Efficacy of Using Intermittent Theta Burst Stimulation to Treat Negative Symptoms in Patients with Schizophrenia—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Eligibility Criteria
2.2. Data Extraction and Risk of Bias Assessment
2.3. Statistical Methods
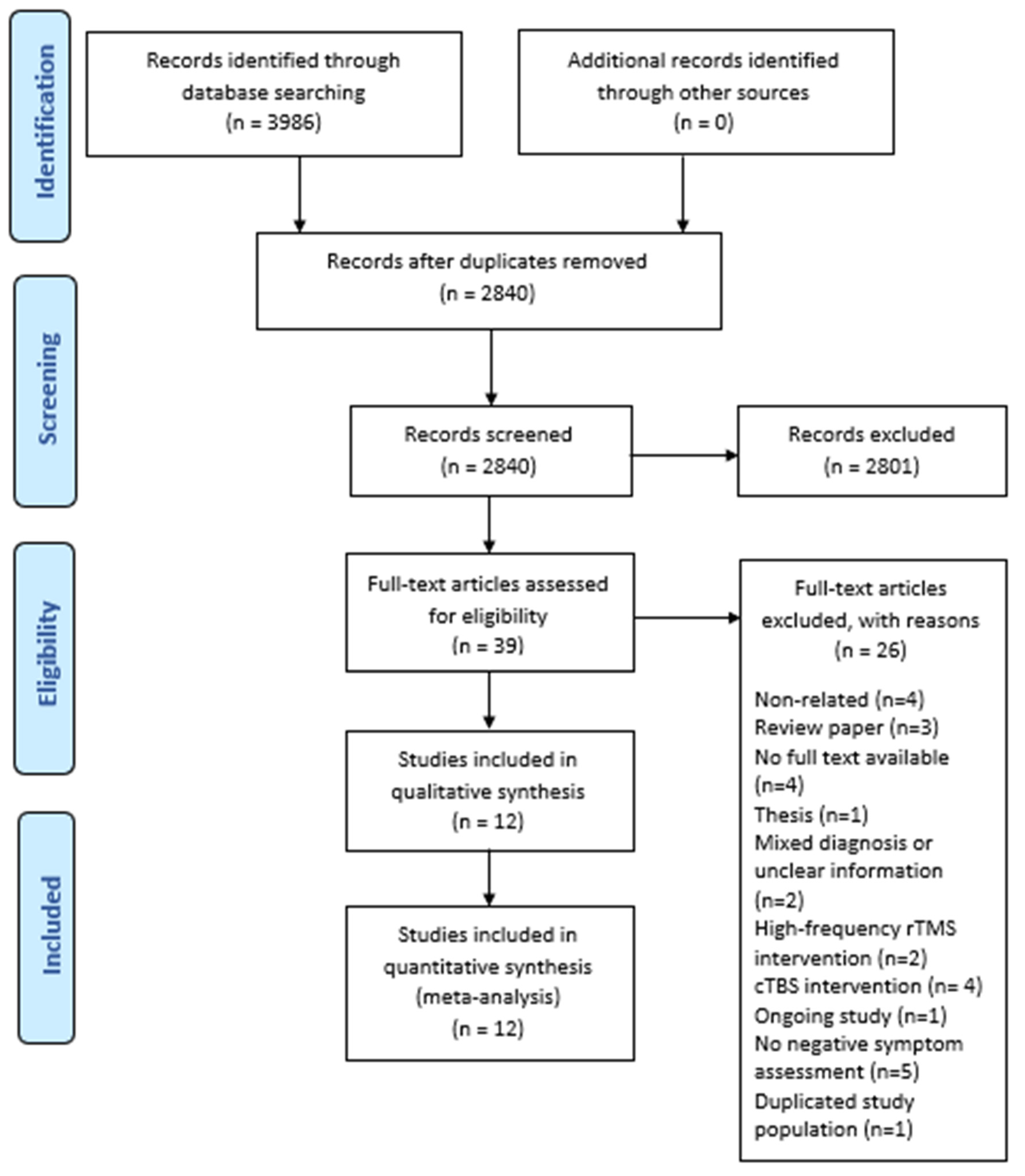
3. Results
3.1. Study Design and Patient Characteristics
3.2. iTBS Treatment Parameters
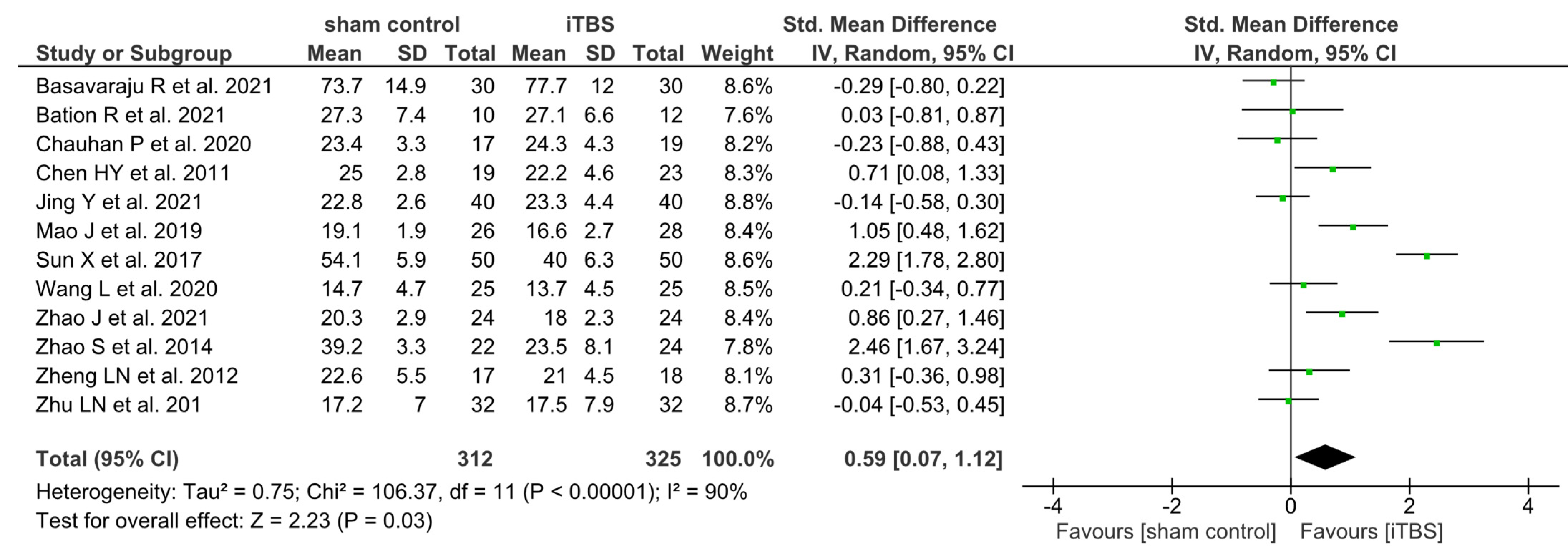
3.3. Treatment Efficacy of iTBS on Negative Symptoms
3.4. Subgroup Analysis of the Impact of iTBS Treatment Parameters on Negative Symptoms
3.5. Treatment Efficacy of iTBS on Positive Symptoms and Depressive Symptoms
3.6. Dropout Rate and Adverse Events
3.7. Quality Assessment of the Included Studies
4. Discussion
4.1. Principal Quantitative Findings
4.2. Study Strengths and Limitations
4.3. Quality Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Buchanan, R.W.; Ross, D.E.; Carpenter, W.T., Jr. A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiatry 2001, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Milev, P.; Ho, B.C.; Arndt, S.; Andreasen, N.C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry 2005, 162, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Remington, G.; Foussias, G.; Fervaha, G.; Agid, O.; Takeuchi, H.; Lee, J.; Hahn, M. Treating Negative Symptoms in Schizophrenia: An Update. Curr. Treat. Options Psychiatry 2016, 3, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Wobrock, T.; Guse, B.; Cordes, J.; Wölwer, W.; Winterer, G.; Gaebel, W.; Langguth, B.; Landgrebe, M.; Eichhammer, P.; Frank, E.; et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: A sham-controlled, randomized multicenter trial. Biol. Psychiatry 2015, 77, 979–988. [Google Scholar] [CrossRef]
- Cole, J.C.; Green Bernacki, C.; Helmer, A.; Pinninti, N.; O’Reardon, J.P. Efficacy of Transcranial Magnetic Stimulation (TMS) in the Treatment of Schizophrenia: A Review of the Literature to Date. Innov. Clin. Neurosci. 2015, 12, 12–19. [Google Scholar]
- Dougall, N.; Maayan, N.; Soares-Weiser, K.; McDermott, L.M.; McIntosh, A. Transcranial magnetic stimulation (TMS) for schizophrenia. Cochrane Database Syst. Rev. 2015, 2015, Cd006081. [Google Scholar] [CrossRef]
- Osoegawa, C.; Gomes, J.S.; Grigolon, R.B.; Brietzke, E.; Gadelha, A.; Lacerda, A.L.T.; Dias, Á.M.; Cordeiro, Q.; Laranjeira, R.; de Jesus, D.; et al. Non-invasive brain stimulation for negative symptoms in schizophrenia: An updated systematic review and meta-analysis. Schizophr. Res. 2018, 197, 34–44. [Google Scholar] [CrossRef]
- Prikryl, R.; Kucerova, H.P. Can repetitive transcranial magnetic stimulation be considered effective treatment option for negative symptoms of schizophrenia? J. ECT 2013, 29, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Gan, H.; Pang, J.; Li, H.; Wang, J.; Li, C. Efficacy Towards Negative Symptoms and Safety of Repetitive Transcranial Magnetic Stimulation Treatment for Patients with Schizophrenia: A Systematic Review. Shanghai Arch. Psychiatry 2017, 29, 61–76. [Google Scholar] [CrossRef]
- Lorentzen, R.; Nguyen, T.D.; McGirr, A.; Hieronymus, F.; Østergaard, S.D. The efficacy of transcranial magnetic stimulation (TMS) for negative symptoms in schizophrenia: A systematic review and meta-analysis. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W. Toward establishing a therapeutic window for rTMS by theta burst stimulation. Neuron 2005, 45, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Pilato, F.; Dileone, M.; Profice, P.; Oliviero, A.; Mazzone, P.; Insola, A.; Ranieri, F.; Meglio, M.; Tonali, P.A.; et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J. Physiol. 2008, 586, 3871–3879. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Pilato, F.; Saturno, E.; Oliviero, A.; Dileone, M.; Mazzone, P.; Insola, A.; Tonali, P.; Ranieri, F.; Huang, Y. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J. Physiol. 2005, 565, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Carpenter, L.; Aaronson, S.; Hutton, T.M.; Mina, M.; Pages, K.; Verdoliva, S.; West, W.S.; Sackeim, H. Comparison of clinical outcomes with two Transcranial Magnetic Stimulation treatment protocols for major depressive disorder. Brain Stimul. 2021, 14, 173–180. [Google Scholar] [CrossRef]
- Giam, A.; Chen, L.; Lisa, H.; Gill, S.; Clarke, P.; Ng, F.; Galletly, C.; Fitzgerald, P. Comparing theta burst stimulation with standard left high frequency transcranial magnetic stimulation in the treatment of depression in a randomized controlled study: A preliminary comparison study. J. Affect. Disord. Rep. 2021, 5, 100162. [Google Scholar] [CrossRef]
- Bulteau, S.; Laurin, A.; Pere, M.; Fayet, G.; Thomas-Ollivier, V.; Deschamps, T.; Auffray-Calvier, E.; Bukowski, N.; Vanelle, J.M.; Sébille, V.; et al. Intermittent theta burst stimulation (iTBS) versus 10 Hz high-frequency repetitive transcranial magnetic stimulation (rTMS) to alleviate treatment-resistant unipolar depression: A randomized controlled trial (THETA-DEP). Brain Stimul. 2022, 15, 870–880. [Google Scholar] [CrossRef]
- Cole, E.J.; Phillips, A.L.; Bentzley, B.S. Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. Am. J. Psychiatry 2022, 179, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and theoretical foundations. Br. J. Psychiatry. Suppl. 1989, 155, 49–58. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin. Res. Ed.) 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J. Clin. Psychiatry 2020, 81, 20f13681. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: London, UK, 1988. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. Ed.) 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Basavaraju, R.; Ithal, D.; Thanki, M.V.; Ramalingaiah, A.H.; Thirthalli, J.; Reddy, R.P.; Brady, R.O., Jr.; Halko, M.A.; Bolo, N.R.; Keshavan, M.S.; et al. Intermittent theta burst stimulation of cerebellar vermis enhances fronto-cerebellar resting state functional connectivity in schizophrenia with predominant negative symptoms: A randomized controlled trial. Schizophr. Res. 2021, 238, 108–120. [Google Scholar] [CrossRef]
- Bation, R.; Magnin, C.; Poulet, E.; Mondino, M. Intermittent theta burst stimulation for negative symptoms of schizophrenia-A double-blind, sham-controlled pilot study. Npj Schizophr. 2017, 7, 10. [Google Scholar] [CrossRef]
- Chauhan, P.; Garg, S. Efficacy of Intensive Cerebellar Intermittent Theta Burst Stimulation (iCiTBS) in Treatment-Resistant Schizophrenia: A Randomized Placebo-Controlled Study. Clin. Neurophysiol. 2021, 20, 116–123. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhang, Z.J.; Wang, J.J.; Cheng, Y.M.; Xiang, Z.Q.; Shi, S.X.; Sheng, J.H. Effect of adjunctive treatment with repetitive transcranial magnetic stimulation on exploratory eye movements and negative symptoms in schizophrenic patients: A randomized, double-blind, sham-controlled study. Shanghai Arch. Psychiatry 2011, 23, 200–206. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Zhu, M.; Zhu, N.; Huang, Y.; Gong, H. Efficacy of intermittent theta burst stimulation on social cognition in patients with schizophrenia. Chin. J. Nerv. Ment. Dis. 2021, 47, 540–545. [Google Scholar] [CrossRef]
- Mao, J.; Yi, F.; Mei, J. Effects of repetitive transcranial magnetic stimulation with theta burst stimulation paradigm on negative symptoms and social functions in patients with chronic schizophrenia. J. Psychiatry 2019, 32, 183–187. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, J.; Zhang, J.; Huang, Y.; Shi, D.H.; Yan, C.Y.; Shen, Y.M.; Yang, Z.D.; Liu, H.J. A study on the efficacy of repetitive transcranial magnetic stimulation (rTMS) with different protocols in the treatment of the negative symptoms of schizophrenia. J. Epileptol. Electroneurophysiol. 2017, 26, 210–212. [Google Scholar]
- Wang, L.; Chen, X.; Wu, Y.; He, K.; Xu, F.; Xiao, G.; Hu, P.; Qiu, B.; Ji, G.J.; Wang, K. Intermittent theta burst stimulation (iTBS) adjustment effects of schizophrenia: Results from an exploratory outcome of a randomized double-blind controlled study. Schizophr. Res. 2020, 216, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guo, Y.S.; Li, M.N.; Gao, J.; Fang, X.Y.; Zou, C.; Chen, J.; Zhang, F.; Zhang, X. Effects of theta burst stimulation mode repetitive transcranial magnetic stimulation on negative symptoms and cognitive function in elderly patients with chronic schizophrenia. Chin. J. Behav. Med. Brain Sci. 2021, 30, 577–583. [Google Scholar] [CrossRef]
- Zhao, S.; Kong, J.; Li, S.; Tong, Z.; Yang, C.; Zhong, H. Randomized controlled trial of four protocols of repetitive transcranial magnetic stimulation for treating the negative symptoms of schizophrenia. Shanghai Arch. Psychiatry 2014, 26, 15–21. [Google Scholar] [CrossRef]
- Zheng, L.N.; Guo, Q.; Li, H.; Li, C.B.; Wang, J.J. Effects of repetitive transcranial magnetic stimulation with different paradigms on the cognitive function and psychotic symptoms of schizophrenia patients. J. Peking Univ. (Health Sci.) 2012, 44, 732–736. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, W.; Zhu, Y.; Mu, X.; Zhang, Q.; Wang, Y.; Cai, J.; Xie, B. Cerebellar theta burst stimulation for the treatment of negative symptoms of schizophrenia: A multicenter, double-blind, randomized controlled trial. Psychiatry Res. 2021, 305, 114204. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ugawa, Y. Adverse events of tDCS and tACS: A review. Clin. Neurophysiol. Pract. 2017, 2, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Brownjohn, P.W.; Reynolds, J.N.; Matheson, N.; Fox, J.; Shemmell, J.B. The effects of individualized theta burst stimulation on the excitability of the human motor system. Brain Stimul. 2014, 7, 260–268. [Google Scholar] [CrossRef]
- Goldsworthy, M.R.; Vallence, A.M.; Hodyl, N.A.; Semmler, J.G.; Pitcher, J.B.; Ridding, M.C. Probing changes in corticospinal excitability following theta burst stimulation of the human primary motor cortex. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2016, 127, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Hill, A.T.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Use of theta-burst stimulation in changing excitability of motor cortex: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Hebel, T.; Langguth, B.; Schecklmann, M.; Schoisswohl, S.; Staudinger, S.; Schiller, A.; Ustohal, L.; Sverak, T.; Horky, M.; Kasparek, T.; et al. Rationale and study design of a trial to assess rTMS add-on value for the amelioration of negative symptoms of schizophrenia (RADOVAN). Contemp. Clin. Trials Commun. 2022, 26, 100891. [Google Scholar] [CrossRef]
- Song, J.; Liu, D.; Zhang, M.; Wang, H.; Tan, S. Intermittent theta burst stimulation (iTBS) combined with working memory training to improve cognitive function in schizophrenia: Study protocol for a randomized controlled trial. Trials 2020, 21, 683. [Google Scholar] [CrossRef]
- Forum on Neuroscience and Nervous System Disorders; Board on Health Sciences Policy; Institute of Medicine; The National Academies of Sciences, Engineering; MedicineDisorders. Enabling Discovery, Development, and Translation of Treatments for Cognitive Dysfunction in Depression: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2015. [Google Scholar]
- Upthegrove, R.; Marwaha, S.; Birchwood, M. Depression and Schizophrenia: Cause, Consequence, or Trans-diagnostic Issue? Schizophr. Bull. 2016, 43, 240–244. [Google Scholar] [CrossRef]
- Williams, N.R.; Sudheimer, K.D.; Bentzley, B.S.; Pannu, J.; Stimpson, K.H.; Duvio, D.; Cherian, K.; Hawkins, J.; Scherrer, K.H.; Vyssoki, B.; et al. High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain 2018, 141, e18. [Google Scholar] [CrossRef]
- Ning, Y.; Zheng, S.; Feng, S.; Zhang, B.; Jia, H. Potential Locations for Non-Invasive Brain Stimulation in Treating Schizophrenia: A Resting-State Functional Connectivity Analysis. Front. Neurol. 2021, 12, 766736. [Google Scholar] [CrossRef]
- Brady, R.O., Jr.; Gonsalvez, I.; Lee, I.; Öngür, D.; Seidman, L.J.; Schmahmann, J.D.; Eack, S.M.; Keshavan, M.S.; Pascual-Leone, A.; Halko, M.A. Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. Am. J. Psychiatry 2019, 176, 512–520. [Google Scholar] [CrossRef]
- Parker, K.L.; Narayanan, N.S.; Andreasen, N.C. The therapeutic potential of the cerebellum in schizophrenia. Front. Syst. Neurosci. 2014, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.P.Y.; Abram, S.V.; Ford, J.M. Cerebellar stimulation in schizophrenia: A systematic review of the evidence and an overview of the methods. Front. Psychiatry 2022, 13, 1069488. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Rogasch, N.C.; Hoy, K.E.; Sullivan, C.M.; Cash, R.F.H.; Fitzgerald, P.B. Impact of different intensities of intermittent theta burst stimulation on the cortical properties during TMS-EEG and working memory performance. Hum. Brain Mapp. 2018, 39, 783–802. [Google Scholar] [CrossRef]
- Demirtas-Tatlidede, A.; Freitas, C.; Cromer, J.R.; Safar, L.; Ongur, D.; Stone, W.S.; Seidman, L.J.; Schmahmann, J.D.; Pascual-Leone, A. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr. Res. 2010, 124, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Enriquez-Geppert, S.; Knegtering, H.; Dlabac-de Lange, J.J. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: Meta-analysis of controlled trials. Neurosci. Biobehav. Rev. 2018, 89, 111–118. [Google Scholar] [CrossRef]
- Tranulis, C.; Guéguen, B.; Pham-Scottez, A.; Vacheron, M.N.; Cabelguen, G.; Costantini, A.; Valero, G.; Galinovski, A. Motor threshold in transcranial magnetic stimulation: Comparison of three estimation methods. Neurophysiol. Clin. Clin. Neurophysiol. 2006, 36, 1–7. [Google Scholar] [CrossRef]
- Westin, G.G.; Bassi, B.D.; Lisanby, S.H.; Luber, B. Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: Safety implications. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2014, 125, 142–147. [Google Scholar] [CrossRef]
- Goh, K.K.; Chen, C.H.; Wu, T.H.; Chiu, Y.H.; Lu, M.L. Efficacy and safety of intermittent theta-burst stimulation in patients with schizophrenia: A meta-analysis of randomized sham-controlled trials. Front Pharmacol 2022, 13, 944437. [Google Scholar] [CrossRef]
- Hoppenrath, K.; Funke, K. Time-course of changes in neuronal activity markers following iTBS-TMS of the rat neocortex. Neurosci. Lett. 2013, 536, 19–23. [Google Scholar] [CrossRef]
- Volz, L.J.; Benali, A.; Mix, A.; Neubacher, U.; Funke, K. Dose-dependence of changes in cortical protein expression induced with repeated transcranial magnetic theta-burst stimulation in the rat. Brain Stimul 2013, 6, 598–606. [Google Scholar] [CrossRef]
- Desforges, M.; Hadas, I.; Mihov, B.; Morin, Y.; Rochette Braün, M.; Lioumis, P.; Zomorrodi, R.; Théoret, H.; Lepage, M.; Daskalakis, Z.J.; et al. Dose-response of intermittent theta burst stimulation of the prefrontal cortex: A TMS-EEG study. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2022, 136, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, M.; Lyu, X.L.; Zhang, L.L.; Du, X.D.; Hung, G.C. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: Findings from a randomized controlled trial. Psychiatry Res. 2016, 240, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Duprat, R.; Desmyter, S.; Rudi de, R.; van Heeringen, K.; Van den Abbeele, D.; Tandt, H.; Bakic, J.; Pourtois, G.; Dedoncker, J.; Vervaet, M.; et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: A fast road to remission? J. Affect. Disord. 2016, 200, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.; Edwards, D.; Eldaief, M.; Pascual-Leone, A. Safety of theta burst transcranial magnetic stimulation: A systematic review of the literature. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2011, 28, 67–74. [Google Scholar] [CrossRef]
- Tseng, P.T.; Zeng, B.S.; Hung, C.M.; Liang, C.S.; Stubbs, B.; Carvalho, A.F.; Brunoni, A.R.; Su, K.P.; Tu, Y.K.; Wu, Y.C.; et al. Assessment of Noninvasive Brain Stimulation Interventions for Negative Symptoms of Schizophrenia: A Systematic Review and Network Meta-analysis. JAMA Psychiatry 2022, 79, 770–779. [Google Scholar] [CrossRef]
- Leucht, S.; Barabássy, Á.; Laszlovszky, I.; Szatmári, B.; Acsai, K.; Szalai, E.; Harsányi, J.; Earley, W.; Németh, G. Linking PANSS negative symptom scores with the Clinical Global Impressions Scale: Understanding negative symptom scores in schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 1589–1596. [Google Scholar] [CrossRef]

| Author | Year | Country | Patient Type | Randomization Method | Patient Blind | Assessor Blind | Sham Type | Baseline Selection of Negative Symptoms | Primary Measurement Scale | Total Enrolment | Treatment Assignment | Age, Mean (SD), Year | Education, Mean (SD), Year | Duration of Illness, Mean (SD), Year | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Basavaraju R et al. [29] | 2021 | India | Outpatient and inpatient | Computerized algorithm | Yes | Yes | Sham coil | SANS severity ≥ 3 | SANS | 60 | iTBS | 31.2 (10.0) ∆ | 11.5 (3.7) ∆ | 8.4 (5.6) ∆ |
| Sham | 34.2 (8.1) ∆ | 11.1 (3.9) | 10.9 (8.0) | ||||||||||||
| 2 | Bation R et al. [30] | 2021 | France | Outpatient | Computerized algorithm | Yes | Yes | Sham coil | PANSS-N ≥ 20, 2 items ≥ 4 | PANSS-N | 22 | iTBS | 42.3 (9.4) | 11.5 (2.5) | 15.0 (5.9) |
| Sham | 41.6 (12.6) | 12.1 (2.8) | 17.1 (15.4) | ||||||||||||
| 3 | Chauhan P et al. [31] | 2020 | India | Inpatient | Block randomization | Yes | Yes | Sham coil | NA | PANSS-N | 36 | iTBS | 41.7 (8.9) | ~8.3 * | 16.1 (5.5) |
| Sham | 39.4 (8.2) | ~9.6 * | 13.0 (7.0) | ||||||||||||
| 4 | Chen HY et al. [32] | 2011 | China | Inpatient | Computerized algorithm | Yes | Yes | Similar sound | PANSS-N ≥ 20 | PANSS-N | 46 | iTBS | 37.4 (11.8) | 12.0 (2.2) | NA |
| Sham | 39.7 (13.3) | 11.0 (2.6) | NA | ||||||||||||
| 5 | Jin Y et al. [33] | 2021 | China | Inpatient | Computerized algorithm | Yes | Yes | 180° | NA | PANSS-N | 80 | iTBS | 48.7 (9.7) | 7.2 (2.5) | 8.9 (4.0) |
| Sham | 47.8 (10.6) | 6.4 (2.4) | 8.4 (4.3) | ||||||||||||
| 6 | Mao J et al. [34] | 2019 | China | Inpatient | NA | NA | NA | NA | PANSS-N ≥ 18 | PANSS-N | 60 | iTBS | 52.8 (7.1) | 9.1 (1.3) | 27.7 (9.2) |
| Sham | 53.5 (5.5) | 9.3 (1.8) | 27.5 (9.9) | ||||||||||||
| 7 | Sun X et al. [35] | 2018 | China | Inpatient | NA | Yes | Yes | No treatment | PANSS-N ≥ 20, lasted for at least 6 weeks | SANS | 100 | iTBS | 51.2 (11.4) | NA | NA |
| Sham | 50.9 (12.1) | NA | NA | ||||||||||||
| 8 | Wang L et al. [36] | 2020 | China | Outpatient | Coin toss | Yes | Yes | Sham coil | NA | PANSS-N | 58 | iTBS | 24.0 (4.4) | 12.1 (2.6) | 5.1 (3.8) |
| Sham | 26.6 (9.0) | 12.1 (2.7) | 4.9 (5.3) | ||||||||||||
| 9 | Zhao J et al. [37] | 2021 | China | Inpatient | Random number table | NA | NA | 90° | NA | PANSS-N | 52 | iTBS | 62.5 (3. 3) | 9.3 (2.3) | 31.3 (8.9) |
| Sham | 64.0 (3. 6) | 9.5 (2.4) | 35.0 (8.9) | ||||||||||||
| 10 | Zhao S et al. [38] | 2014 | China | Outpatient and inpatient | Random number table | Yes | Yes | 180° | PANSS-N ≥ 20, with at least one of the negative symptom scores > 3 | PANSS-N | 48 | iTBS | 47.7 (11.8) | 12.9 (0.9) | NA |
| Sham | 46.7 (13.1) | 13.8 (0.1) | NA | ||||||||||||
| 11 | Zheng LN et al. [39] | 2012 | China | Inpatient | Computerized algorithm | Yes | Yes | 180° | NA | PANSS-N | 39 | iTBS | 55.6 (5.8) | ~10.2 * | 32.9 (8.1) |
| Sham | 56.4 (9.3) | ~9.6 * | 31.7 (7.2) | ||||||||||||
| 12 | Zhu L et al. [40] | 2021 | China | Inpatient | Odd–even number sequence | Yes | Yes | 180°/90° | NA | PANSS-N | 64 | iTBS | 35.2 (7.1) ∆ | 10.9 (3.2) | 15.4 (7.8) |
| Sham | 35.3 (6.1) ∆ | 10.2 (3.9) | 15.8 (6.5) |
| Author | TMS Machine | MT Method | Target Site | Intensity | iTBS Sessions/Day | Inner Train Frequency (Hz) | Inter Train Frequency (Hz) | Inter Train Interval (s) | Total Number of Sessions | Number of Pulses/Sessions | Total Number of Pulses | Concurrent Antipsychotics | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Basavaraju R et al. [29] | MagPro X100 | Rossini–Rothwell MEP | Midline cerebellum | 100% MT | 2 | 50 | 5 | 8 | 10 | 600 | 6000 | No change in medication |
| 2 | Bation R et al. [30] | MagPro X100 | Visual observation | L-DLPFC | 80% MT | 2 | 50 | 5 | 8 | 20 | 990 | 19,800 | No change in medication |
| 3 | Chauhan P et al. [31] | MagPro-R30 | Rossini–Rothwell MEP | Midline cerebellum (cerebellar) | 80% MT | 2 | 50 | 5 | 8 | 10 | 600 | 6000 | No change in medication |
| 4 | Chen HY et al. [32] | MagPro X100 | NA | L-DLPFC | 80% MT | 1 | 50 | NA | NA | 20 | 2400 | 48,000 | No change in medication |
| 5 | Jin Y et al. [33] | JunJiang RT-100 | Visual observation | L-DLPFC | 100% MT | 1 | NA | NA | NA | 20 | NA | NA | No change in medication |
| 6 | Mao J et al. [34] | MagPro R100 | NA | L-DLPFC | 80% MT | 1 | NA | NA | NA | 20 | NA | NA | No change in medication |
| 7 | Sun X et al. [35] | NA | NA | L-DLPFC | 80% MT | 1 | NA | 5 | 120 | 40 | 2400 | 96,000 | NA |
| 8 | Wang L et al. [36] | MagStim Rapid2 | Sequential testing MEP | L-DLPFC | 80% MT | 3 | 50 | 5 | 8 | 42 | 600 | 25,200 | No change in medication |
| 9 | Zhao J et al. [37] | NA | NA | L-DLPFC | 100% MT | 1 | 50 | 5 | NA | 20 | 600 | 12,000 | No change in medication |
| 10 | Zhao S et al. [38] | MagPro X100 | Rossini–Rothwell MEP | L-DLPFC | 80% MT | 1 | 50 | 5 | NA | 20 | 2400 | 48,000 | No change in medication |
| 11 | Zheng LN et al. [39] | MagPro X100 | NA | L-DLPFC | 80% MT | 1 | 50 | 5 | 8 | 5 | 1200 | 6000 | No change in medication |
| 12 | Zhu LN et al. [40] | MagPro X100/CCY-I | NA | Midline cerebellum (cerebellar) | 100% MT | 1 | 50 | 5 | 8 | 10 | 600 | 6000 | No change in medication |
| Subgroup Attributes | Pooled Effect Size | Total Number of Patients | ||||
|---|---|---|---|---|---|---|
| SMD | 95% CI | p-Value | I2 | |||
| 1 | Studies with baseline prominent negative symptoms | 1.04 | 0.11, 1.97 | 0.030 | 93% | 324 |
| 2 | Stimulation site: L-DLPFC | 0.86 | 0.24, 1.48 | 0.007 | 90% | 477 |
| Stimulation site: cerebellum | −0.18 | −0.49, 0.13 | 0.270 | 0% | 160 | |
| 3 | 80% MT | 0.86 | 0.15, 1.56 | 0.020 | 90% | 385 |
| 100% MT | 0.07 | −0.38, 0.53 | 0.750 | 69% | 252 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Goh, S.E.; Lee, J.J.; Vanniasingham, S.D.; Brunelin, J.; Lee, J.; Tor, P.C. Efficacy of Using Intermittent Theta Burst Stimulation to Treat Negative Symptoms in Patients with Schizophrenia—A Systematic Review and Meta-Analysis. Brain Sci. 2024, 14, 18. https://doi.org/10.3390/brainsci14010018
Tan X, Goh SE, Lee JJ, Vanniasingham SD, Brunelin J, Lee J, Tor PC. Efficacy of Using Intermittent Theta Burst Stimulation to Treat Negative Symptoms in Patients with Schizophrenia—A Systematic Review and Meta-Analysis. Brain Sciences. 2024; 14(1):18. https://doi.org/10.3390/brainsci14010018
Chicago/Turabian StyleTan, Xiaowei, Shih Ee Goh, Jonathan Jie Lee, Sean David Vanniasingham, Jérôme Brunelin, Jimmy Lee, and Phern Chern Tor. 2024. "Efficacy of Using Intermittent Theta Burst Stimulation to Treat Negative Symptoms in Patients with Schizophrenia—A Systematic Review and Meta-Analysis" Brain Sciences 14, no. 1: 18. https://doi.org/10.3390/brainsci14010018
APA StyleTan, X., Goh, S. E., Lee, J. J., Vanniasingham, S. D., Brunelin, J., Lee, J., & Tor, P. C. (2024). Efficacy of Using Intermittent Theta Burst Stimulation to Treat Negative Symptoms in Patients with Schizophrenia—A Systematic Review and Meta-Analysis. Brain Sciences, 14(1), 18. https://doi.org/10.3390/brainsci14010018





