Cognitive Skills and DNA Methylation Are Correlating in Healthy and Novice College Students Practicing Preksha Dhyāna Meditation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Participants and PM Meditation Protocol
2.3. Test Assessments and Data Management
2.4. Blood Withdrawal, DNA Extraction, and Microarrays Analysis
2.5. Genome-Wide DNA Methylation and Quality Control and Downstream Analysis
2.6. Correlation Study
2.7. Further Statistical Analysis
3. Results
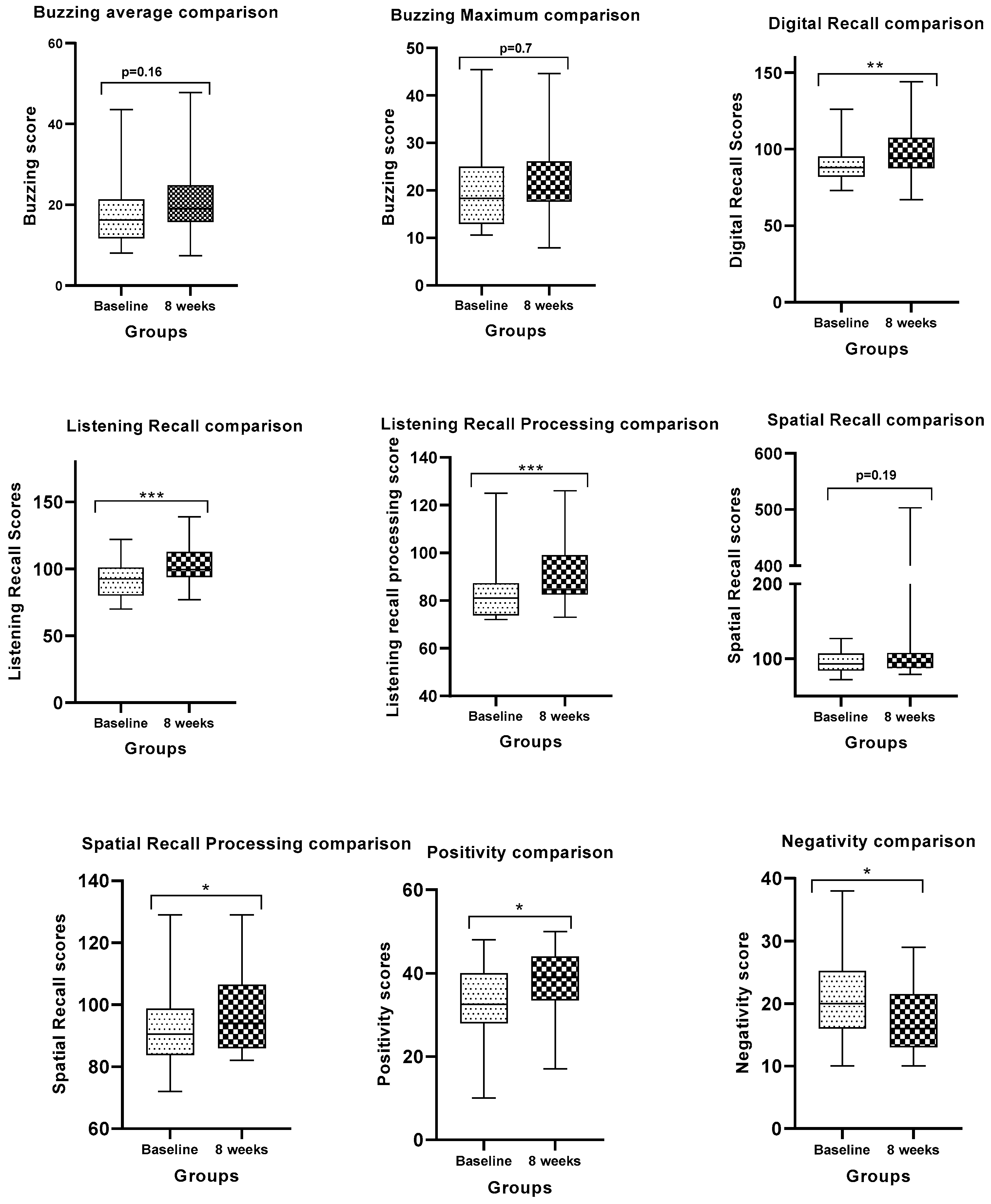
3.1. Cognitive Skills Assessments of the Different Meditation Groups
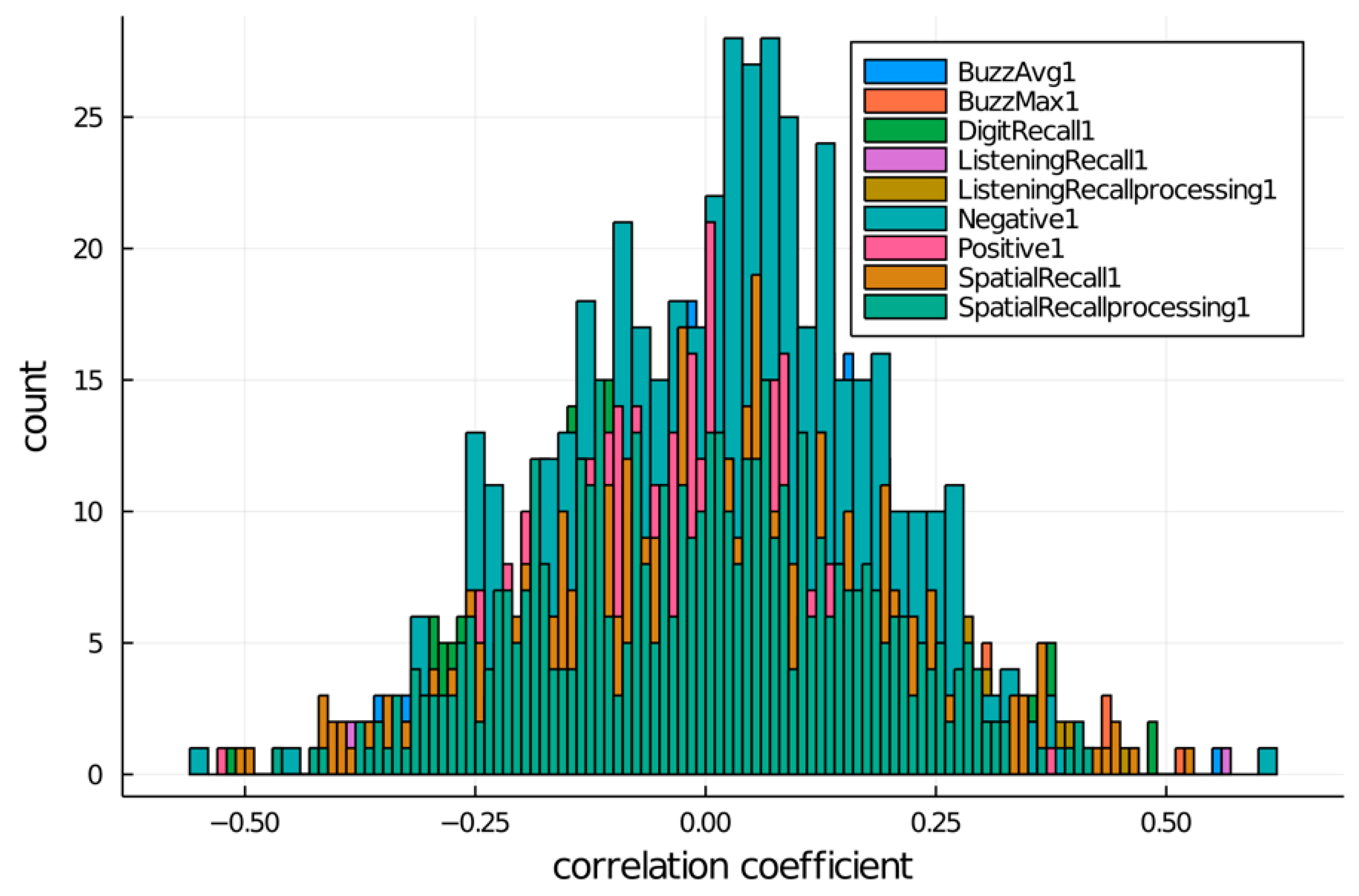
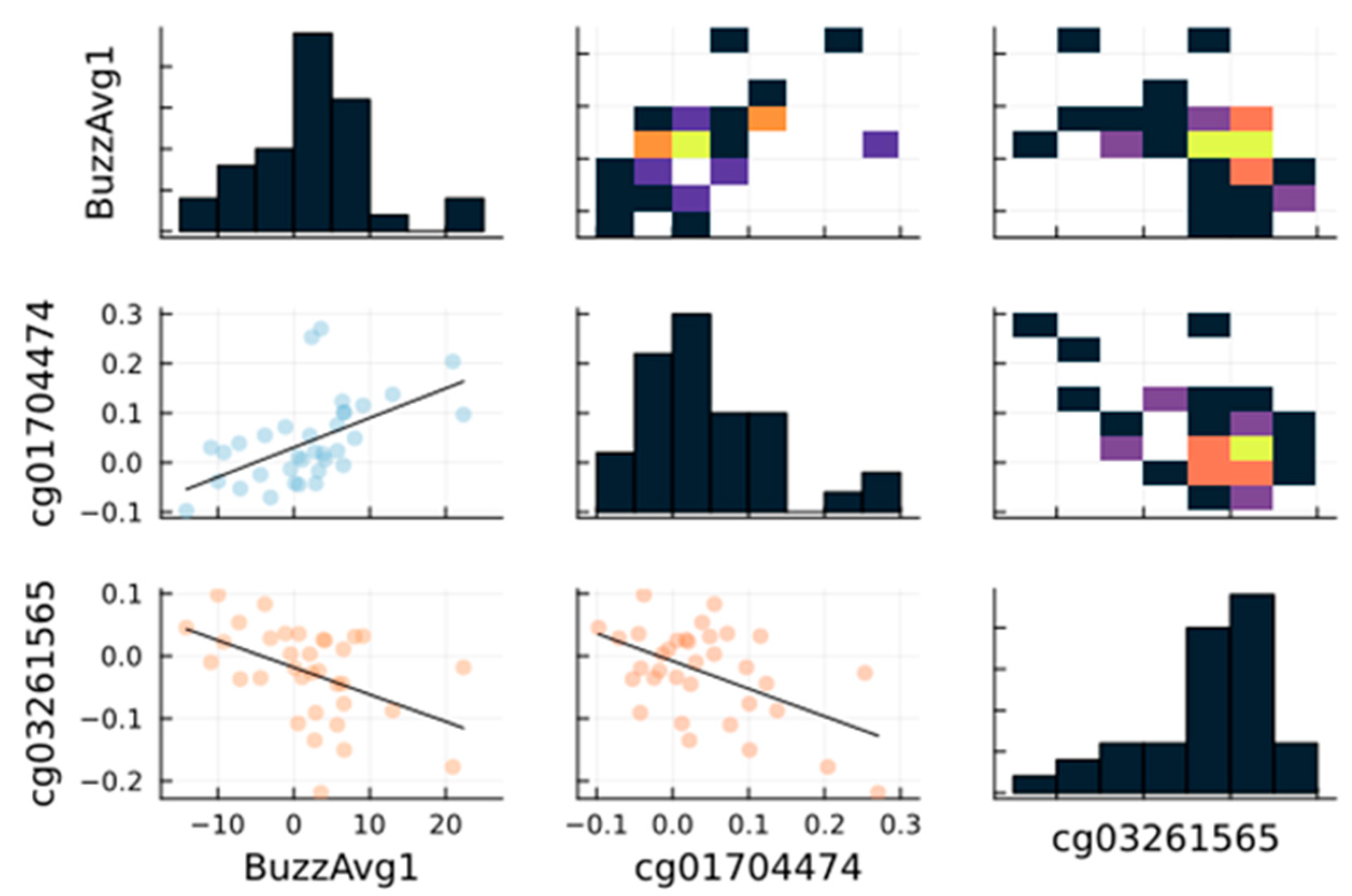
3.2. Correlation between the Cognitive Skills and the 470 Differentially Methylated Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felder, J.N.; Dimidjian, S.; Segal, Z. Collaboration in mindfulness-based cognitive therapy. J. Clin. Psychol. 2012, 68, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, R.B.; Minhajuddin, A.; Borman, P.D.; Dunlap, L.; Segal, Z.V.; Kidner, C.L.; Friedman, E.S. Cognitive reactivity, dysfunctional attitudes, and depressive relapse and recurrence in cognitive therapy responders. Behav. Res. Ther. 2012, 50, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zylowska, L.; Ackerman, D.L.; Yang, M.H.; Futrell, J.L.; Horton, N.L.; Hale, T.S.; Pataki, C.; Smalley, S.L. Mindfulness meditation training in adults and adolescents with ADHD: A feasibility study. J. Atten. Disord. 2008, 11, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Speca, M.; Carlson, L.E.; Goodey, E.; Angen, M. A randomized, wait-list controlled clinical trial: The effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom. Med. 2000, 62, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, A.B.; Chopp-Hurley, J.N.; Brenneman, E.C.; Karampatos, S.; Wiebenga, E.G.; Adachi, J.D.; Noseworthy, M.D.; Maly, M.R. Efficacy of a biomechanically-based yoga exercise program in knee osteoarthritis: A randomized controlled trial. PLoS ONE 2018, 13, e0195653. [Google Scholar] [CrossRef] [PubMed]
- Mahapragya, A. The Mirror of The Self: Author’s Preface; Jain Vishva Bharati Institute: Ladnun, India, 1995. [Google Scholar]
- Pragya, S.U.; Mehta, N.D.; Abomoelak, B.; Uddin, P.; Veeramachaneni, P.; Mehta, N.; Moore, S.; Jean-Francois, M.; Garcia, S.; Mehta, D.I. Effects of Combining Meditation Techniques on Short-Term Memory, Attention, and Affect in Healthy College Students. Front. Psychol. 2021, 12, 607573. [Google Scholar] [CrossRef] [PubMed]
- Abomoelak, B.; Pragya, S.U.; Griswold, A.J.; Mehta, N.; Uddin, P.; Veeramachaneni, P.; Mehta, N.; Pragya, S.C.; El Enshasy, H.A.; Mehta, D. Preksha Dhyana meditation induces alterations at the transcriptome level in novice and healthy college students. Saudi J. Biol. Sci. 2022, 29, 2299–2305. [Google Scholar] [CrossRef]
- Pragya, S.U.; Pragya, S.C.; Griswold, A.J.; Gu, E.; Mehta, N.D.; Uddin, P.; Veeramachaneni, P.; Mehta, N.; Mehta, D.; Abomoelak, B. Preksha Dhyana Meditation Effect on the DNA Methylation Signature in College Students. J. Integr. Complement. Med. 2023, 29, 224–233. [Google Scholar] [CrossRef]
- Mehta, V.; Mehta, A.; Patel, S.; Irastorza, L.; Rizvi, S.A.; Abomoelak, B.; Mehta, N.; Mehta, D. Efficacy of Short Course of Preksha Dhyana for Functional Abdominal Pain Disorder in a Busy Pediatric Clinic. Front. Pediatr. 2021, 9, 646686. [Google Scholar] [CrossRef]
- Qu, S.; Olafsrud, S.M.; Meza-Zepeda, L.A.; Saatcioglu, F. Rapid gene expression changes in peripheral blood lymphocytes upon practice of a comprehensive yoga program. PLoS ONE 2013, 8, e61910. [Google Scholar] [CrossRef]
- Bhasin, M.K.; Dusek, J.A.; Chang, B.H.; Joseph, M.G.; Denninger, J.W.; Fricchione, G.L.; Benson, H.; Libermann, T.A. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS ONE 2013, 8, e62817. [Google Scholar] [CrossRef] [PubMed]
- Househam, A.M.; Peterson, C.T.; Mills, P.J.; Chopra, D. The Effects of Stress and Meditation on the Immune System, Human Microbiota, and Epigenetics. Adv. Mind-Body Med. 2017, 31, 10–25. [Google Scholar] [PubMed]
- Filipe, M.G.; Magalhaes, S.; Veloso, A.S.; Costa, A.F.; Ribeiro, L.; Araújo, P.; Castro, S.L.; Limpo, T. Exploring the Effects of Meditation Techniques Used by Mindfulness-Based Programs on the Cognitive, Social-Emotional, and Academic Skills of Children: A Systematic Review. Front. Psychol. 2021, 12, 660650. [Google Scholar] [CrossRef] [PubMed]
- Wielgosz, J.; Goldberg, S.B.; Kral, T.R.A.; Dunne, J.D.; Davidson, R.J. Mindfulness Meditation and Psychopathology. Annu. Rev. Clin. Psychol. 2019, 15, 285–316. [Google Scholar] [CrossRef] [PubMed]
- Zautra, A.J.; Davis, M.C.; Reich, J.W.; Nicassario, P.; Tennen, H.; Finan, P.; Kratz, A.; Parrish, B.; Irwin, M.R. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J. Consult. Clin. Psychol. 2008, 76, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Alloway, T.P. Working memory, reading, and mathematical skills in children with developmental coordination disorder. J. Exp. Child Psychol. 2007, 96, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.F.; Kozasa, E.H.; da Silva, M.A.; Alves, T.M.; Louza, M.R.; Pompeia, S. Mindfulness Meditation Improves Mood, Quality of Life, and Attention in Adults with Attention Deficit Hyperactivity Disorder. BioMed Res. Int. 2015, 2015, 962857. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lemire, M.; Choufani, S.; Butcher, D.T.; Grafodatskaya, D.; Zanke, B.W.; Gallinger, S.; Hudson, T.J.; Weksberg, R. Discovery of cross-reactive probes and polymorphic CpGs in the Illumina Infinium HumanMethylation450 microarray. Epigenetics 2013, 8, 203–209. [Google Scholar] [CrossRef]
- Salas, L.A.; Koestler, D.C.; Butler, R.A.; Hansen, H.M.; Wiencke, J.K.; Kelsey, K.T.; Christensen, B.C. An optimized library for reference-based deconvolution of whole-blood biospecimens assayed using the Illumina HumanMethylationEPIC BeadArray. Genome Biol. 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Koestler, D.C.; Jones, M.J.; Usset, J.; Christensen, B.C.; Butler, R.A.; Kobor, M.S.; Wiencke, J.K.; Kelsey, K.T. Improving cell mixture deconvolution by identifying optimal DNA methylation libraries (IDOL). BMC Bioinform. 2016, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Kibbe, W.A.; Lin, S.M. lumi: A pipeline for processing Illumina microarray. Bioinformatics 2008, 24, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Teschendorff, A.E.; Marabita, F.; Lechner, M.; Bartlett, T.; Tegner, J.; Gomez-Cabrero, D.; Beck, S. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 2013, 29, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Maksimovic, J.; Phipson, B.; Oshlack, A. A cross-package Bioconductor workflow for analysing methylation array data. F1000Research 2016, 5, 1281. [Google Scholar] [CrossRef]
- Hedberg-Oldfors, C.; Mitra, S.; Molinaro, A.; Visuttijai, K.; Fogelstrand, L.; Oldfors, A.; Sterky, F.H.; Darin, N. Ribonuclease inhibitor 1 (RNH1) deficiency cause congenital cataracts and global developmental delay with infection-induced psychomotor regression and anemia. Eur. J. Hum. Genet. 2023, 31, 887–894. [Google Scholar] [CrossRef]
- Maria, A.G.; Azevedo, B.; Settas, N.; Hannah-Shmouni, F.; Stratakis, C.A.; Faucz, F.R. USP13 genetics and expression in a family with thyroid cancer. Endocrine 2022, 77, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, G.; Zhang, W.; Qin, B.; Ye, Z.; Shi, H.; Zhao, X.; Chen, Y.; Song, B.; Mei, Z.; et al. USP13: Multiple Functions and Target Inhibition. Front. Cell Dev. Biol. 2022, 10, 875124. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Gutierrez, G.J. USP13 modulates the stability of the APC/C adaptor CDH1. Mol. Biol. Rep. 2022, 49, 4079–4087. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Choi, H.; Ware, A.D.; Morillo, B.C.; Wang, H.; Bouker, K.B.; Lu, X.; Waldman, T.; Han, C. USP13 promotes development and metastasis of high-grade serous ovarian carcinoma in a novel mouse model. Oncogene 2022, 41, 1974–1985. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, J.; Qiao, L.; Zhao, W. Deubiquitinase USP13 promotes the epithelial-mesenchymal transition and metastasis in gastric cancer by maintaining Snail protein. Pathol. Res. Pract. 2022, 229, 153705. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; He, L.; Yang, J.; Pei, F.; Li, W.; Liu, S.; Chen, Z.; Xie, G.; Xu, B.; et al. ZNF516 suppresses EGFR by targeting the CtBP/LSD1/CoREST complex to chromatin. Nat. Commun. 2017, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- RBMS3 versus ZNF516: Interaction between SNPs in two genes impacts on BMD. Bonekey Rep. 2013, 2, 376. [CrossRef][Green Version]
- Brebi, P.; Maldonado, L.; Noordhuis, M.G.; Ili, C.; Leal, P.; Garcia, P.; Brait, M.; Ribas, J.; Michailidi, C.; Perez, J.; et al. Genome-wide methylation profiling reveals Zinc finger protein 516 (ZNF516) and FK-506-binding protein 6 (FKBP6) promoters frequently methylated in cervical neoplasia, associated with HPV status and ethnicity in a Chilean population. Epigenetics 2014, 9, 308–317. [Google Scholar] [CrossRef]
- Yang, T.L.; Guo, Y.; Li, J.; Zhang, L.; Shen, H.; Li, S.M.; Li, S.K.; Tian, Q.; Liu, Y.-J.; Papasian, C.J.; et al. Gene-gene interaction between RBMS3 and ZNF516 influences bone mineral density. J. Bone Min. Res. 2013, 28, 828–837. [Google Scholar] [CrossRef]
- Meijer, A.J.M.; Diepstraten, F.A.; Langer, T.; Broer, L.; Domingo, I.K.; Clemens, E.; Uitterlinden, A.G.; de Vries, A.C.H.; van Grotel, M.; Vermeij, W.P.; et al. TCERG1L allelic variation is associated with cisplatin-induced hearing loss in childhood cancer, a PanCareLIFE study. NPJ Precis. Oncol. 2021, 5, 64. [Google Scholar] [CrossRef]
- Mukai, M.; Ikegami, K.; Sugiura, Y.; Takeshita, K.; Nakagawa, A.; Setou, M. Recombinant mammalian tubulin polyglutamylase TTLL7 performs both initiation and elongation of polyglutamylation on beta-tubulin through a random sequential pathway. Biochemistry 2009, 48, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, K.; Mukai, M.; Tsuchida, J.; Heier, R.L.; Macgregor, G.R.; Setou, M. TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. J. Biol. Chem. 2006, 281, 30707–30716. [Google Scholar] [CrossRef] [PubMed]
- Audebert, S.; Koulakoff, A.; Berwald-Netter, Y.; Gros, F.; Denoulet, P.; Edde, B. Developmental regulation of polyglutamylated alpha- and beta-tubulin in mouse brain neurons. J. Cell Sci. 1994, 107 Pt 8, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Yahya, D.N.; Guad, R.M.; Wu, Y.S.; Gan, S.H.; Gopinath, S.C.; Zakariah, H.A.; Rashid, R.A.; Sim, M.S. SLC1A2 Gene Polymorphism Influences Methamphetamine-Induced Psychosis. J. Pers. Med. 2023, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Sharp, S.I.; McQuillin, A. Association of rare variation in the glutamate receptor gene SLC1A2 with susceptibility to bipolar disorder and schizophrenia. Eur. J. Hum. Genet. 2015, 23, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Jimenez, F.J.; Alonso-Navarro, H.; Martinez, C.; Zurdo, M.; Turpín-Fenoll, L.; Millán-Pascual, J.; Adeva-Bartolomé, T.; Cubo, E.; Navacerrada, F.; Rojo-Sebastián, A.; et al. The solute carrier family 1 (glial high affinity glutamate transporter), member 2 gene, SLC1A2, rs3794087 variant and assessment risk for restless legs syndrome. Sleep Med. 2014, 15, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Deng, N.T.; Ramnarayanan, K.; Huang, B.; Oh, H.K.; Leong, S.H.; Lim, S.S.; Tan, I.B.; Ooi, C.H.; Wu, J.; et al. CD44-SLC1A2 gene fusions in gastric cancer. Sci. Transl. Med. 2011, 3, 77ra30. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Ohnuma, T.; Karibe, J.; Shibata, N.; Maeshima, H.; Baba, H.; Hatano, T.; Hanzawa, R.; Arai, H. No genetic association between the SLC1A2 gene and Japanese patients with schizophrenia. Neurosci. Lett. 2009, 463, 223–227. [Google Scholar] [CrossRef]
- Deng, X.; Shibata, H.; Ninomiya, H.; Tashiro, N.; Iwata, N.; Ozaki, N.; Fukumaki, Y. Association study of polymorphisms in the excitatory amino acid transporter 2 gene (SLC1A2) with schizophrenia. BMC Psychiatry 2004, 4, 21. [Google Scholar] [CrossRef]
- Li, X.; Francke, U. Assignment of the gene SLC1A2 coding for the human glutamate transporter EAAT2 to human chromosome 11 bands p13-p12. Cytogenet. Cell Genet. 1995, 71, 212–213. [Google Scholar] [CrossRef]
- Ludwig, D.; Carter, J.; Smith, J.R.; Borsani, G.; Barlati, S.; Hafizi, S. Functional characterisation of human cells harbouring a novel t(2p;7p) translocation involving TNS3 and EXOC6B genes. BMC Med. Genet. 2013, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.A.; Gorecki, D.C.; Mein, C.A.; Ljungberg, B.; Hafizi, S. CpG dinucleotide-specific hypermethylation of the TNS3 gene promoter in human renal cell carcinoma. Epigenetics 2013, 8, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Borsani, G.; Piovani, G.; Zoppi, N.; Bertini, V.; Bini, R.; Notarangelo, L.; Barlati, S. Cytogenetic and molecular characterization of a de-novo t(2p;7p) translocation involving TNS3 and EXOC6B genes in a boy with a complex syndromic phenotype. Eur. J. Med. Genet. 2008, 51, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Serman, T.M.; Gack, M.U. FBXO38 Drives PD-1 to Destruction. Trends Immunol. 2019, 40, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, X.; Guo, X.; Jiang, S.; Chen, T.; Hu, Z.; Liu, H.; Bai, Y.; Xue, M.; Hu, R.; et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature 2018, 564, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Dong, L.; Zeng, L.; Yang, R.; Xu, J.; Wu, Y.; Xu, R.; Tao, H.; Zhang, N. Two-stage comprehensive evaluation of genetic susceptibility of common variants in FBXO38, AP3B2 and WHAMM to severe chronic periodontitis. Sci. Rep. 2015, 5, 17882. [Google Scholar] [CrossRef] [PubMed]
- Sumner, C.J.; d’Ydewalle, C.; Wooley, J.; Fawcett, K.A.; Hernandez, D.; Gardiner, A.R.; Kalmar, B.; Baloh, R.H.; Gonzalez, M.; Züchner, S.; et al. A dominant mutation in FBXO38 causes distal spinal muscular atrophy with calf predominance. Am. J. Hum. Genet. 2013, 93, 976–983. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, J.S.; Li, F.; Wang, J.; Deng, Z.; White, M.A.; Osborne, D.G.; Phillips-Krawczak, C.; Gomez, T.S.; Li, H.; et al. Structural and mechanistic insights into regulation of the retromer coat by TBC1d5. Nat. Commun. 2016, 7, 13305. [Google Scholar] [CrossRef]
- Popovic, D.; Dikic, I. TBC1D5 and the AP2 complex regulate ATG9 trafficking and initiation of autophagy. EMBO Rep. 2014, 15, 392–401. [Google Scholar] [CrossRef]
- Narboux-Neme, N.; Goiame, R.; Mattei, M.G.; Cohen-Tannoudji, M.; Wassef, M. Integration of H-2Z1, a somatosensory cortex-expressed transgene, interferes with the expression of the Satb1 and Tbc1d5 flanking genes and affects the differentiation of a subset of cortical interneurons. J. Neurosci. 2012, 32, 7287–7300. [Google Scholar] [CrossRef]
- Seaman, M.N.; Harbour, M.E.; Tattersall, D.; Read, E.; Bright, N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J. Cell Sci. 2009, 122 Pt 14, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Kurahara, H.; Bohl, C.; Natsugoe, S.; Nishizono, Y.; Harihar, S.; Sharma, R.; Iwakuma, T.; Welch, D.R. Suppression of pancreatic cancer growth and metastasis by HMP19 identified through genome-wide shRNA screen. Int. J. Cancer 2016, 139, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Szatmari, E.M.; Moran, C.; Cohen, S.; Jacob, A.; Parra-Bueno, P.; Kamasawa, N.; Guerrero-Given, D.; Klein, M.; Stackman, R.; Yasuda, R. ADAP1/Centaurin-alpha1 Negatively Regulates Dendritic Spine Function and Memory Formation in the Hippocampus. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Van Duzer, A.; Taniguchi, S.; Elhance, A.; Tsujikawa, T.; Oshimori, N. ADAP1 promotes invasive squamous cell carcinoma progression and predicts patient survival. Life Sci. Alliance 2019, 2, e201900582. [Google Scholar] [CrossRef] [PubMed]
- Giguere, H.; Dumont, A.A.; Berthiaume, J.; Oliveira, V.; Laberge, G.; Auger-Messier, M. ADAP1 limits neonatal cardiomyocyte hypertrophy by reducing integrin cell surface expression. Sci. Rep. 2018, 8, 13605. [Google Scholar] [CrossRef] [PubMed]
- Reisdorph, R.; Littrell-Miller, B.; Powell, R.; Reisdorph, N. A mass spectrometry based predictive strategy reveals ADAP1 is phosphorylated at tyrosine 364. Rapid Commun. Mass Spectrom. 2018, 32, 1173–1180. [Google Scholar] [CrossRef]
- Stricker, R.; Reiser, G. Functions of the neuron-specific protein ADAP1 (centaurin-alpha1) in neuronal differentiation and neurodegenerative diseases, with an overview of structural and biochemical properties of ADAP1. Biol. Chem. 2014, 395, 1321–1340. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Wu, H.; Zhang, F.; Liu, G.; Wu, J.; Peng, X.; Mei, S. Polymorphisms in PEG10 and PPP1R9A genes are associated with porcine carcass and meat quality traits. Anim. Genet. 2016, 47, 270. [Google Scholar] [CrossRef]
- Konopaske, G.T.; Subburaju, S.; Coyle, J.T.; Benes, F.M. Altered prefrontal cortical MARCKS and PPP1R9A mRNA expression in schizophrenia and bipolar disorder. Schizophr. Res. 2015, 164, 100–108. [Google Scholar] [CrossRef]
- Jiang, C.D.; Li, S.; Deng, C.Y. Assessment of genomic imprinting of PPP1R9A, NAP1L5 and PEG3 in pigs. Genetika 2011, 47, 537–542. [Google Scholar] [CrossRef]
- Zhang, F.W.; Deng, C.Y.; He, H.J.; Gu, N.; Han, Z.-B.; Chen, Y.; Wu, Q. Molecular cloning, mRNA expression and imprinting status of PEG3, NAP1L5 and PPP1R9A genes in pig. Genes Genet. Syst. 2011, 86, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Makino, S.; Minagawa, S.; Smith, A.C.; Bamforth, J.S.; Stanier, P.; Preece, M.; Parker-Katiraee, L.; Paton, T.; Oshimura, M.; et al. Genomic imprinting of PPP1R9A encoding neurabin I in skeletal muscle and extra-embryonic tissues. J. Med. Genet. 2004, 41, 601–608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- The PLoS ONE Editors. Retraction: Modulatory Role of PYY in Transport and Metabolism of Cholesterol in Intestinal Epithelial Cells. PLoS ONE 2022, 17, e0267059. [Google Scholar]
- Sanford, D.; Luong, L.; Vu, J.P.; Oh, S.; Gabalski, A.; Lewis, M.; Pisegna, J.R.; Germano, P. The VIP/VPAC1R Pathway Regulates Energy and Glucose Homeostasis by Modulating GLP-1, Glucagon, Leptin and PYY Levels in Mice. Biology 2022, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Ghelman, J.; Grewing, L.; Windener, F.; Albrecht, S.; Zarbock, A.; Kuhlmann, T. SKAP2 as a new regulator of oligodendroglial migration and myelin sheath formation. Glia 2021, 69, 2699–2716. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Xu, S.; Adams, W.; Leong, J.M.; Bunnell, S.C.; Mansour, M.K.; Sykes, D.B.; Mecsas, J. Neutrophils require SKAP2 for reactive oxygen species production following C-type lectin and Candida stimulation. iScience 2021, 24, 102871. [Google Scholar] [CrossRef] [PubMed]
- Takagane, K.; Umakoshi, M.; Itoh, G.; Kuriyama, S.; Goto, A.; Tanaka, M. SKAP2 suppresses inflammation-mediated tumorigenesis by regulating SHP-1 and SHP-2. Oncogene 2022, 41, 1087–1099. [Google Scholar] [CrossRef]
- Bata, N.; Chaikuad, A.; Bakas, N.A.; Limpert, A.S.; Lambert, L.J.; Sheffler, D.J.; Berger, L.M.; Liu, G.; Yuan, C.; Wang, L.; et al. Inhibitors of the Hippo Pathway Kinases STK3/MST2 and STK4/MST1 Have Utility for the Treatment of Acute Myeloid Leukemia. J. Med. Chem. 2022, 65, 1352–1369. [Google Scholar] [CrossRef]


|
Buzz
Average | Buzz Max | Digit Recall | Listening Recall | Listening Recall Processing | Negative | Positive |
Spatial
Recall |
Spatial
Recall Processing | ||
|---|---|---|---|---|---|---|---|---|---|---|
| max positive | 0.55 | 0.52 | 0.49 | 0.57 | 0.45 | 0.62 | 0.40 | 0.52 | 0.42 | |
| p-value | 7.07 × 10−4 | 1.79 × 10−3 | 3.37 × 10−3 | 4.81 × 10−4 | 7.55 × 10−3 | 9.41 × 10−5 | 1.87 × 10−2 | 1.61 × 10−3 | 1.42 × 10−2 | |
| site | cg01704474 | cg01704474 | cg19060557 | cg23140777 | cg12128316 | cg17095850 | cg06148656 | cg05990364 | cg03333699 | |
| Chromosome | chr11 | chr11 | chr3 | chr9 | chr5 | chr11 | chr5 | chr5 | chr7 | |
| Position | 504918 | 504918 | 179399839 | 125457429 | 157137992 | 35311522 | 147808721 | 173493084 | 966569 | |
| max negative | −0.47 | −0.42 | −0.52 | −0.47 | −0.36 | −0.56 | −0.53 | −0.50 | −0.46 | |
| p-value | 5.26 × 10−3 | 1.33 × 10−2 | 1.77 × 10−3 | 5.44 × 10−3 | 3.60 × 10−2 | 5.78 × 10−4 | 1.41 × 10−3 | 2.46 × 10−3 | 5.81 × 10−3 | |
| site | cg03261565 | cg03261565 | cg13049398 | cg06938601 | cg23561053 | cg23768860 | cg13566979 | cg22717379 | cg00730266 | |
| Chromosome | chr10 | chr10 | chr18 | chr10 | chr1 | chr7 | chr3 | chr4 | chr7 | |
| Position | 29312799 | 29312799 | 74157682 | 132942686 | 84465000 | 47472944 | 17712381 | 147939863 | 94537716 |
| CG Site | Buzz Average | Buzz Max | Digit Recall | Listening Recall | Listening Recall Processing | Negative | Positive | Spatial Recall | Spatial Recall Processing | Total Count |
|---|---|---|---|---|---|---|---|---|---|---|
| cg26094004 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 4 |
| cg03362824 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 3 |
| cg14862307 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 3 |
| cg23632416 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 3 |
| cg23191941 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 3 |
| cg12128316 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 3 |
| Skill | W | p-Value | Normal |
|---|---|---|---|
| Buzz Average | 0.9972 | 0.6258 | true |
| Buzz Max | 0.9989 | 0.9930 | true |
| Digit Recall | 0.9869 | 0.0003 | false |
| Listening Recall | 0.9957 | 0.2282 | true |
| Listening Recall Processing | 0.9952 | 0.1592 | true |
| Negative | 0.9958 | 0.2411 | true |
| Positive | 0.9977 | 0.7672 | true |
| Spatial Recall | 0.9983 | 0.9267 | true |
| Spatial Recall Processing | 0.9944 | 0.0850 | true |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abomoelak, B.; Prather, R.; Pragya, S.U.; Pragya, S.C.; Mehta, N.D.; Uddin, P.; Veeramachaneni, P.; Mehta, N.; Young, A.; Kapoor, S.; et al. Cognitive Skills and DNA Methylation Are Correlating in Healthy and Novice College Students Practicing Preksha Dhyāna Meditation. Brain Sci. 2023, 13, 1214. https://doi.org/10.3390/brainsci13081214
Abomoelak B, Prather R, Pragya SU, Pragya SC, Mehta ND, Uddin P, Veeramachaneni P, Mehta N, Young A, Kapoor S, et al. Cognitive Skills and DNA Methylation Are Correlating in Healthy and Novice College Students Practicing Preksha Dhyāna Meditation. Brain Sciences. 2023; 13(8):1214. https://doi.org/10.3390/brainsci13081214
Chicago/Turabian StyleAbomoelak, Bassam, Ray Prather, Samani U. Pragya, Samani C. Pragya, Neelam D. Mehta, Parvin Uddin, Pushya Veeramachaneni, Naina Mehta, Amanda Young, Saumya Kapoor, and et al. 2023. "Cognitive Skills and DNA Methylation Are Correlating in Healthy and Novice College Students Practicing Preksha Dhyāna Meditation" Brain Sciences 13, no. 8: 1214. https://doi.org/10.3390/brainsci13081214
APA StyleAbomoelak, B., Prather, R., Pragya, S. U., Pragya, S. C., Mehta, N. D., Uddin, P., Veeramachaneni, P., Mehta, N., Young, A., Kapoor, S., & Mehta, D. (2023). Cognitive Skills and DNA Methylation Are Correlating in Healthy and Novice College Students Practicing Preksha Dhyāna Meditation. Brain Sciences, 13(8), 1214. https://doi.org/10.3390/brainsci13081214





