Individualized Vibrotactile Neurofeedback Training in Patients with Chronic Bilateral Vestibulopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Interventions
- -
- Standing on two legs with eyes open/closed;
- -
- Standing on one leg with eyes open/closed;
- -
- Eight tandem steps (one foot in front of the other) with eyes open;
- -
- Standing with two legs on a foam support surface (height 10 cm; density 25 kg/m3) with eyes open/closed;
- -
- Standing on one leg on a foam support surface;
- -
- Eight tandem steps on a foam support surface;
- -
- Walking 3 m while rotating the head;
- -
- Walking 3 m while vertically pitching the head in rhythm;
- -
- Walking 3 m forward with eyes open/closed;
- -
- Walking over four barriers (height 26 cm with an inter-barrier distance of 1 m).

2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
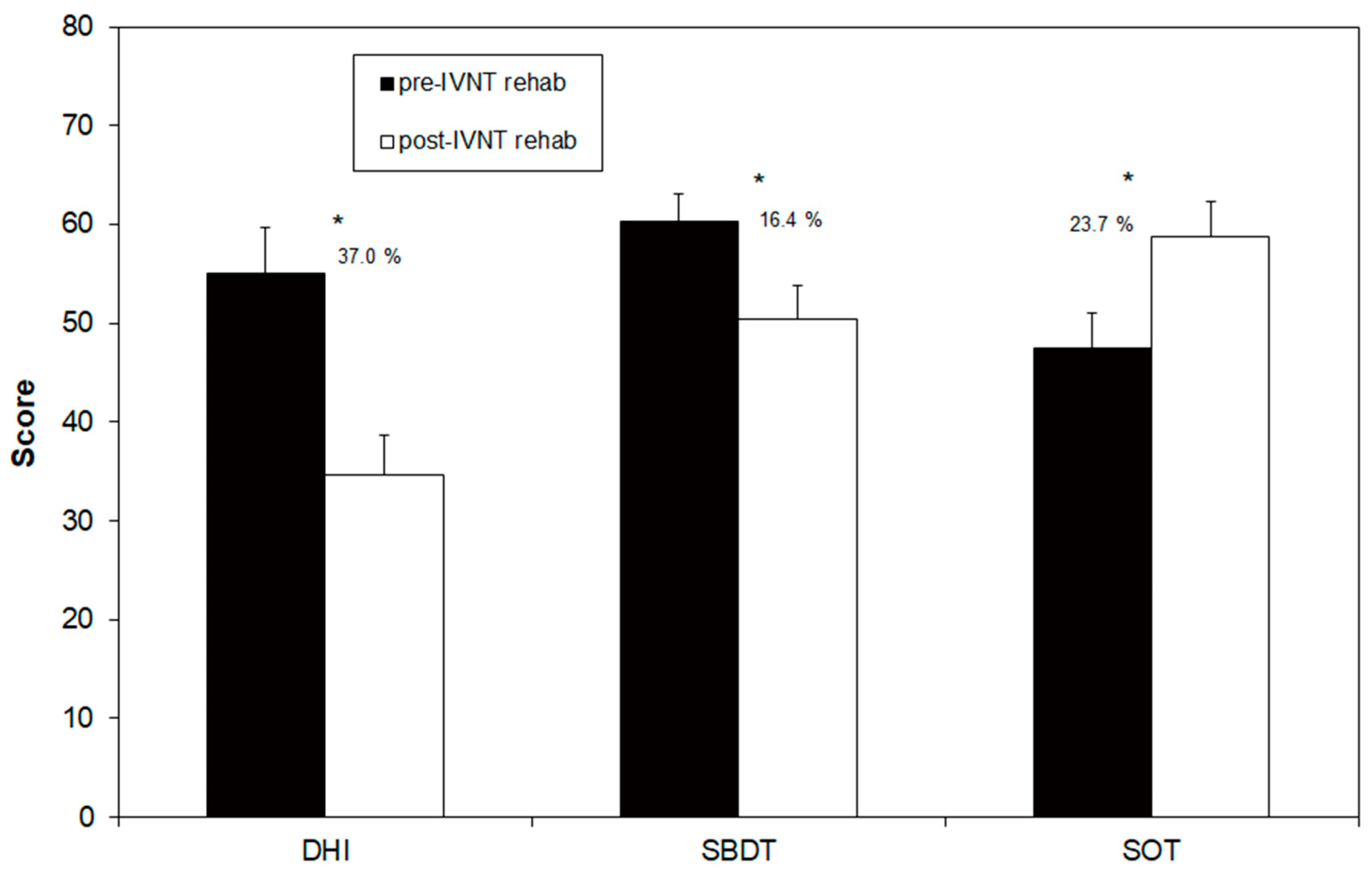
3.1. Comparison of the Pre-Post Rehabilitation Measures
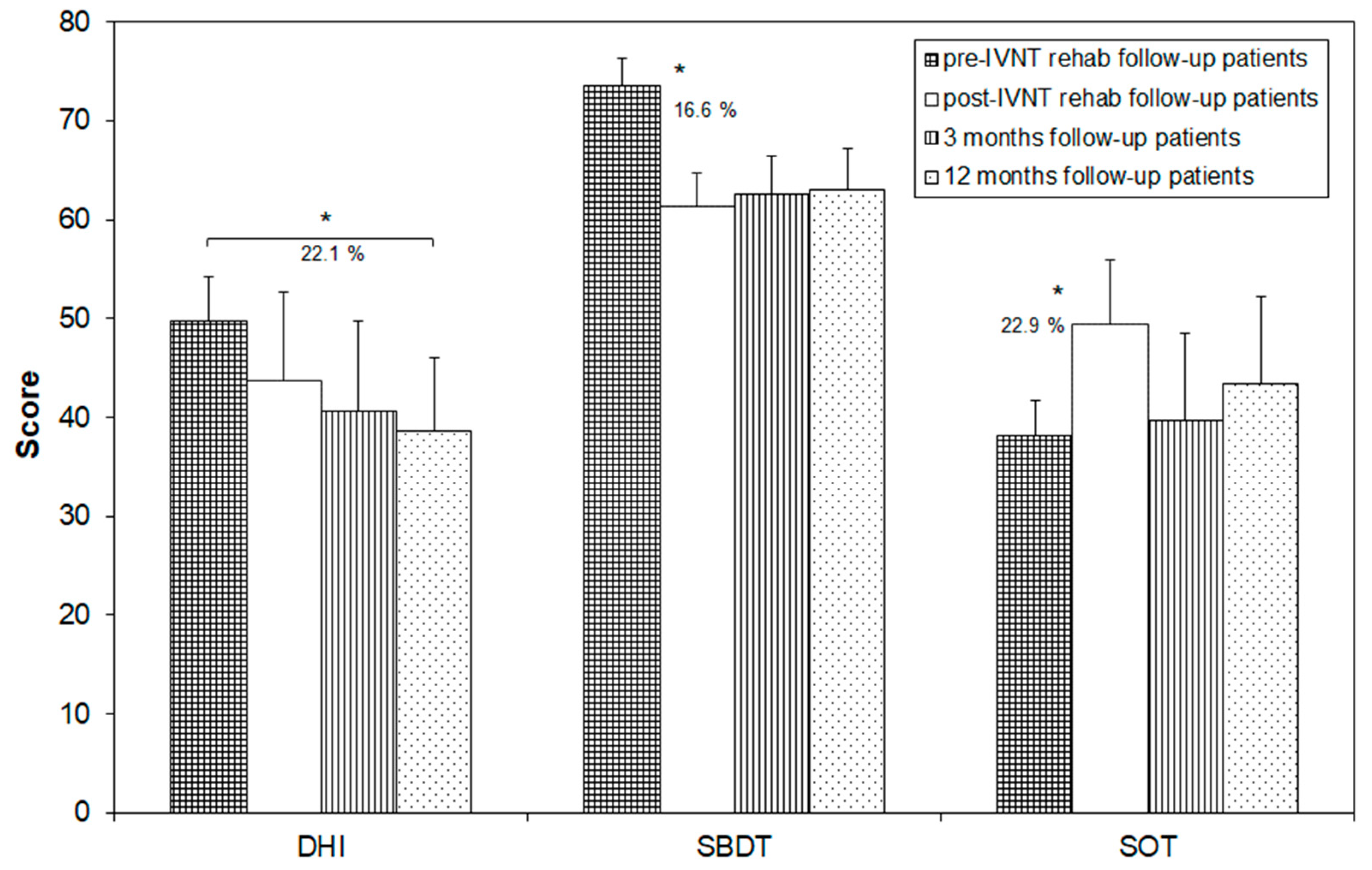
3.2. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ward, B.K.; Agrawal, Y.; Hoffman, H.J.; Carey, J.P.; Della Santina, C.C. Prevalence and impact of bilateral vestibular hypofunction: Results from the 2008 US National Health Interview Survey. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Piper, K.S.; Juhl, C.B.; Andersen, H.E.; Christensen, J.; Søndergaard, K. Prevalence of bilateral vestibulopathy among older adults above 65 years on the indication of vestibular impairment and the association with Dynamic Gait Index and Dizziness Handicap Inventory. Disabil. Rehabil. 2023, 45, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Wuehr, M.; Decker, J.; Schenkel, F.; Jahn, K.; Schniepp, R. Impact on daily mobility and risk of falling in bilateral vestibulopathy. J. Neurol. 2022, 269, 5746–5754. [Google Scholar] [CrossRef]
- Herdman, S.J.; Blatt, P.; Schubert, M.C.; Tusa, R.J. Falls in patients with vestibular deficits. Am. J. Otol. 2000, 21, 847–851. [Google Scholar] [PubMed]
- Skarzynska, M.B.; Król, B.; Czajka, L. Ototoxicity as a side-effect of drugs: Literature review. J. Hear Sci. 2020, 10, 9–19. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, H.J. Bilateral vestibulopathy: The causes, diagnosis, and treatments. Curr. Opin. Neurol. 2022, 35, 98–106. [Google Scholar] [CrossRef]
- Medendorp, W.P.; Alberts, B.B.G.T.; Verhagen, W.I.M.; Koppen, M.; Selen, L.P.J. Psychophysical Evaluation of Sensory Reweighting in Bilateral Vestibulopathy. Front. Neurol. 2018, 25, 377. [Google Scholar] [CrossRef]
- Telian, S.A.; Shepard, N.T.; Smith-Wheelock, M.; Hoberg, M. Bilateral vestibular paresis: Diagnosis and treatment. Otolaryngol. Head Neck Surg. 1991, 104, 67–71. [Google Scholar] [CrossRef]
- Herdman, S.J.; Hall, C.D.; Maloney, B.; Knight, S.; Ebert, M.; Lowe, J. Variables associated with outcome in patients with bilateral vestibular hypofunction: Preliminary study. J. Vestib. Res. 2015, 25, 185–194. [Google Scholar] [CrossRef]
- Gillespie, M.B.; Minor, L.B. Prognosis in bilateral vestibular hypofunction. Laryngoscope 1999, 109, 35–41. [Google Scholar] [CrossRef]
- Brown, K.E.; Whitney, S.L.; Wrisley, D.M.; Furman, J.M. Physical Therapy outcome for persons with bilateral vestibular loss. Laryngoscope 2001, 111, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Porciuncula, F.; Johnson, C.C.; Glickman, L.B. The effect of vestibular rehabilitation on adults with bilateral vestibular hypofunction: A systematic review. J. Vestib. Res. 2012, 22, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Krebs, D.E.; Gill-Body, K.M.; Parker, S.W.; Ramirez, J.V.; Wernick-Robinson, M. Vestibular rehabilitation: Useful but not universally so. Otolaryngol. Head Neck Surg. 2003, 128, 240–250. [Google Scholar]
- McLaren, R.; Smith, P.F.; Taylor, R.L.; Ravindran, S.; Rashid, U.; Taylor, D. Efficacy of nGVS to improve postural stability in people with bilateral vestibulopathy: A systematic review and meta-analysis. Front. Neurosci. 2022, 16, 1010239. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Hillier, S.L. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst. Rev. 2015, 13, CD005397. [Google Scholar] [CrossRef]
- Basta, D.; Rossi-Izquierdo, M.; Soto-Varela, A.; Greters, M.E.; Bittar, R.S.; Steinhagen-Thiessen, E.; Eckardt, R.; Harada, T.; Goto, F.; Ogawa, K.; et al. Efficacy of a vibrotactile neurofeedback training in stance and gait conditions for the treatment of balance deficits -a double-blinded, placebo-controlled multicenter study. Otol. Neurotol. 2011, 32, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Izquierdo, M.; Ernst, A.; Soto-Varela, A.; Santos-Pérez, S.; Faraldo-García, A.; Sesar-Ignacio, A.; Basta, D. Vibrotactile neurofeedback balance training in patients with Parkinson’s disease: Reducing the number of falls. Gait Posture 2013, 37, 195–200. [Google Scholar] [CrossRef]
- Brugnera, C.; Bittar, R.S.M.; Greters, M.E.; Basta, D. Effects of vibrotactile vestibular substitution on vestibular rehabilitation—Preliminary study. Brazil. J. Otolaryngol. 2015, 81, 616–621. [Google Scholar] [CrossRef]
- Soto-Varela, A.; Rossi-Izquierdo, M.; Del-Río-Valeiras, M.; Faraldo-García, A.; Vaamonde-Sánchez-Andrade, I.; Lirola-Delgado, A.; Santos-Pérez, S. Vestibular rehabilitation with mobile posturography as a “low-cost” alternative to vestibular rehabilitation with computerized dynamic posturography, in old people with imbalance: A randomized clinical trial. Aging Clin. Exp. Res. 2021, 33, 2807–2819. [Google Scholar] [CrossRef]
- Strupp, M.; Kim, J.S.; Murofushi, T.; Straumann, D.; Jen, J.C.; Rosengren, S.M.; Della Santina, C.C.; Kingma, H. Bilateral vestibulopathy: Diagnostic criteria Consensus document of the Classification Committee of the Bárány Society. J. Vestib. Res. 2017, 27, 177–189. [Google Scholar] [CrossRef]
- Basta, D.; Rossi-Izquierdo, M.; Soto-Varela, A.; Ernst, A. Mobile posturography: Posturographic analysis of daily-life mobility. Otol. Neurotol. 2013, 34, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Kurre, A.; van Gool, C.J.; Bastiaenen, C.H.; Gloor-Juzi, T.; Straumann, D.; de Bruin, E.D. Translation, cross-cultural adaptation and reliability of the German version of the dizziness handicap inventory. Otol. Neurotol. 2009, 30, 359–367. [Google Scholar] [CrossRef]
- Nashner, L.M. Computerized Dynamic Posturography. In Practical Management of the Dizzy Patient; Goebel, J.A., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 143–170. [Google Scholar]
- Jahn, K.; Saul, A.K.; Elstner, M.; Sapa, K.; Kellerer, S. Vestibular rehabilitation therapy and Nintendo Wii balance board training both improve postural control in bilateral vestibulopathy. J. Neurol. 2018, 265, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Eder, J.; Kellerer, S.; Amberger, T.; Keywan, A.; Dlugaiczyk, J.; Wuehr, M.; Jahn, K. Combining vestibular rehabilitation with noisy galvanic vestibular stimulation for treatment of bilateral vestibulopathy. J. Neurol. 2022, 269, 5731–5737. [Google Scholar] [CrossRef] [PubMed]
| Patient | DHI Pre-IVNT | DHI Post-IVNT | Delta DHI Post-Pre |
|---|---|---|---|
| 1 | 70 | 44 | −26 |
| 2 | 96 | 76 | −20 |
| 3 | 72 | 62 | −10 |
| 4 | 28 | 36 | 8 |
| 5 | 22 | 24 | 2 |
| 6 | 52 | 28 | −24 |
| 7 | 76 | 26 | −50 |
| 8 | 74 | 24 | −50 |
| 9 | 24 | 12 | −12 |
| 10 | 64 | 28 | −36 |
| 11 | 42 | 22 | −20 |
| 12 | 26 | 14 | −12 |
| 13 | 54 | 41 | −13 |
| 14 | 42 | 16 | −26 |
| 15 | 28 | 24 | −4 |
| 16 | 58 | 40 | −18 |
| 17 | 62 | 16 | −46 |
| 18 | 52 | 18 | −34 |
| 19 | 72 | 60 | −12 |
| 20 | 64 | 68 | 4 |
| 21 | 42 | 28 | −14 |
| 22 | 92 | 56 | −36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basta, D.; Rossi-Izquierdo, M.; Wonneberger, K.; Brugnera, C.; Bittar, R.S.M.; Greters, M.E.; Ernst, A.; Soto-Varela, A. Individualized Vibrotactile Neurofeedback Training in Patients with Chronic Bilateral Vestibulopathy. Brain Sci. 2023, 13, 1219. https://doi.org/10.3390/brainsci13081219
Basta D, Rossi-Izquierdo M, Wonneberger K, Brugnera C, Bittar RSM, Greters ME, Ernst A, Soto-Varela A. Individualized Vibrotactile Neurofeedback Training in Patients with Chronic Bilateral Vestibulopathy. Brain Sciences. 2023; 13(8):1219. https://doi.org/10.3390/brainsci13081219
Chicago/Turabian StyleBasta, Dietmar, Marcos Rossi-Izquierdo, Kai Wonneberger, Cibele Brugnera, Roseli Saraiva Moreira Bittar, Mário Edvin Greters, Arne Ernst, and Andrés Soto-Varela. 2023. "Individualized Vibrotactile Neurofeedback Training in Patients with Chronic Bilateral Vestibulopathy" Brain Sciences 13, no. 8: 1219. https://doi.org/10.3390/brainsci13081219
APA StyleBasta, D., Rossi-Izquierdo, M., Wonneberger, K., Brugnera, C., Bittar, R. S. M., Greters, M. E., Ernst, A., & Soto-Varela, A. (2023). Individualized Vibrotactile Neurofeedback Training in Patients with Chronic Bilateral Vestibulopathy. Brain Sciences, 13(8), 1219. https://doi.org/10.3390/brainsci13081219







