang, L.; Jensen, O.; Van Den Brink, D.; Weder, N.; Schoffelen, J.; Magyari, L.; Hagoort, P.; Bastiaansen, M. Beta oscillations relate to the N400m during language comprehension. Hum. Brain Mapp. 2012, 33, 2898–2912. [Google Scholar] [CrossRef]
- Bornkessel-Schlesewsky, I.; Schlesewsky, M. An alternative perspective on “semantic P600” effects in language comprehension. Brain Res. Rev. 2008, 59, 55–73. [Google Scholar] [CrossRef]
- Wang, L.; Kuperberg, G.R. Better together: Integrating multivariate with univariate methods, and MEG with EEG to study language comprehension. Lang. Cogn. Neurosci. 2023, 39, 991–1019. [Google Scholar] [CrossRef]
- Bambini, V.; Ranieri, G.; Bischetti, L.; Scalingi, B.; Bertini, C.; Ricci, I.; Schaeken, W.; Canal, P. The costs of multimodal metaphors: Comparing ERPs to figurative expressions in verbal and verbo-pictorial formats. Discourse Process. 2024, 61, 44–68. [Google Scholar] [CrossRef]
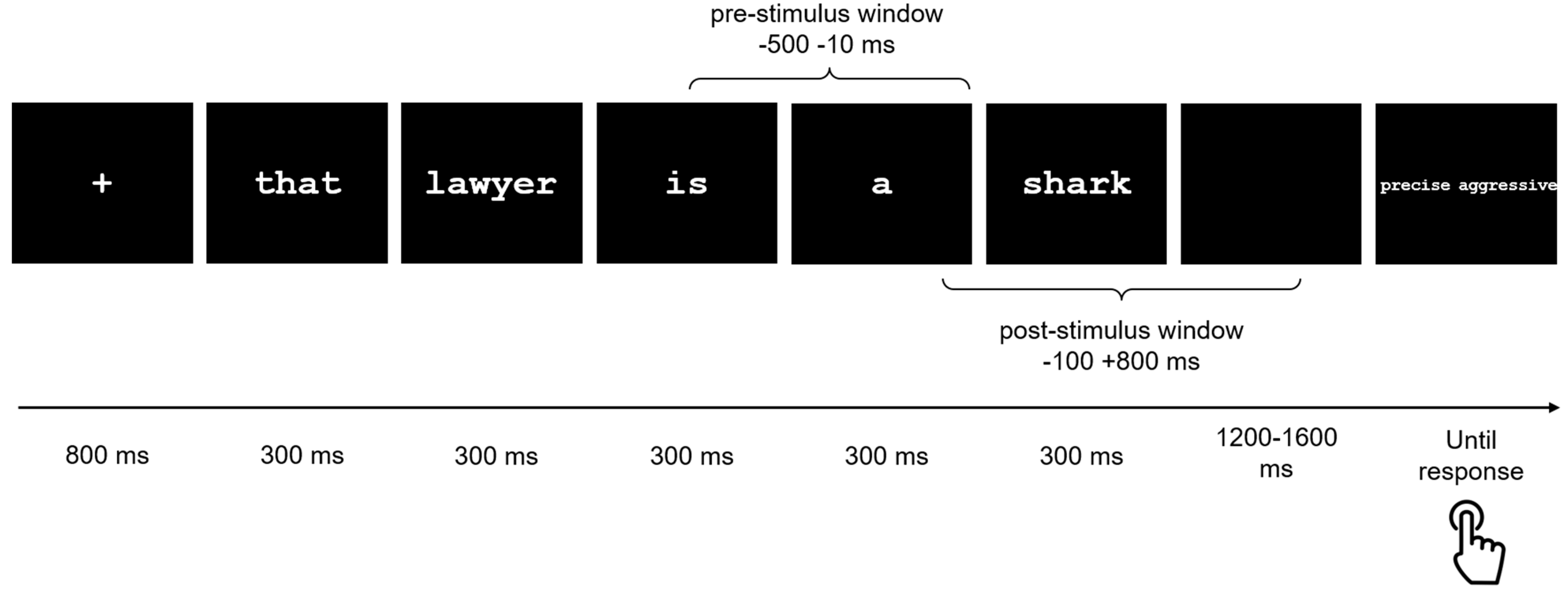

| Condition | Stimulus | Related Adjective | Unrelated Adjective |
|---|---|---|---|
| Literal | That weapon is a bomb | Explosive | Difficult |
| Literal | That animal is a bear | Unsociable | Fitting |
| Literal | That tree is an oak | Strong | Nautical |
| Metaphor | That show is a bomb | Explosive | Difficult |
| Metaphor | That sailor is a bear | Unsociable | Fitting |
| Metaphor | That marriage is an oak | Strong | Nautical |
| Filler | That city is a metropolis | Expanded | Credible |
| Filler | That cup is a trophy | Prestigious | Friendly |
| Filler | That appliance is a blender | Homely | Inebriating |
| ROI | x | y | z |
|---|---|---|---|
| Broca | −51 | 9 | 12 |
| lMTG | −59 | −34 | −13 |
| lSTG | −56 | −10 | −11 |
| lTPJ | −45 | −70 | 37 |
| rTPJ | 45 | 57 | 39 |
| lTPJ | rTPJ | |||
|---|---|---|---|---|
| F (edf) | p Value | F (edf) | p Value | |
| Interaction: time, power, literal condition | 0.186 (1.244) | 0.654 | 1.879 (2.392) | 0.119 |
| Interaction: time, power, metaphor condition | 3.046 (1.007) | 0.080 | 0.754 (1.005) | 0.385 |
| Broca | lMTG | lSTG | ||||
|---|---|---|---|---|---|---|
| F (edf) | p Value | F (edf) | p Value | F (edf) | p Value | |
| Interaction: time, lTPJ power, literal condition | 4.683 (1.008) | 0.031 * | 6.150 (1.023) | 0.014 * | 2.811 (5.297) | 0.006 * |
| Interaction: time, lTPJ power, metaphor condition | 2.156 (2.984) | 0.069 | 0.837 (9.84) | 0.632 | 1.113 (1.008) | 0.289 |
| Interaction: time, rTPJ power, literal condition | 0.572 (3.295) | 0.729 | 0.658 (2.523) | 0.607 | 3.397 (2.735) | 0.021 * |
| Interaction: time, rTPJ power, metaphor condition | 1.319 (8.879) | 0.195 | 1.947 (5.859) | 0.059 | 3.441 (1.836) | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lago, S.; Zago, S.; Bambini, V.; Arcara, G. Pre-Stimulus Activity of Left and Right TPJ in Linguistic Predictive Processing: A MEG Study. Brain Sci. 2024, 14, 1014. https://doi.org/10.3390/brainsci14101014
Lago S, Zago S, Bambini V, Arcara G. Pre-Stimulus Activity of Left and Right TPJ in Linguistic Predictive Processing: A MEG Study. Brain Sciences. 2024; 14(10):1014. https://doi.org/10.3390/brainsci14101014
Chicago/Turabian StyleLago, Sara, Sara Zago, Valentina Bambini, and Giorgio Arcara. 2024. "Pre-Stimulus Activity of Left and Right TPJ in Linguistic Predictive Processing: A MEG Study" Brain Sciences 14, no. 10: 1014. https://doi.org/10.3390/brainsci14101014
APA StyleLago, S., Zago, S., Bambini, V., & Arcara, G. (2024). Pre-Stimulus Activity of Left and Right TPJ in Linguistic Predictive Processing: A MEG Study. Brain Sciences, 14(10), 1014. https://doi.org/10.3390/brainsci14101014






