Neurophysiological Parameters Influencing Sleep–Wake Discrepancy in Insomnia Disorder: A Preliminary Analysis on Alpha Rhythm during Sleep Onset
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Polysomnography
2.3. Sleep Diary
2.4. Sleep State Misperception Indices
2.5. Electrophysiological Indices
2.5.1. Alpha Density
2.5.2. Latency-to-Sleep Stages
2.5.3. REM Arousal Density
2.5.4. Sleep Transition Probability
2.6. Statistical Analysis
3. Results
3.1. Demographical and Clinical Features
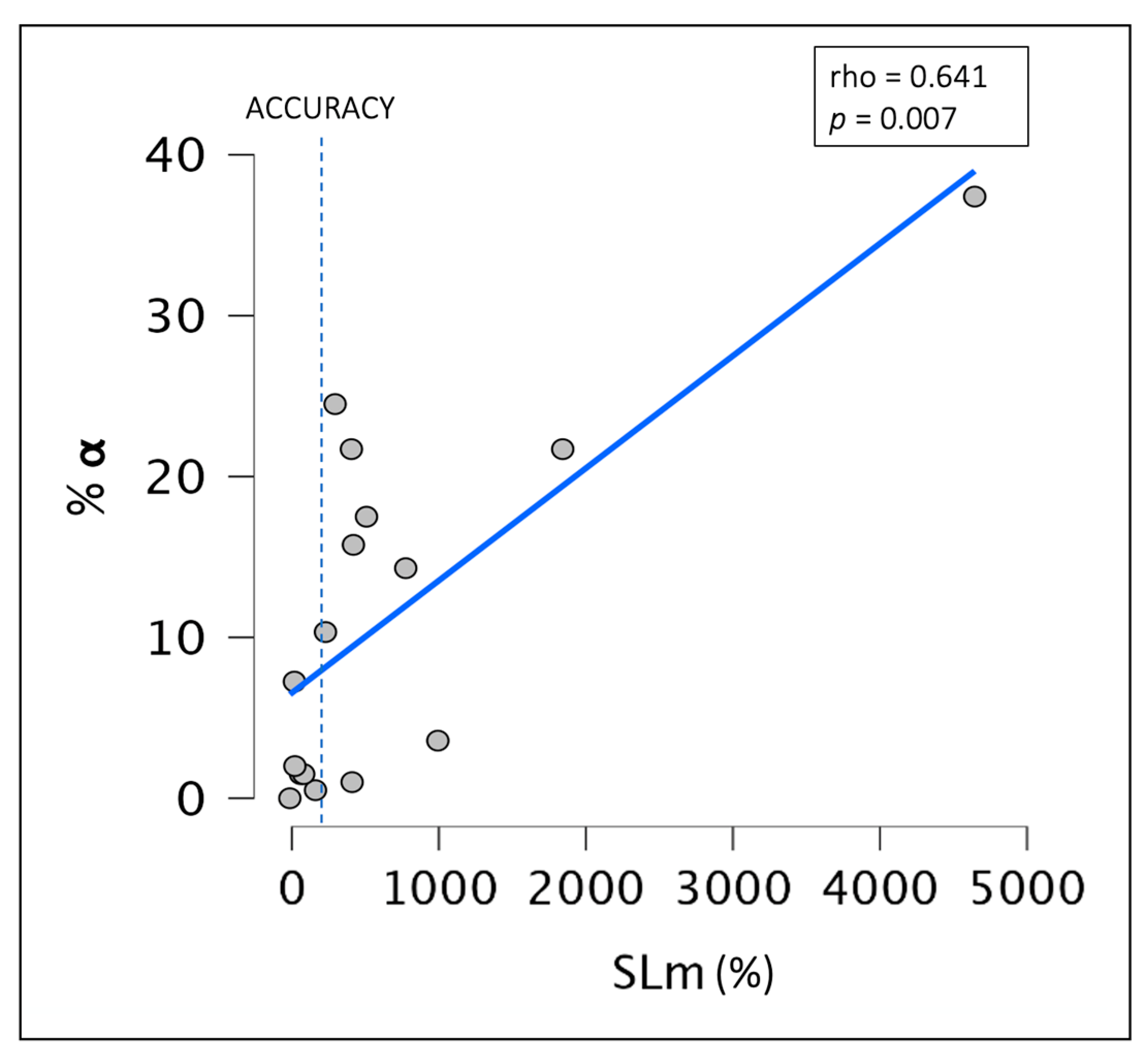
3.2. Linear Regression Analysis
3.3. Relationship between Objective Sleep Indices and the Degree of SSM
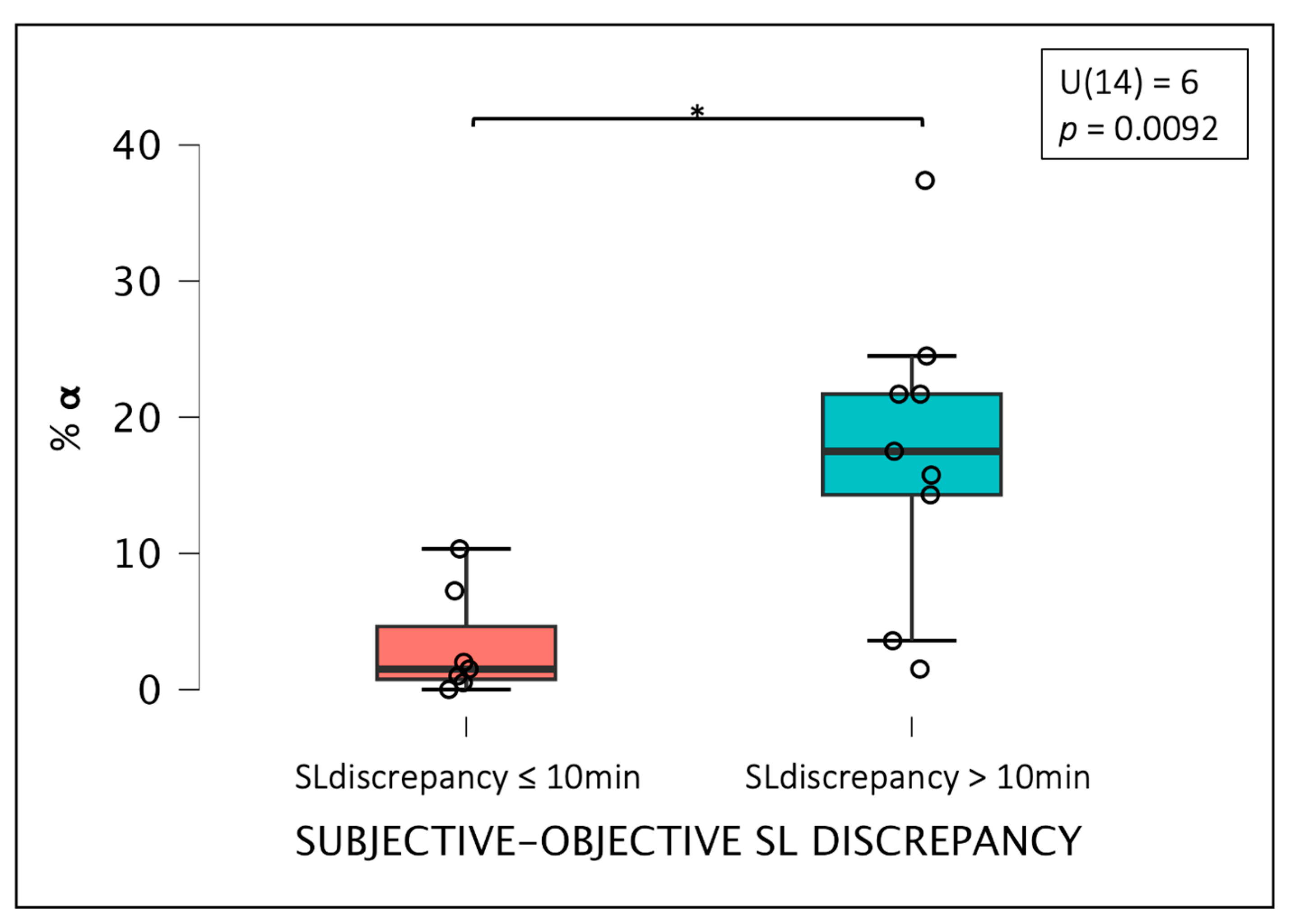
3.4. Differences between Groups Based on the Degree of Misperception
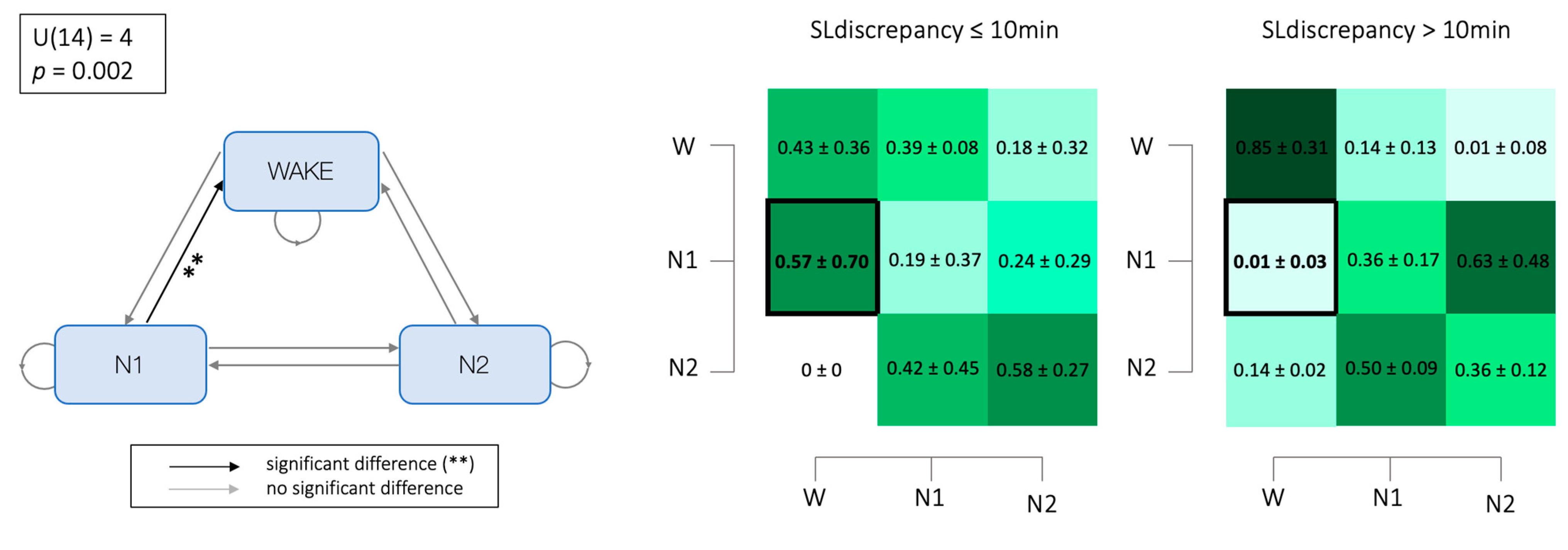
3.5. Markovian Matrices
4. Discussion
5. Conclusions, Limitations, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Perlis, M.L.; Posner, D.; Riemann, D.; Bastien, C.H.; Teel, J.; Thase, M.J.L. Sleep and Sleep Disorders 2 Insomnia. Lancet 2022, 400, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Espie, C.A.; Altena, E.; Arnardottir, E.S.; Baglioni, C.; Bassetti, C.L.A.; Bastien, C.; Berzina, N.; Bjorvatn, B.; Dikeos, D.; et al. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J. Sleep Res. 2023, 32, e14035. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A.J.; Caruso, L.S.; Glovinsky, P.B. A behavioral perspective on insomnia treatment. Psychiatr. Clin. N. Am. 1987, 10, 541–553. [Google Scholar] [CrossRef]
- Riemann, D.; Benz, F.; Dressle, R.J.; Espie, C.A.; Johann, A.F.; Blanken, T.F.; Leerssen, J.; Wassing, R.; Henry, A.L.; Kyle, S.D.; et al. Insomnia disorder: State of the science and challenges for the future. J. Sleep Res. 2022, 31, e13604. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.H.; Ceklic, T.; St-Hilaire, P.; Desmarais, F.; Pérusse, A.D.; Lefrançois, J.; Pedneault-Drolet, M. Insomnia and sleep misperception. Pathol. Biol. 2014, 62, 241–251. [Google Scholar] [CrossRef]
- Harvey, A.G.; Tang, N.K. (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychol. Bull. 2012, 138, 77–101. [Google Scholar] [CrossRef]
- Castelnovo, A.; Ferri, R.; Punjabi, N.M.; Castronovo, V.; Garbazza, C.; Zucconi, M.; Ferini-Strambi, L.; Manconi, M. The paradox of paradoxical insomnia: A theoretical review towards a unifying evidence-based definition. Sleep Med. Rev. 2019, 44, 70–82. [Google Scholar] [CrossRef]
- Dressle, R.J.; Riemann, D. Hyperarousal in insomnia disorder: Current evidence and potential mechanisms. J. Sleep Res. 2023, 32, e13928. [Google Scholar] [CrossRef]
- Mercer, J.; Bootzin, R.; Lack, L. Insomniacs’ perception of wake instead of sleep. Sleep 2002, 25, 564–571. [Google Scholar] [CrossRef]
- Conte, F.; Malloggi, S.; De Rosa, O.; Di Iorio, I.; Romano, F.; Giganti, F.; Ficca, G. Sleep Continuity, Stability and Cyclic Organization Are Impaired in Insomniacs: A Case–Control Study. Int. J. Environ. Res. Public Health 2023, 20, 1240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, G.; Shao, Y.; Yao, P.; Liu, J.; Zhou, S.; Hu, S.; Xu, J.; Guo, Y.; Sun, H.; et al. Sleep discrepancy is associated with alterations in the salience network in patients with insomnia disorder: An EEG-fMRI study. NeuroImage Clin. 2022, 35, 103111. [Google Scholar] [CrossRef]
- Marcuse, L.V.; Fields, M.C.; Yoo, J. The normal adult EEG. In Rowan’s Primer of EEG, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 39–66. [Google Scholar]
- Perlis, M.L.; Merica, H.; Smith, M.T.; Giles, D.E. Beta EEG activity and insomnia. Sleep Med. Rev. 2001, 5, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Lecci, S.; Cataldi, J.; Betta, M.; Bernardi, G.; Heinzer, R.; Siclari, F. EEG changes associated with subjective under- and overestimation of sleep duration. Sleep 2020, 43, 94. [Google Scholar] [CrossRef]
- Siclari, F.; Tononi, G. Local aspects of sleep and wakefulness. Curr. Opin. Neurobiol. 2017, 44, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; De Gennaro, L. Going Local: Insights from EEG and Stereo-EEG Studies of the Human Sleep-Wake Cycle. Curr. Top. Med. Chem. 2011, 11, 2423–2437. [Google Scholar] [CrossRef]
- Riedner, B.A.; Goldstein, M.R.; Plante, D.T.; Rumble, M.E.; Ferrarelli, F.; Tononi, G.; Benca, R.M. Regional Patterns of Elevated Alpha and High-Frequency Electroencephalographic Activity during Nonrapid Eye Movement Sleep in Chronic Insomnia: A Pilot Study. Sleep 2016, 39, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lian, J.; Wang, K.; Wen, J.; Luo, Y. Changes in the cortical network during sleep stage transitions. J. Neurosci. Res. 2023, 101, 20–33. [Google Scholar] [CrossRef]
- Hermans, L.W.A.; Leufkens, T.R.; van Gilst, M.M.; Weysen, T.; Ross, M.; Anderer, P.; Overeem, S.; Vermeeren, A. Sleep EEG characteristics associated with sleep onset misperception. Sleep Med. 2019, 57, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hermans, L.W.A.; van Gilst, M.M.; Regis, M.; van den Heuvel, L.C.E.; Langen, H.; van Mierlo, P.; Krijn, R.; Hoondert, B.; Maass, H.; van Dijk, J.P.; et al. Modeling sleep onset misperception in insomnia. Sleep 2020, 12, 43. [Google Scholar] [CrossRef]
- Aminoff, M.J. Aminoff’s Electrodiagnosis in Clinical Neurology, 6th ed.; W.B. Saunders: Philadelphia, PA, USA, 2012; Section II; pp. 37–84. [Google Scholar]
- Schwabedal, J.T.; Riedl, M.; Penzel, T.; Wessel, N. Alpha-wave frequency characteristics in health and insomnia during sleep. J. Sleep Res. 2016, 25, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Mohammadi, H.; Khazaie, H. Alpha-wave Characteristics in Psychophysiological Insomnia. J. Med. Signals Sens. 2019, 9, 259–266. [Google Scholar]
- Wei, Y.; Colombo, M.A.; Ramautar, J.R.; Blanken, T.F.; van der Werf, Y.D.; Spiegelhalder, K.; Feige, B.; Riemann, D.; Van Someren, E.J.W. Sleep Stage Transition Dynamics Reveal Specific Stage 2 Vulnerability in Insomnia. Sleep 2017, 40, zsx117. [Google Scholar] [CrossRef]
- Feige, B.; Benz, F.; Dressle, R.J.; Riemann, D. Insomnia and REM sleep instability. J. Sleep Res. 2023, 32, e14032. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Spiegelhalder, K.; Nissen, C.; Hirscher, C.; Baglioni, C.; Feige, B. REM sleep instability—A new pathway for insomnia? Pharmacopsychiatry 2012, 45, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Castelnovo, A.; Ferri, R.; Galbiati, A.; Rossi, A.; Zucconi, M.; Castronovo, V.; Ferini-Strambi, L.; Manconi, M. Extreme sleep state misperception: From psychopathology to objective-subjective sleep measures. Int. J. Psychophysiol. 2021, 167, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Klem, G.H. The ten-twenty electrode system of the international federation. the internanional federation of clinical nenrophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar] [PubMed]
- Lee, Y.J.; Lee, J.Y.; Cho, J.H.; Choi, J.H. Interrater reliability of sleep stage scoring: A meta-analysis. J. Clin. Sleep Med. 2022, 18, 193–202. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.L.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events; Version 2.6; American Academy of Sleep Medicine: Darien, IL, USA, 2020. [Google Scholar]
- Spedicato, G. Discrete Time Markov Chains with R. R J. 2017, 9, 84–104. [Google Scholar] [CrossRef]
- Häggström, O. Finite Markov Chains and Algorithmic Applications; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Kim, J.W.; Lee, J.S.; Robinson, P.A.; Jeong, D.U. Markov analysis of sleep dynamics. Phys. Rev. Lett. 2009, 102, 178104. [Google Scholar] [CrossRef] [PubMed]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.; Ly, A.; Gronau, Q.F.; Šmíra, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Maltezos, A.; Perrault, A.A.; Walsh, N.A.; Phillips, E.-M.; Gong, K.; Tarelli, L.; Smith, D.; Cross, N.E.; Pomares, F.B.; Gouin, J.-P.; et al. Methodological approach to sleep state misperception in insomnia disorder: Comparison between multiple nights of actigraphy recordings and a single night of polysomnography recording. medRxiv 2023. [Google Scholar] [CrossRef]
- Dillon, H.R.; Lichstein, K.L.; Dautovich, N.D.; Taylor, D.J.; Riedel, B.W.; Bush, A.J. Variability in Self-Reported Normal Sleep Across the Adult Age Span. J. Gerontol. 2015, 70, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Broughton, R.; Hasan, J. Quantitative topographic electroencephalographic mapping during drowsiness and sleep onset. J. Clin. Neurophysiol. 1995, 12, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.; Kim, M.; Hong, S.; Park, K. Driver drowsiness detection using the in-ear EEG. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4646–4649. [Google Scholar]
- Asadi-Pooya, A.A.; Sperling, M.R. Normal Awake, Drowsy, and Sleep EEG Patterns That Might Be Overinterpreted as Abnormal. J. Clin. Neurophysiol. 2019, 36, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Andrillon, T.; Solelhac, G.; Bouchequet, P.; Romano, F.; Le Brun, M.P.; Brigham, M.; Chennaoui, M.; Léger, D. Revisiting the value of polysomnographic data in insomnia: More than meets the eye. Sleep Med. 2020, 66, 184–200. [Google Scholar] [CrossRef]
- Krystal, A.D.; Edinger, J.D.; Wohlgemuth, W.K.; Marsh, G.R. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep 2002, 25, 630–640. [Google Scholar]
- Zhao, W.; Van Someren, E.J.; Li, C.; Chen, X.; Gui, W.; Tian, Y.; Liu, Y.; Lei, X. EEG spectral analysis in insomnia disorder: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 59, 101457. [Google Scholar] [CrossRef]
- St-Jean, G.; Turcotte, I.; Pérusse, A.D.; Bastien, C.H. REM and NREM power spectral analysis on two consecutive nights in psychophysiological and paradoxical insomnia sufferers. Int. J. Psychophysiol. 2013, 89, 181–194. [Google Scholar] [CrossRef]
- Stephan, A.M.; Siclari, F. Reconsidering sleep perception in insomnia: From misperception to mismeasurement. J. Sleep Res. 2023, 32, e14028. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ren, R.; Lei, F.; Zhang, Y.; Vitiello, M.V.; Tang, X. Elevated beta activity in the nighttime sleep and multiple sleep latency electroencephalograms of chronic insomnia patients. Front. Neurosci. 2022, 16, 1045934. [Google Scholar] [CrossRef]
- Maes, J.; Verbraecken, J.; Willemen, M.; De Volder, I.; Van Gastel, A.; Michiels, N.; Verbeek, I.; Vandekerckhove, M.; Wuyts, J.; Haex, B.; et al. Sleep misperception, EEG characteristics and autonomic nervous system activity in primary insomnia: A retrospective study on polysomnographic data. Int. J. Psychophysiol. 2014, 91, 163–171. [Google Scholar] [CrossRef]
- Hsiao, F.C.; Tsai, P.J.; Wu, C.W.; Yang, C.M.; Lane, T.J.; Lee, H.C.; Chen, L.C.; Lee, W.K.; Lu, L.H.; Wu, Y.Z. The neurophysiological basis of the discrepancy between objective and subjective sleep during the sleep onset period: An EEG-fMRI study. Sleep 2018, 41, zsy056. [Google Scholar] [CrossRef] [PubMed]
- Hertenstein, E.; Nissen, C.; Riemann, D.; Feige, B.; Baglioni, C.; Spiegelhalder, K. The exploratory power of sleep effort, dysfunctional beliefs and arousal for insomnia severity and polysomnography-determined sleep. J. Sleep Res. 2015, 24, 399–406. [Google Scholar] [CrossRef]
- Bastien, C.H.; St-Jean, G.; Morin, C.M.; Turcotte, I.; Carrier, J. Chronic psychophysiological insomnia: Hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep 2008, 31, 887–898. [Google Scholar] [CrossRef]
- Turcotte, I.; St-Jean, G.; Bastien, C.H. Are individuals with paradoxical insomnia more hyperaroused than individuals with psychophysiological insomnia? Event-related potentials measures at the peri-onset of sleep. Int. J. Psychophysiol. 2011, 81, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Broomfield, N.; Espie, C. Towards a valid, reliable measure of sleep effort. J. Sleep Res. 2005, 14, 401–407. [Google Scholar] [CrossRef]
- Borges, C.; Ellis, J.G.; Ruivo Marques, D. The Role of Sleep Effort as a Mediator Between Anxiety and Depression. Psychol. Rep. 2023, 2023, 332941221149181. [Google Scholar] [CrossRef]
- Espie, C.A.; Broomfield, N.M.; MacMahon, K.M.; Macphee, L.M.; Taylor, L.M. The attention-intention-effort pathway in the development of psychophysiologic insomnia: A theoretical review. Sleep Med. Rev. 2006, 10, 215–245. [Google Scholar] [CrossRef]
- Lo, E.; Tsai, H.; Yang, A.C. Sleep Instability in Paradoxical Insomnia is Associated with Perception of Sleep. medRxiv 2022. [Google Scholar] [CrossRef]
- Benz, F.; Riemann, D.; Feige, B. Dreaming and Insomnia: Link between Physiological REM Parameters and Mentation Characteristics. Brain Sci. 2020, 10, 378. [Google Scholar] [CrossRef]
- Stephan, A.M.; Lecci, S.; Cataldi, J.; Siclari, F. Conscious experiences and high-density EEG patterns predicting subjective sleep depth. Curr. Biol. 2021, 31, 5487–5500. [Google Scholar] [CrossRef]
- Feige, B.; Al-Shajlawi, A.; Nissen, C.; Voderholzer, U.; Hornyak, M.; Spiegelhalder, K.; Kloepfer, C.; Perlis, M.; Riemann, D. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J. Sleep Res. 2008, 17, 180–190. [Google Scholar] [CrossRef]
- Forget, D.; Morin, C.M.; Bastien, C.H. The role of the spontaneous and evoked k-complex in good-sleeper controls and in individuals with insomnia. Sleep 2011, 34, 1251–1260. [Google Scholar]
- Younes, M.; Raneri, J.; Hanly, P. Staging Sleep in Polysomnograms: Analysis of Inter-Scorer Variability. J. Clin. Sleep Med. 2016, 12, 885–894. [Google Scholar] [CrossRef] [PubMed]


| n = 16 | % | ||||
| Male | 12 | 75% | |||
| On medication | 10 | 62.5% | |||
| Mean (Mean ± SD) | |||||
| Age (years) | 51.3 ± 13.34 | ||||
| Disease duration a (years) | 13.14 ± 6.62 | ||||
| Objective parameters (Mean ± SD) | Subjective parameters (Mean ± SD) | U-test | p | Effect Size (rank-biserial correlation) | |
| SL (min) | 11.69 ± 10.55 | 34.12 ± 32.60 | 186.5 | 0.028 | −0.926 |
| TST (min) | 417.12 ± 65.92 | 379.37 ± 129.12 | 2.1 | 0.044 | −0.768 |
| WASO (min) | 58.91 ± 47.63 | 37.12 ± 46.44 | 81 | 0.049 | −0.562 |
| ℷN1 (min) | 15.66 ± 14.97 | -- | |||
| ℷN3 (min) | 60.09 ± 54.44 | -- | |||
| ℷK-Complex (min) | 26.73 ± 18.22 | -- | |||
| Alpha density (%) | 11.28 ± 0.11 | -- | |||
| REM arousal density (%) | 0.38 ± 0.19 | -- | |||
| SLm (%) | 753.41 ± 1240.56 | -- | |||
| TSTm (%) | 90.99 ± 26.30 | -- | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berra, F.; Fasiello, E.; Zucconi, M.; Casoni, F.; De Gennaro, L.; Ferini-Strambi, L.; Galbiati, A. Neurophysiological Parameters Influencing Sleep–Wake Discrepancy in Insomnia Disorder: A Preliminary Analysis on Alpha Rhythm during Sleep Onset. Brain Sci. 2024, 14, 97. https://doi.org/10.3390/brainsci14010097
Berra F, Fasiello E, Zucconi M, Casoni F, De Gennaro L, Ferini-Strambi L, Galbiati A. Neurophysiological Parameters Influencing Sleep–Wake Discrepancy in Insomnia Disorder: A Preliminary Analysis on Alpha Rhythm during Sleep Onset. Brain Sciences. 2024; 14(1):97. https://doi.org/10.3390/brainsci14010097
Chicago/Turabian StyleBerra, Francesca, Elisabetta Fasiello, Marco Zucconi, Francesca Casoni, Luigi De Gennaro, Luigi Ferini-Strambi, and Andrea Galbiati. 2024. "Neurophysiological Parameters Influencing Sleep–Wake Discrepancy in Insomnia Disorder: A Preliminary Analysis on Alpha Rhythm during Sleep Onset" Brain Sciences 14, no. 1: 97. https://doi.org/10.3390/brainsci14010097
APA StyleBerra, F., Fasiello, E., Zucconi, M., Casoni, F., De Gennaro, L., Ferini-Strambi, L., & Galbiati, A. (2024). Neurophysiological Parameters Influencing Sleep–Wake Discrepancy in Insomnia Disorder: A Preliminary Analysis on Alpha Rhythm during Sleep Onset. Brain Sciences, 14(1), 97. https://doi.org/10.3390/brainsci14010097







