Skilled Performers Show Right Parietal Lateralization during Anticipation of Volleyball Attacks
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
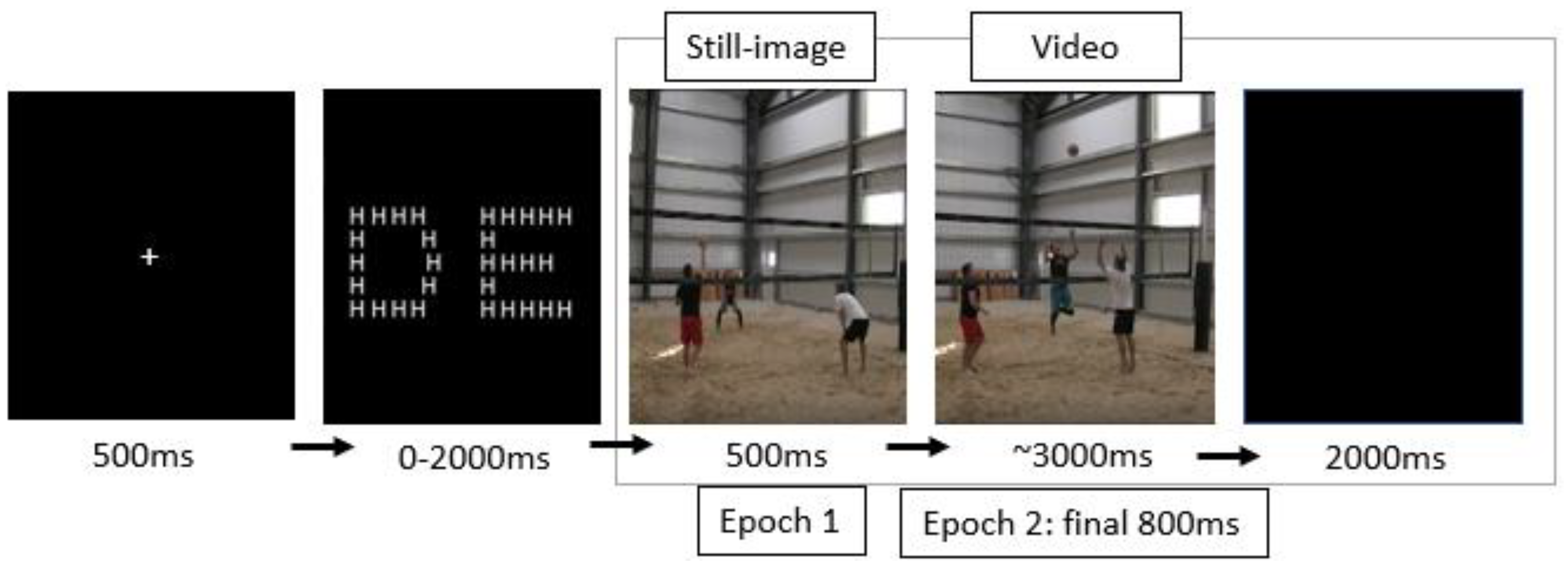
2.2. Stimuli
2.3. Procedures
2.4. Measurements
2.5. Data Analysis
3. Results
3.1. Volleyball Task Performance
3.2. Brain Activity
4. Discussion
4.1. Right Parietal Lateralization in Skilled Performers
4.2. Preparatory Period Versus Motion Processing Period
4.3. Superior Performance with Global Priming
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirai, M.; Senju, A. The two-process theory of biological motion processing. Neurosci. Biobehav. Rev. 2020, 111, 114–124. [Google Scholar] [CrossRef]
- Chang, D.H.F.; Troje, N.F. Characterizing global and local mechanisms in biological motion perception. J. Vis. 2009, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Hiraki, K. An event-related potentials study of biological motion perception in human infants. Cogn. Brain Res. 2005, 22, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Simion, F.; Regolin, L.; Bulf, H. A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci. USA 2008, 105, 809–813. [Google Scholar] [CrossRef]
- Williams, A.M.; Jackson, R.C. Anticipation and Decision Making in Sport, 1st ed.; Routledge: Abingdon, UK, 2019. [Google Scholar]
- Breslin, G.; Hodges, N.J.; Williams, M.A. Effect of Information Load and Time on Observational Learning. Res. Q. Exerc. Sport 2009, 80, 480–490. [Google Scholar] [CrossRef]
- Williams, A.M.; Huys, R.; Cañal-Bruland, R.; Hagemann, N. The dynamical information underpinning anticipation skill. Hum. Mov. Sci. 2009, 28, 362–370. [Google Scholar] [CrossRef]
- Smeeton, N.J.; Hüttermann, S.; Williams, A.M. Postural cues, biological motion perception, and anticipation in sport. In Anticipation and Decision Making in Sport, 1st ed.; Routledge: Abingdon, UK, 2019; pp. 16–18. [Google Scholar]
- Chang, D.H.F.; Troje, N.F. Acceleration carries the local inversion effect in biological motion perception. J. Vis. 2009, 9, 19. [Google Scholar] [CrossRef]
- Huys, R.; Cañal-Bruland, R.; Hagemann, N.; Beek, P.J.; Smeeton, N.J.; Williams, A.M. Global Information Pickup Underpins Anticipation of Tennis Shot Direction. J. Mot. Behav. 2009, 41, 158–171. [Google Scholar] [CrossRef]
- Bertenthal, B.I.; Pinto, J. Global Processing of Biological Motions. Psychol. Sci. 1994, 5, 221–225. [Google Scholar] [CrossRef]
- Gao, Z.; Flevaris, A.V.; Robertson, L.C.; Bentin, S. Priming global and local processing of composite faces: Revisiting the processing-bias effect on face perception. Atten. Percept. Psychophys. 2011, 73, 1477–1486. [Google Scholar] [CrossRef]
- Schooler, J.W. Verbalization produces a transfer inappropriate processing shift. Appl. Cogn. Psychol. 2002, 16, 989–997. [Google Scholar] [CrossRef]
- Meissner, C.A.; Brigham, J.C.; Kelley, C.M. The influence of retrieval processes in verbal overshadowing. Mem. Cogn. 2001, 29, 176–186. [Google Scholar] [CrossRef]
- Schooler, J.W.; Engstler-Schooler, T.Y. Verbal overshadowing of visual memories: Some things are better left unsaid. Cogn. Psychol. 1990, 22, 36–71. [Google Scholar] [CrossRef] [PubMed]
- Perfect, T.J.; Weston, N.J.; Dennis, I.; Snell, A. Short article: The effects of precedence on Navon-induced processing bias in face recognition. Q. J. Exp. Psychol. 2008, 61, 1479–1486. [Google Scholar] [CrossRef]
- Hills, P.J.; Lewis, M.B. Temporal Limitation of Navon Effect on Face Recognition. Percept. Mot. Ski. 2007, 104, 501–509. [Google Scholar] [CrossRef]
- Rhodes, G.; Brake, S.; Taylor, K.; Tan, S. Expertise and configural coding in face recognition. Br. J. Psychol. 1989, 80, 313–331. [Google Scholar] [CrossRef]
- Azer, L.; Zhang, W. Composite Face Effect Predicts Configural Encoding in Visual Short-Term Memory. Front. Psychol. 2019, 10, 2753. [Google Scholar] [CrossRef] [PubMed]
- Olulade, O.A.; Seydell-Greenwald, A.; Chambers, C.E.; Turkeltaub, P.E.; Dromerick, A.W.; Berl, M.M.; Gaillard, W.D.; Newport, E.L. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. USA 2020, 117, 23477–23483. [Google Scholar] [CrossRef]
- Robertson, L.; Lamb, M.R.; Knight, R. Effects of lesions of temporal-parietal junction on perceptual and attentional processing in humans. J. Neurosci. 1988, 8, 3757–3769. [Google Scholar] [CrossRef]
- Romei, V.; Thut, G.; Mok, R.M.; Schyns, P.G.; Driver, J. Causal implication by rhythmic transcranial magnetic stimulation of alpha frequency in feature-based local vs. global attention. Eur. J. Neurosci. 2012, 35, 968–974. [Google Scholar] [CrossRef]
- Flevaris, A.V.; Robertson, L.C. Spatial frequency selection and integration of global and local information in visual processing: A selective review and tribute to Shlomo Bentin. Neuropsychologia 2016, 83, 192–200. [Google Scholar] [CrossRef]
- Volberg, G.; Kliegl, K.; Hanslmayr, S.; Greenlee, M.W. EEG alpha oscillations in the preparation for global and local processing predict behavioral performance. Hum. Brain Mapp. 2009, 30, 2173–2183. [Google Scholar] [CrossRef]
- Lamb, M.R.; Robertson, L.C.; Knight, R.T. Component mechanisms underlying the processing of hierarchically organized patterns: Inferences from patients with unilateral cortical lesions. J. Exp. Psychol. Learn. Mem. Cogn. 1990, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Flevaris, A.V.; Bentin, S.; Robertson, L.C. Attentional selection of relative SF mediates global versus local processing: Evidence from EEG. J. Vis. 2011, 11, 11. [Google Scholar] [CrossRef][Green Version]
- Gable, P.A.; Poole, B.D.; Cook, M.S. Asymmetrical hemisphere activation enhances global–local processing. Brain Cogn. 2013, 83, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.; Nešić, M. Contralateral Hemisphere Activation by Unilateral Hand Contraction: ReExamining Global and Local Attention. Percept. Mot. Ski. 2018, 125, 438–450. [Google Scholar] [CrossRef]
- Cross-Villasana, F.; Gröpel, P.; Doppelmayr, M.; Beckmann, J. Unilateral Left-Hand Contractions Produce Widespread Depression of Cortical Activity after Their Execution. PLoS ONE 2015, 10, e0145867. [Google Scholar] [CrossRef] [PubMed]
- Delis, D.C.; Robertson, L.C.; Efron, R. Hemispheric specialization of memory for visual hierarchical stimuli. Neuropsychologia 1986, 24, 205–214. [Google Scholar] [CrossRef]
- Liu, L.; Luo, H. Behavioral oscillation in global/local processing: Global alpha oscillations mediate global precedence effect. J. Vis. 2019, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Pitchford, B.; Arnell, K.M. Resting EEG in alpha and beta bands predicts individual differences in attentional breadth. Conscious. Cogn. 2019, 75, 102803. [Google Scholar] [CrossRef]
- Costa, S.; Berchicci, M.; Bianco, V.; Croce, P.; Di Russo, F.; Quinzi, F.; Bertollo, M.; Zappasodi, F. Brain dynamics of visual anticipation during spatial occlusion tasks in expert tennis players. Psychol. Sport Exerc. 2023, 65, 102335. [Google Scholar] [CrossRef]
- Pagnotta, M.F.; Pascucci, D.; Plomp, G. Selective attention involves a feature-specific sequential release from inhibitory gating. Neuroimage 2022, 246, 118782. [Google Scholar] [CrossRef]
- Riddle, J.; Hwang, K.; Cellier, D.; Dhanani, S.; D’Esposito, M. Causal Evidence for the Role of Neuronal Oscillations in Top–Down and Bottom–Up Attention. J. Cogn. Neurosci. 2019, 31, 768–779. [Google Scholar] [CrossRef]
- MacLean, M.H.; Arnell, K.M.; Cote, K.A. Resting EEG in alpha and beta bands predicts individual differences in attentional blink magnitude. Brain Cogn. 2012, 78, 218–229. [Google Scholar] [CrossRef]
- DeCouto, B.S.; Smeeton, N.J.; Williams, A.M. Skill and experience impact global and local biological motion processing. Neuropsychologica 2023, in press. [Google Scholar]
- Spitzer, B.; Haegens, S. Beyond the Status Quo: A Role for Beta Oscillations in Endogenous Content (Re)Activation. Eneuro 2017, 4, ENEURO.0170-17. [Google Scholar] [CrossRef]
- Pagnotta, M.F.; Pascucci, D.; Plomp, G. Nested oscillations and brain connectivity during sequential stages of feature-based attention. Neuroimage 2020, 223, 117354. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef]
- Lewis, M.B.; Dawkins, G. Local Navon letter processing affects skilled behavior: A golf-putting experiment. Psychon. Bull. Rev. 2014, 22, 420–428. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K.; Peirce, J.; Gray, J.R.; et al. PsychoPy2: Experiments in behavior made easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef]
- Lewis, M.B.; Mills, C.; Hills, P.J.; Weston, N. Navon Letters Affect Face Learning and Face Retrieval. Exp. Psychol. 2009, 56, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Jasper, H.H. The ten twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Boisgontier, M.P.; Cheval, B. The anova to mixed model transition. Neurosci. Biobehav. Rev. 2016, 68, 1004–1005. [Google Scholar] [CrossRef]
- Kristensen, M.; Hansen, T.; Goswami, N.; Batzel, J.J.; Hinghofer-Szalkay, H.; Curran-Everett, D.; Mortensen, K.E.; Godtliebsen, F.; Revhaug, A.; Juel, C. Statistical analyses of repeated measures in physiological research: A tutorial. Adv. Physiol. Educ. 2004, 28, 2–14. [Google Scholar] [CrossRef]
- Schielzeth, H.; Dingemanse, N.J.; Nakagawa, S.; Westneat, D.F.; Allegue, H.; Teplitsky, C.; Réale, D.; Dochtermann, N.A.; Garamszegi, L.Z.; Araya-Ajoy, Y.G. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol. Evol. 2020, 11, 1141–1152. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Package “Emmeans”; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Wickham, H. ggplot2: Elegant Plots for Ata Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Grolemund, G. Tidyverse. 2020. Available online: https://www.tidyverse.org/ (accessed on 4 January 2022).
- Scheeringa, R.; Fries, P.; Petersson, K.-M.; Oostenveld, R.; Grothe, I.; Norris, D.G.; Hagoort, P.; Bastiaansen, M.C. Neuronal Dynamics Underlying High- and Low-Frequency EEG Oscillations Contribute Independently to the Human BOLD Signal. Neuron 2011, 69, 572–583. [Google Scholar] [CrossRef]
- Arif, Y.; Spooner, R.K.; Wiesman, A.I.; Embury, C.M.; Proskovec, A.L.; Wilson, T.W. Modulation of attention networks serving reorientation in healthy aging. Aging 2020, 12, 12582–12597. [Google Scholar] [CrossRef] [PubMed]
- Orgs, G.; Dombrowski, J.-H.; Heil, M.; Jansen-Osmann, P. Expertise in dance modulates alphabeta event-related desynchronization during action observation. Eur. J. Neurosci. 2008, 27, 3380–3384. [Google Scholar] [CrossRef]
- Denis, D.; Rowe, R.; Williams, A.M.; Milne, E. The role of cortical sensorimotor oscillations in action anticipation. Neuroimage 2017, 146, 1102–1114. [Google Scholar] [CrossRef]
- Li, L.; Smith, D.M. Neural Efficiency in Athletes: A Systematic Review. Front. Behav. Neurosci. 2021, 15, 167. [Google Scholar] [CrossRef]
- Del Percio, C.; Franzetti, M.; De Matti, A.J.; Noce, G.; Lizio, R.; Lopez, S.; Soricelli, A.; Ferri, R.; Pascarelli, M.T.; Rizzo, M.; et al. Football Players Do Not Show “Neural Efficiency” in Cortical Activity Related to Visuospatial Information Processing During Football Scenes: An EEG Mapping Study. Front. Psychol. 2019, 10, 433621. [Google Scholar] [CrossRef]
- Haufler, A.J.; Spalding, T.W.; Maria, D.S.; Hatfield, B.D. Neuro-cognitive activity during a self-paced visuospatial task: Comparative EEG profiles in marksmen and novice shooters. Biol. Psychol. 2000, 53, 131–160. [Google Scholar] [CrossRef] [PubMed]
- Janelle, C.M.; Hillman, C.H.; Apparies, R.J.; Murray, N.P.; Meili, L.; Fallon, E.A.; Hatfield, B.D. Expertise differences in cortical activation and gaze behavior during rifle shooting. J. Sport Exerc. Psychol. 2000, 22, 167–182. [Google Scholar] [CrossRef]
- Hillman, C.H.; Apparies, R.J.; Janelle, C.M.; Hatfield, B.D. An electrocortical comparison of executed and rejected shots in skilled marksmen. Biol. Psychol. 2000, 52, 71–83. [Google Scholar] [CrossRef]
- Hatfield, B.D.; Landers, D.M.; Ray, W.J. Cognitive Processes During Self-Paced Motor Performance: An Electroencephalographic Profile of Skilled Marksmen. J. Sport Psychol. 1984, 6, 42–59. [Google Scholar] [CrossRef]
- Deeny, S.P.; Haufler, A.J.; Saffer, M.; Hatfield, B.D. Electroencephalographic Coherence During Visuomotor Performance: A Comparison of Cortico-Cortical Communication in Experts and Novices. J. Mot. Behav. 2009, 41, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Masters, R.; Maxwell, J. The theory of reinvestment. Int. Rev. Sport Exerc. Psychol. 2008, 1, 160–183. [Google Scholar] [CrossRef]
- Schläppi-Lienhard, O.; Hossner, E.-J. Decision making in beach volleyball defense: Crucial factors derived from interviews with top-level experts. Psychol. Sport Exerc. 2015, 16, 60–73. [Google Scholar] [CrossRef]
- Balser, N.; Lorey, B.; Pilgramm, S.; Stark, R.; Bischoff, M.; Zentgraf, K.; Williams, A.M.; Munzert, J. Prediction of human actions: Expertise and task-related effects on neural activation of the action observation network. Hum. Brain Mapp. 2014, 35, 4016–4034. [Google Scholar] [CrossRef]
- Balser, N.; Lorey, B.; Pilgramm, S.; Naumann, T.; Kindermann, S.; Stark, R.; Zentgraf, K.; Williams, A.M.; Munzert, J. The influence of expertise on brain activation of the action observation network during anticipation of tennis and volleyball serves. Front. Hum. Neurosci. 2014, 8, 568. [Google Scholar] [CrossRef]
- Babiloni, C.; Marzano, N.; Infarinato, F.; Iacoboni, M.; Rizza, G.; Aschieri, P.; Cibelli, G.; Soricelli, A.; Eusebi, F.; Del Percio, C. “Neural efficiency” of experts’ brain during judgment of actions: A high-resolution EEG study in elite and amateur karate athletes. Behav. Brain Res. 2010, 207, 466–475. [Google Scholar] [CrossRef]
- Kamiński, J.; Brzezicka, A.; Gola, M.; Wróbel, A. Beta band oscillations engagement in human alertness process. Int. J. Psychophysiol. 2012, 85, 125–128. [Google Scholar] [CrossRef]
- Battaglini, L.; Ghiani, A.; Casco, C.; Ronconi, L. Parietal tACS at beta frequency improves vision in a crowding regime. Neuroimage 2020, 208, 116451. [Google Scholar] [CrossRef]
- Ronconi, L.; Marotti, R.B. Awareness in the crowd: Beta power and alpha phase of prestimulus oscillations predict object discrimination in visual crowding. Conscious. Cogn. 2017, 54, 36–46. [Google Scholar] [CrossRef]
- Iwatsuki, T.; Wright, P. Relationships among Movement Reinvestment, Decision-Making Reinvestment, and Perceived Choking. Int. J. Coach. Sci. 2016, 10, 25–35. Available online: https://www.dbpia.co.kr/Journal/articleDetail?nodeId=NODE06602976 (accessed on 14 September 2020).
- Jackson, R.C.; Ashford, K.J.; Norsworthy, G. Attentional Focus, Dispositional Reinvestment, and Skilled Motor Performance under Pressure. J. Sport Exerc. Psychol. 2006, 28, 49–68. [Google Scholar] [CrossRef]
- Kal, E.; van der Kamp, J.; Houdijk, H. External attentional focus enhances movement automatization: A comprehensive test of the constrained action hypothesis. Hum. Mov. Sci. 2013, 32, 527–539. [Google Scholar] [CrossRef]
- Smeeton, N.J.; Williams, A.M.; Hodges, N.J.; Ward, P. The Relative Effectiveness of Various Instructional Approaches in Developing Anticipation Skill. J. Exp. Psychol. Appl. 2005, 11, 98–110. [Google Scholar] [CrossRef]
- Anticipating deceptive movements in rugby union: The role of reinvestment. J. Sport Exerc. Sci. 2019, 3, 14–20. [CrossRef]
- Mann, D.L.; Abernethy, B.; Farrow, D. Visual information underpinning skilled anticipation: The effect of blur on a coupled and uncoupled in situ anticipatory response. Atten. Percept. Psychophys. 2010, 72, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Causer, J.; Smeeton, N.J.; Williams, A.M. Expertise differences in anticipatory judgements during a temporally and spatially occluded task. PLoS ONE 2017, 12, e0171330. [Google Scholar] [CrossRef]



| DV | Fixed Effect | df | F | p | η2 | R2 |
|---|---|---|---|---|---|---|
| Performance | Skill | 1, 32 | 17.02 | <0.001 | 0.167 | 0.504 |
| Condition | 1, 32 | 4.56 | 0.041 | 0.051 | ||
| Occlusion | 1, 32 | 72.15 | <0.001 | 0.459 | ||
| Skill × Condition | 1, 32 | <0.01 | 0.950 | <0.001 | ||
| Skill × Occlusion | 1, 32 | 1.11 | 0.300 | 0.013 | ||
| Condition × Occlusion | 1, 31 | 0.02 | 0.894 | <0.001 | ||
| Skill × Condition × Hemisphere | 1, 31 | 0.27 | 0.608 | 0.003 |
| DV | Epoch | Fixed Effect | df | F | p | η2 | R2 |
|---|---|---|---|---|---|---|---|
| Alpha Power | 1 | Skill | 1, 31 | 0.96 | 0.336 | 0.025 | 0.184 |
| Hemisphere | 1, 31 | 13.05 | 0.001 | 0.257 | |||
| Condition | 1, 30 | 0.02 | 0.891 | 0.001 | |||
| Skill × Hemisphere | 1, 31 | 11.18 | 0.002 | 0.228 | |||
| Skill × Condition | 1, 30 | 0.25 | 0.624 | 0.006 | |||
| Condition × Hemisphere | 1, 30 | 1.26 | 0.271 | 0.032 | |||
| Skill × Condition × Hemisphere | 1, 30 | 1.04 | 0.316 | 0.027 | |||
| 2 | Skill | 1, 31 | 1.43 | 0.240 | 0.033 | 0.147 | |
| Hemisphere | 1, 31 | 26.58 | <0.001 | 0.385 | |||
| Condition | 1, 30 | 0.18 | 0.678 | 0.004 | |||
| Skill × Hemisphere | 1, 31 | 14.15 | 0.001 | 0.250 | |||
| Skill × Condition | 1, 30 | 3.41 | 0.075 | 0.074 | |||
| Condition × Hemisphere | 1, 30 | 0.15 | 0.697 | 0.004 | |||
| Skill × Condition × Hemisphere | 1, 30 | 1.57 | 0.220 | 0.036 | |||
| Beta Power | 1 | Skill | 1, 31 | 2.73 | 0.108 | 0.066 | 0.304 |
| Hemisphere | 1, 31 | 36.16 | <0.001 | 0.481 | |||
| Condition | 1, 30 | 3.07 | 0.090 | 0.073 | |||
| Skill × Hemisphere | 1, 31 | 5.67 | 0.024 | 0.127 | |||
| Skill × Condition | 1, 30 | 0.02 | 0.899 | <0.001 | |||
| Condition × Hemisphere | 1, 30 | 0.12 | 0.736 | 0.003 | |||
| Skill × Condition × Hemisphere | 1, 30 | 0.06 | 0.807 | 0.002 | |||
| 2 | Skill | 1, 31 | 2.00 | 0.167 | 0.048 | 0.241 | |
| Hemisphere | 1, 31 | 35.14 | <0.001 | 0.472 | |||
| Condition | 1, 30 | 1.09 | 0.306 | 0.027 | |||
| Skill × Hemisphere | 1, 31 | 5.81 | 0.022 | 0.129 | |||
| Skill × Condition | 1, 30 | 0.43 | 0.515 | 0.011 | |||
| Condition × Hemisphere | 1, 30 | 0.30 | 0.591 | 0.007 | |||
| Skill × Condition × Hemisphere | 1, 30 | 0.28 | 0.603 | 0.007 | |||
| Beta–Alpha Power | 1 | Skill | 1, 31 | 0.95 | 0.337 | 0.026 | 0.128 |
| Hemisphere | 1, 31 | 14.25 | 0.001 | 0.288 | |||
| Condition | 1, 30 | 1.61 | 0.214 | 0.044 | |||
| Skill × Hemisphere | 1, 31 | 0.58 | 0.452 | 0.016 | |||
| Skill × Condition | 1, 30 | 0.07 | 0.792 | 0.002 | |||
| Condition × Hemisphere | 1, 30 | 0.50 | 0.483 | 0.014 | |||
| Skill × Condition × Hemisphere | 1, 30 | 0.51 | 0.482 | 0.014 | |||
| 2 | Skill | 1, 31 | <0.01 | 0.967 | <0.001 | 0.046 | |
| Hemisphere | 1, 31 | 8.30 | 0.007 | 0.182 | |||
| Condition | 1, 30 | 1.08 | 0.307 | 0.028 | |||
| Skill × Hemisphere | 1, 31 | 0.33 | 0.568 | 0.009 | |||
| Skill × Condition | 1, 30 | 0.11 | 0.742 | 0.003 | |||
| Condition × Hemisphere | 1, 30 | 1.30 | 0.263 | 0.034 | |||
| Skill × Condition × Hemisphere | 1, 30 | 0.12 | 0.729 | 0.003 |
| Dependent Variable | Effect/Interaction | Factor Levels | Post Hoc Comparison | β | SE | p | d |
|---|---|---|---|---|---|---|---|
| Alpha Power Epoch 1 | Skill × Hemisphere | Left Hemisphere | Skilled ≈ Less Skilled | 0.066 | 0.077 | 0.520 | 0.15 |
| Right Hemisphere | Skilled < Less Skilled | 0.190 | 0.077 | 0.032 | 0.44 | ||
| Skilled | Left H > Right H | 0.267 | 0.057 | <0.001 | 0.83 | ||
| Less Skilled | Left H ≈ Right H | 0.010 | 0.055 | 0.852 | 0.03 | ||
| Alpha Power Epoch 2 | Skill × Hemisphere | Left Hemisphere | Skilled ≈ Less Skilled | 0.005 | 0.107 | 0.960 | 0.01 |
| Right Hemisphere | Skilled ≈ Less Skilled | 0.241 | 0.107 | 0.060 | 0.40 | ||
| Skilled | Left H > Right H | 0.292 | 0.049 | <0.001 | 1.10 | ||
| Less Skilled | Left H ≈ Right H | 0.046 | 0.047 | 0.453 | 0.17 | ||
| Beta Power Epoch 1 | Skill × Hemisphere | Left Hemisphere | Skilled ≈ Less Skilled | 0.011 | 0.084 | 0.903 | 0.02 |
| Right Hemisphere | Skilled < Less Skilled | 0.218 | 0.084 | 0.022 | 0.46 | ||
| Skilled | Left H > Right H | 0.365 | 0.065 | <0.001 | 1.03 | ||
| Less Skilled | Left H ≈ Right H | 0.158 | 0.063 | 0.022 | 0.45 | ||
| Beta Power Epoch 2 | Skill × Hemisphere | Left Hemisphere | Skilled ≈ Less Skilled | 0.015 | 0.099 | 0.879 | 0.03 |
| Right Hemisphere | Skilled < Less Skilled | 0.226 | 0.099 | 0.036 | 0.41 | ||
| Skilled | Left H > Right H | 0.364 | 0.065 | <0.001 | 1.03 | ||
| Less Skilled | Left H > Right H | 0.154 | 0.063 | 0.036 | 0.43 | ||
| Beta–Alpha Power Epoch 1 | Hemisphere | Left H > Right H | 0.123 | 0.034 | 0.001 | 0.46 | |
| Beta–Alpha Power Epoch 2 | Hemisphere | Left H > Right H | 0.090 | 0.032 | 0.009 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeCouto, B.S.; Smeeton, N.J.; Williams, A.M. Skilled Performers Show Right Parietal Lateralization during Anticipation of Volleyball Attacks. Brain Sci. 2023, 13, 1204. https://doi.org/10.3390/brainsci13081204
DeCouto BS, Smeeton NJ, Williams AM. Skilled Performers Show Right Parietal Lateralization during Anticipation of Volleyball Attacks. Brain Sciences. 2023; 13(8):1204. https://doi.org/10.3390/brainsci13081204
Chicago/Turabian StyleDeCouto, Brady S., Nicholas J. Smeeton, and A. Mark Williams. 2023. "Skilled Performers Show Right Parietal Lateralization during Anticipation of Volleyball Attacks" Brain Sciences 13, no. 8: 1204. https://doi.org/10.3390/brainsci13081204
APA StyleDeCouto, B. S., Smeeton, N. J., & Williams, A. M. (2023). Skilled Performers Show Right Parietal Lateralization during Anticipation of Volleyball Attacks. Brain Sciences, 13(8), 1204. https://doi.org/10.3390/brainsci13081204





