Gamma-Aminobutyric Acid and Glutamate Concentrations in the Striatum and Anterior Cingulate Cortex Not Found to Be Associated with Cognitive Flexibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Experimental Subjects and Ethical Approval
2.1.2. Experimental Design and Procedures
2.2. MRS Data Acquisition and Quantification
2.3. Experimental Tasks
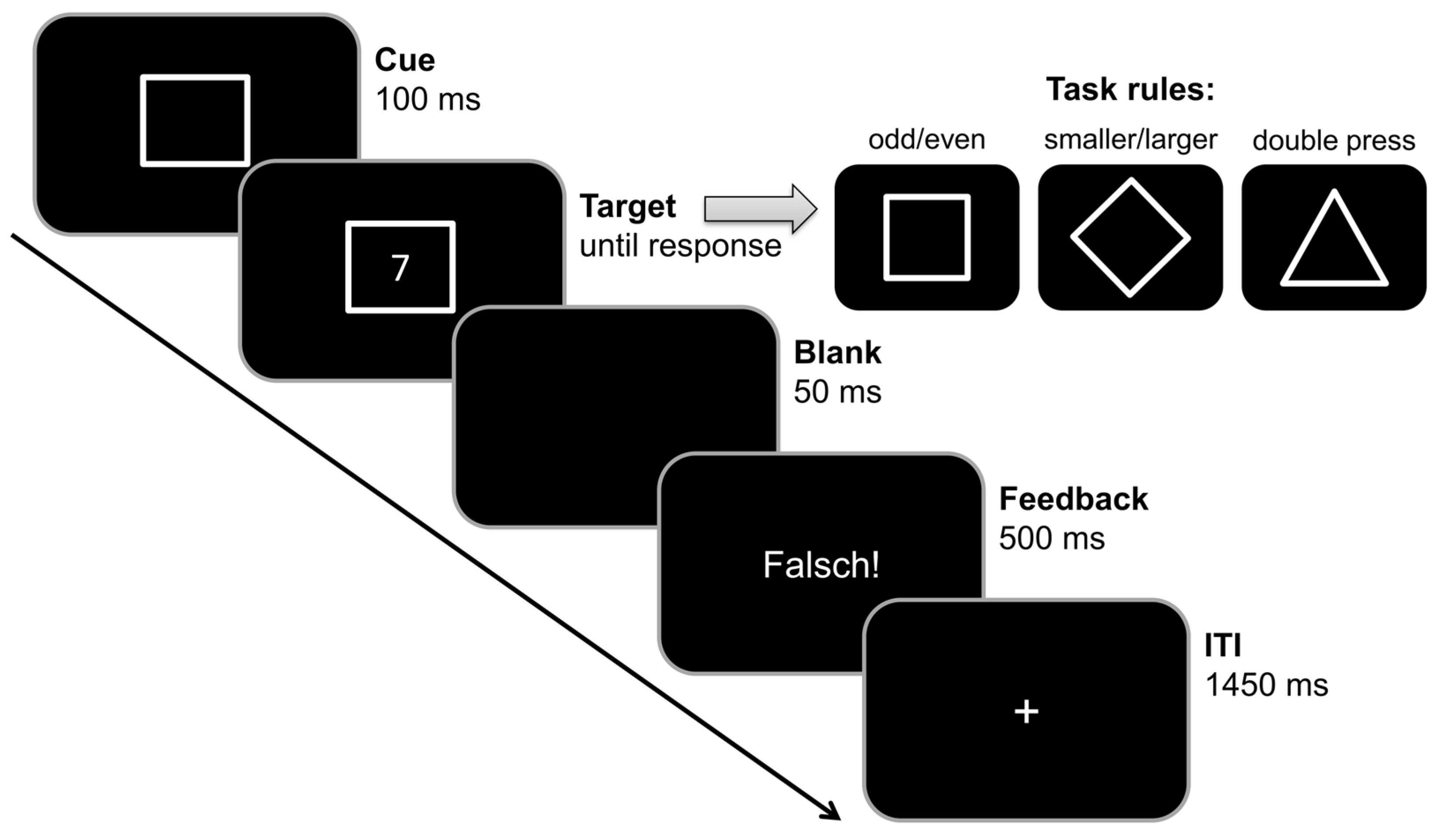
2.3.1. Task Switching Paradigm
2.3.2. Backward Inhibition Paradigm
2.4. Statistics
3. Results
3.1. Exclusion of Participants and Outlier Values
3.1.1. Task Switching Paradigm: Sample Characterization
3.1.2. Task Switching Paradigm: Behavioral Data
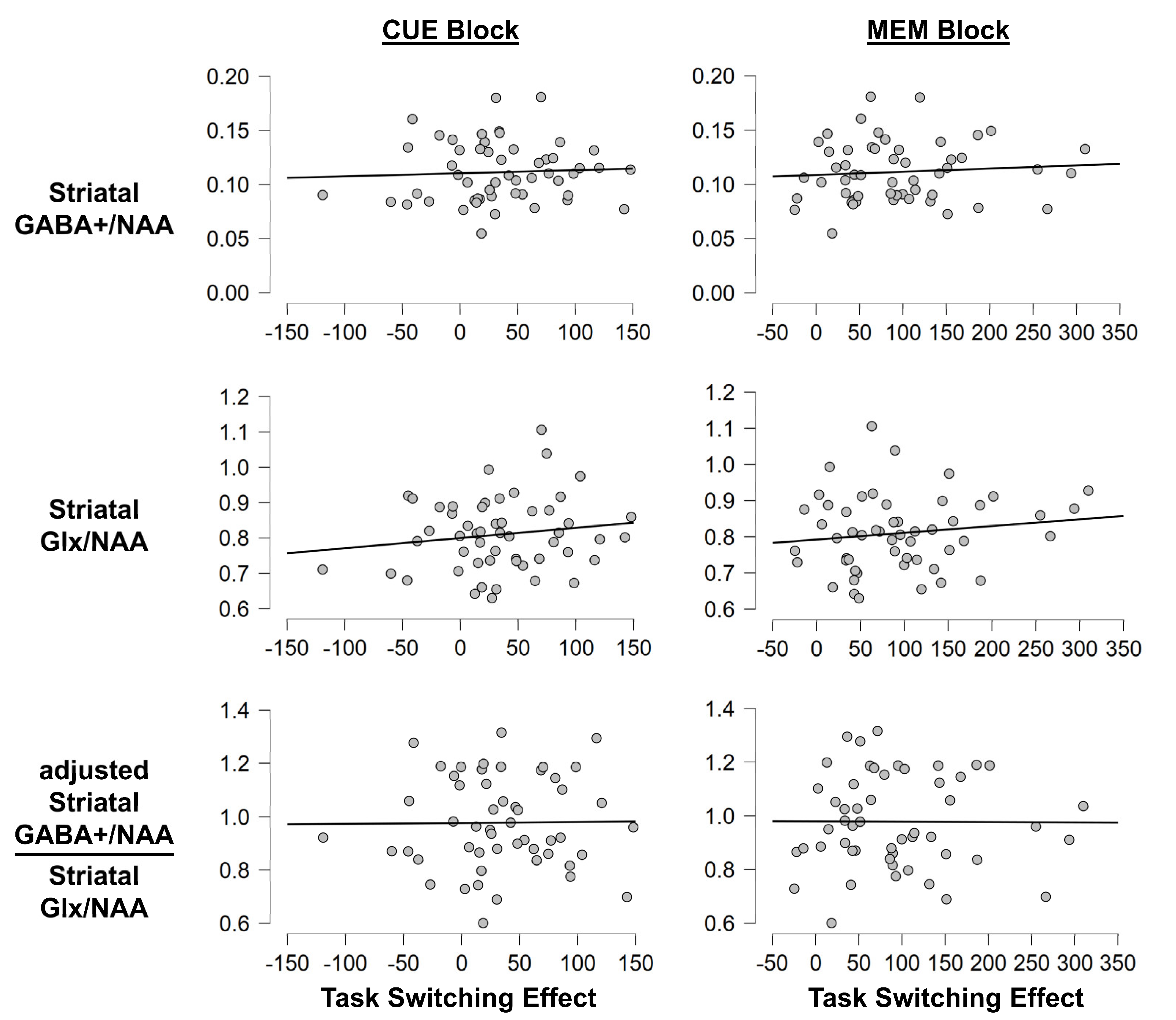
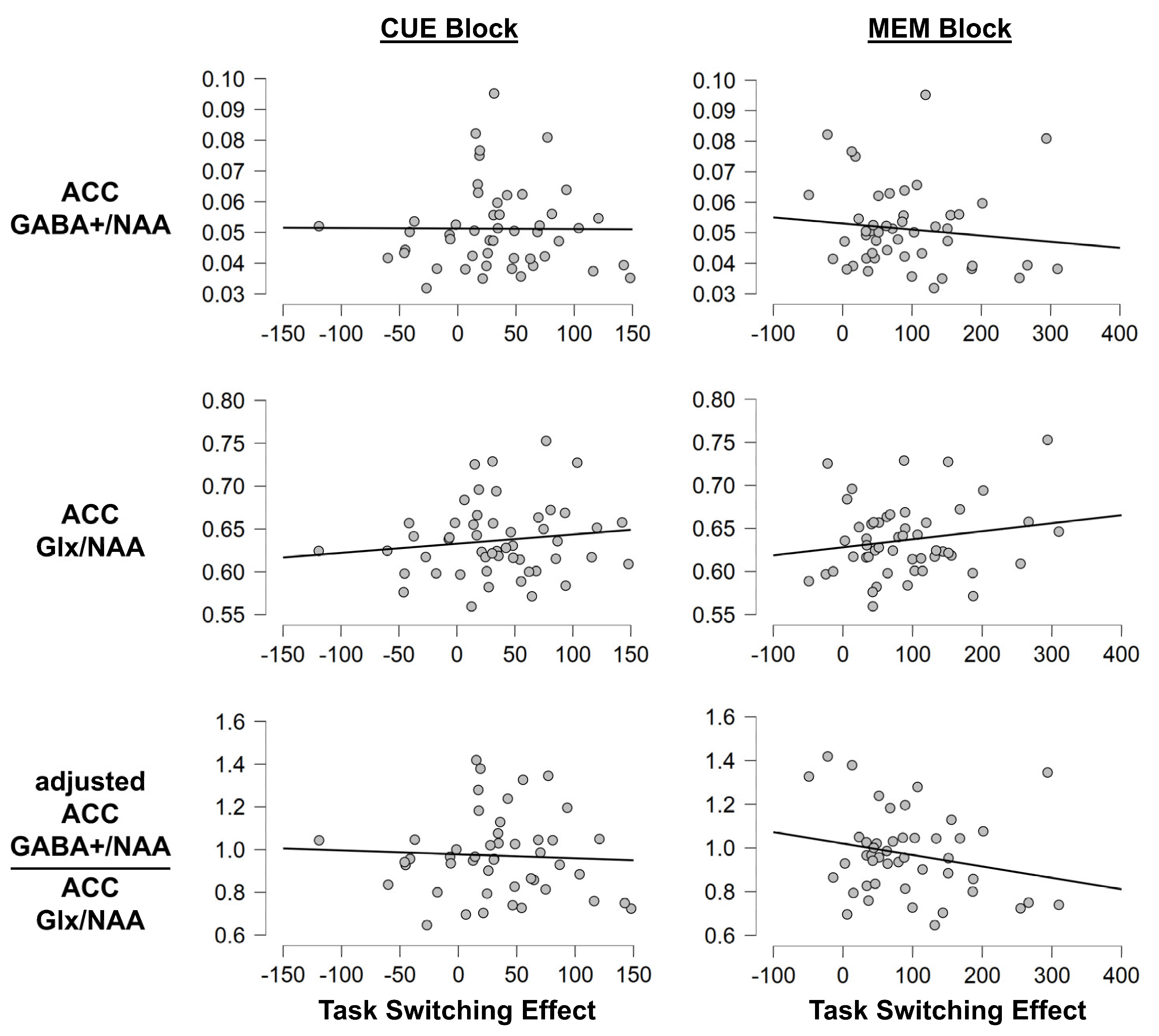
3.1.3. Task Switching Paradigm: MRS Measures
3.1.4. Mayr Switch Paradigm: Sample Characterization
3.1.5. Mayr Switch Paradigm: Behavioral Data
3.1.6. Mayr Switch Paradigm: MRS Measures
3.2. Summary of Main Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Kiesel, A.; Steinhauser, M.; Wendt, M.; Falkenstein, M.; Jost, K.; Philipp, A.M.; Koch, I. Control and Interference in Task Switching—A Review. Psychol. Bull. 2010, 136, 849–874. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Revisiting the Effects of Parkinson’s Disease and Frontal Lobe Lesions on Task Switching: The Role of Rule Reconfiguration. J. Neuropsychol. 2014, 8, 53–74. [Google Scholar] [CrossRef]
- Langley, C.; Gregory, S.; Osborne-Crowley, K.; O’Callaghan, C.; Zeun, P.; Lowe, J.; Johnson, E.B.; Papoutsi, M.; Scahill, R.I.; Rees, G.; et al. Fronto-Striatal Circuits for Cognitive Flexibility in Far from Onset Huntington’s Disease: Evidence from the Young Adult Study. J. Neurol. Neurosurg. Psychiatry 2020, 92, 143–149. [Google Scholar] [CrossRef]
- Marquardt, K.; Josey, M.; Kenton, J.A.; Cavanagh, J.F.; Holmes, A.; Brigman, J.L. Impaired Cognitive Flexibility Following NMDAR-GluN2B Deletion Is Associated with Altered Orbitofrontal-Striatal Function. Neuroscience 2019, 404, 338–352. [Google Scholar] [CrossRef]
- Petruo, V.A.; Zeißig, S.; Schmelz, R.; Hampe, J.; Beste, C. Specific Neurophysiological Mechanisms Underlie Cognitive Inflexibility in Inflammatory Bowel Disease. Sci. Rep. 2017, 7, 13943. [Google Scholar] [CrossRef]
- Trempler, I.; Schiffer, A.-M.; El-Sourani, N.; Ahlheim, C.; Fink, G.R.; Schubotz, R.I. Frontostriatal Contribution to the Interplay of Flexibility and Stability in Serial Prediction. J. Cogn. Neurosci. 2017, 29, 298–309. [Google Scholar] [CrossRef]
- Vaghi, M.M.; Vértes, P.E.; Kitzbichler, M.G.; Apergis-Schoute, A.M.; van der Flier, F.E.; Fineberg, N.A.; Sule, A.; Zaman, R.; Voon, V.; Kundu, P.; et al. Specific Frontostriatal Circuits for Impaired Cognitive Flexibility and Goal-Directed Planning in Obsessive-Compulsive Disorder: Evidence from Resting-State Functional Connectivity. Biol. Psychiatry 2017, 81, 708–717. [Google Scholar] [CrossRef]
- Van Schouwenburg, M.R.; Onnink, A.M.H.; ter Huurne, N.; Kan, C.C.; Zwiers, M.P.; Hoogman, M.; Franke, B.; Buitelaar, J.K.; Cools, R. Cognitive Flexibility Depends on White Matter Microstructure of the Basal Ganglia. Neuropsychologia 2014, 53, 171–177. [Google Scholar] [CrossRef]
- Redgrave, P.; Vautrelle, N.; Reynolds, J.N.J. Functional Properties of the Basal Ganglia’s Re-Entrant Loop Architecture: Selection and Reinforcement. Neuroscience 2011, 198, 138–151. [Google Scholar] [CrossRef]
- Redgrave, P.; Rodriguez, M.; Smith, Y.; Rodriguez-Oroz, M.C.; Lehericy, S.; Bergman, H.; Agid, Y.; DeLong, M.R.; Obeso, J.A. Goal-Directed and Habitual Control in the Basal Ganglia: Implications for Parkinson’s Disease. Nat. Rev. Neurosci. 2010, 11, 760–772. [Google Scholar] [CrossRef]
- Redgrave, P.; Prescott, T.J.; Gurney, K. The Basal Ganglia: A Vertebrate Solution to the Selection Problem? Neuroscience 1999, 89, 1009–1023. [Google Scholar] [CrossRef]
- Bolam, J.P.; Hanley, J.J.; Booth, P.A.; Bevan, M.D. Synaptic Organisation of the Basal Ganglia. J. Anat. 2000, 196 Pt 4, 527–542. [Google Scholar] [CrossRef]
- Plenz, D. When Inhibition Goes Incognito: Feedback Interaction between Spiny Projection Neurons in Striatal Function. Trends Neurosci. 2003, 26, 436–443. [Google Scholar] [CrossRef]
- Beste, C.; Humphries, M.; Saft, C. Striatal Disorders Dissociate Mechanisms of Enhanced and Impaired Response Selection—Evidence from Cognitive Neurophysiology and Computational Modelling. NeuroImage Clin. 2014, 4, 623–634. [Google Scholar] [CrossRef]
- Gurney, K.; Prescott, T.J.; Redgrave, P. A Computational Model of Action Selection in the Basal Ganglia. II. Analysis and Simulation of Behaviour. Biol. Cybern. 2001, 84, 411–423. [Google Scholar] [CrossRef]
- Gurney, K.; Prescott, T.J.; Wickens, J.R.; Redgrave, P. Computational Models of the Basal Ganglia: From Robots to Membranes. Trends Neurosci. 2004, 27, 453–459. [Google Scholar] [CrossRef]
- Humphries, M.D.; Stewart, R.D.; Gurney, K.N. A Physiologically Plausible Model of Action Selection and Oscillatory Activity in the Basal Ganglia. J. Neurosci. 2006, 26, 12921–12942. [Google Scholar] [CrossRef]
- Tomkins, A.; Vasilaki, E.; Beste, C.; Gurney, K.; Humphries, M.D. Transient and Steady-State Selection in the Striatal Microcircuit. Front. Comput. Neurosci. 2014, 7, 192. [Google Scholar] [CrossRef]
- Bar-Gad, I.; Morris, G.; Bergman, H. Information Processing, Dimensionality Reduction and Reinforcement Learning in the Basal Ganglia. Prog. Neurobiol. 2003, 71, 439–473. [Google Scholar] [CrossRef]
- Clarke, H.F.; Dalley, J.W.; Crofts, H.S.; Robbins, T.W.; Roberts, A.C. Cognitive Inflexibility after Prefrontal Serotonin Depletion. Science 2004, 304, 878–880. [Google Scholar] [CrossRef]
- Lapiz, M.D.S.; Morilak, D.A. Noradrenergic Modulation of Cognitive Function in Rat Medial Prefrontal Cortex as Measured by Attentional Set Shifting Capability. Neuroscience 2006, 137, 1039–1049. [Google Scholar] [CrossRef]
- Crofts, H.S.; Dalley, J.W.; Collins, P.; Van Denderen, J.C.; Everitt, B.J.; Robbins, T.W.; Roberts, A.C. Differential Effects of 6-OHDA Lesions of the Frontal Cortex and Caudate Nucleus on the Ability to Acquire an Attentional Set. Cereb. Cortex 2001, 11, 1015–1026. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Murray, G.K.; Robbins, T.W. Learning and Cognitive Flexibility: Frontostriatal Function and Monoaminergic Modulation. Curr. Opin. Neurobiol. 2010, 20, 199–204. [Google Scholar] [CrossRef]
- Tepper, J.M.; Bolam, J.P. Functional Diversity and Specificity of Neostriatal Interneurons. Curr. Opin. Neurobiol. 2004, 14, 685–692. [Google Scholar] [CrossRef]
- Calabresi, P.; Centonze, D.; Gubellini, P.; Marfia, G.A.; Pisani, A.; Sancesario, G.; Bernardi, G. Synaptic Transmission in the Striatum: From Plasticity to Neurodegeneration. Prog. Neurobiol. 2000, 61, 231–265. [Google Scholar] [CrossRef]
- Beste, C.; Saft, C.; Güntürkün, O.; Falkenstein, M. Increased Cognitive Functioning in Symptomatic Huntington’s Disease as Revealed by Behavioral and Event-Related Potential Indices of Auditory Sensory Memory and Attention. J. Neurosci. 2008, 28, 11695–11702. [Google Scholar] [CrossRef]
- Haag, L.; Quetscher, C.; Dharmadhikari, S.; Dydak, U.; Schmidt-Wilcke, T.; Beste, C. Interrelation of Resting State Functional Connectivity, Striatal GABA Levels, and Cognitive Control Processes. Hum. Brain Mapp. 2015, 36, 4383–4393. [Google Scholar] [CrossRef]
- Quetscher, C.; Yildiz, A.; Dharmadhikari, S.; Glaubitz, B.; Schmidt-Wilcke, T.; Dydak, U.; Beste, C. Striatal GABA-MRS Predicts Response Inhibition Performance and Its Cortical Electrophysiological Correlates. Brain Struct. Funct. 2015, 220, 3555–3564. [Google Scholar] [CrossRef]
- Yildiz, A.; Quetscher, C.; Dharmadhikari, S.; Chmielewski, W.; Glaubitz, B.; Schmidt-Wilcke, T.; Edden, R.; Dydak, U.; Beste, C. Feeling Safe in the Plane: Neural Mechanisms Underlying Superior Action Control in Airplane Pilot Trainees--a Combined EEG/MRS Study. Hum. Brain Mapp. 2014, 35, 5040–5051. [Google Scholar] [CrossRef]
- Chudasama, Y.; Robbins, T.W. Functions of Frontostriatal Systems in Cognition: Comparative Neuropsychopharmacological Studies in Rats, Monkeys and Humans. Biol. Psychol. 2006, 73, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Middleton, F.A.; Strick, P.L. Basal Ganglia Output and Cognition: Evidence from Anatomical, Behavioral, and Clinical Studies. Brain Cogn. 2000, 42, 183–200. [Google Scholar] [CrossRef]
- Parvizi, J. Corticocentric Myopia: Old Bias in New Cognitive Sciences. Trends Cogn. Sci. 2009, 13, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Bernácer, J.; Prensa, L.; Giménez-Amaya, J.M. Distribution of GABAergic Interneurons and Dopaminergic Cells in the Functional Territories of the Human Striatum. PLoS ONE 2012, 7, e30504. [Google Scholar] [CrossRef] [PubMed]
- Whissell, P.D.; Cajanding, J.D.; Fogel, N.; Kim, J.C. Comparative Density of CCK- and PV-GABA Cells within the Cortex and Hippocampus. Front. Neuroanat. 2015, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.E.; Sherfey, J.S.; Kopell, N.J.; Whittington, M.A.; LeBeau, F.E.N. Hetereogeneity in Neuronal Intrinsic Properties: A Possible Mechanism for Hub-Like Properties of the Rat Anterior Cingulate Cortex during Network Activity. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Levar, N.; Van Doesum, T.J.; Denys, D.; Van Wingen, G.A. Anterior Cingulate GABA and Glutamate Concentrations Are Associated with Resting-State Network Connectivity. Sci. Rep. 2019, 9, 2116. [Google Scholar] [CrossRef]
- Silveri, M.M.; Sneider, J.T.; Crowley, D.J.; Covell, M.J.; Acharya, D.; Rosso, I.M.; Jensen, J.E. Frontal Lobe γ-Aminobutyric Acid Levels during Adolescence: Associations with Impulsivity and Response Inhibition. Biol. Psychiatry 2013, 74, 296–304. [Google Scholar] [CrossRef]
- Monsell, S. Task Switching. Trends Cogn. Sci. 2003, 7, 134–140. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Hengstler, J.G.; Golka, K.; Falkenstein, M.; Beste, C. The Met-Allele of the BDNF Val66Met Polymorphism Enhances Task Switching in Elderly. Neurobiol. Aging 2011, 32, 2327.e7–2327.e19. [Google Scholar] [CrossRef]
- D’Esposito, M.; Postle, B.R. The Cognitive Neuroscience of Working Memory. Annu. Rev. Psychol. 2015, 66, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Panegyres, P.K. The Contribution of the Study of Neurodegenerative Disorders to the Understanding of Human Memory. QJM 2004, 97, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Dajani, D.R.; Uddin, L.Q. Demystifying Cognitive Flexibility: Implications for Clinical and Developmental Neuroscience. Trends Neurosci. 2015, 38, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Mayr, U.; Keele, S.W. Changing Internal Constraints on Action: The Role of Backward Inhibition. J. Exp. Psychol. Gen. 2000, 129, 4–26. [Google Scholar] [CrossRef]
- Allport, A.; Styles, E.A.; Hsieh, S. Shifting Intentional Set: Exploring the Dynamic Control of Task. In Attention and Performance XV: Conscious and Nonconscious Information Processing; Umiltà, C., Moscovitch, M., Eds.; MIT Press: Cambridge, MA, USA, 1994; pp. 421–452. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Chmielewski, W.X.; Beste, C. Testing Interactive Effects of Automatic and Conflict Control Processes during Response Inhibition—A System Neurophysiological Study. Neuroimage 2017, 146, 1149–1156. [Google Scholar] [CrossRef]
- Heil, M. The Functional Significance of ERP Effects during Mental Rotation. Psychophysiology 2002, 39, 535–545. [Google Scholar] [CrossRef]
- Bensmann, W.; Zink, N.; Werner, A.; Beste, C.; Stock, A.-K. Acute Alcohol Effects on Response Inhibition Depend on Response Automatization, but Not on GABA or Glutamate Levels in the ACC and Striatum. J. Clin. Med. 2020, 9, 481. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Barker, P.B.; Bhattacharyya, P.K.; Brix, M.K.; Buur, P.F.; Cecil, K.M.; Chan, K.L.; Chen, D.Y.-T.; Craven, A.R.; Cuypers, K.; et al. Big GABA: Edited MR Spectroscopy at 24 Research Sites. Neuroimage 2017, 159, 32–45. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Bolam, J.P. The Neuroanatomical Organization of the Basal Ganglia. In Handbook of Basal Ganglia Structure and Function; Steiner, H., Tseng, K.-Y., Eds.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2010; pp. 3–28. [Google Scholar]
- Iversen, L.L. Dopamine Handbook; Oxford University Press: Oxford, UK, 2010; ISBN 978-0-19-537303-5. [Google Scholar]
- Mikkelsen, M.; Rimbault, D.L.; Barker, P.B.; Bhattacharyya, P.K.; Brix, M.K.; Buur, P.F.; Cecil, K.M.; Chan, K.L.; Chen, D.Y.-T.; Craven, A.R.; et al. Big GABA II: Water-Referenced Edited MR Spectroscopy at 25 Research Sites. Neuroimage 2019, 191, 537–548. [Google Scholar] [CrossRef]
- Edden, R.A.E.; Puts, N.A.J.; Harris, A.D.; Barker, P.B.; Evans, C.J. Gannet: A Batch-Processing Tool for the Quantitative Analysis of Gamma-Aminobutyric Acid–Edited MR Spectroscopy Spectra. J. Magn. Reson. Imaging 2014, 40, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Marjańska, M.; Terpstra, M. Influence of Fitting Approaches in LCModel on MRS Quantification Focusing on Age-Specific Macromolecules and the Spline Baseline. NMR Biomed. 2021, 34, e4197. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.; Gussek, P.; Stock, A.-K.; Beste, C. Effects of High-Dose Ethanol Intoxication and Hangover on Cognitive Flexibility. Addict. Biol. 2018, 23, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.; Mückschel, M.; Ziemssen, T.; Beste, C. The Role of Phasic Norepinephrine Modulations during Task Switching: Evidence for Specific Effects in Parietal Areas. Brain Struct. Funct. 2017, 223, 925–940. [Google Scholar] [CrossRef]
- Koch, I.; Gade, M.; Philipp, A.M. Inhibition of Response Mode in Task Switching. Exp. Psychol. 2004, 51, 52–58. [Google Scholar] [CrossRef]
- Zhang, R.; Stock, A.-K.; Fischer, R.; Beste, C. The System Neurophysiological Basis of Backward Inhibition. Brain Struct. Funct. 2016, 221, 4575–4587. [Google Scholar] [CrossRef]
- Zink, N.; Bensmann, W.; Arning, L.; Stock, A.-K.; Beste, C. CHRM2 Genotype Affects Inhibitory Control Mechanisms During Cognitive Flexibility. Mol. Neurobiol. 2019, 56, 6134–6141. [Google Scholar] [CrossRef]
- Koch, I.; Philipp, A.M. Effects of Response Selection on the Task Repetition Benefit in Task Switching. Mem. Cogn. 2005, 33, 624–634. [Google Scholar] [CrossRef]
- Philipp, A.M.; Jolicoeur, P.; Falkenstein, M.; Koch, I. Response Selection and Response Execution in Task Switching: Evidence from a Go-Signal Paradigm. J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 1062. [Google Scholar] [CrossRef]
- Schuch, S.; Koch, I. The Role of Response Selection for Inhibition of Task Sets in Task Shifting. J. Exp. Psychol. Hum. Percept. Perform. 2003, 29, 92–105. [Google Scholar] [CrossRef]
- Steinhauser, M.; Hübner, R. Response-Based Strengthening in Task Shifting: Evidence from Shift Effects Produced by Errors. J. Exp. Psychol. Hum. Percept. Perform. 2006, 32, 517. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, M.; Hübner, R. How Task Errors Affect Subsequent Behavior: Evidence from Distributional Analyses of Task-Switching Effects. Mem. Cogn. 2008, 36, 979–990. [Google Scholar] [CrossRef]
- Vandierendonck, A.; Liefooghe, B.; Verbruggen, F. Task Switching: Interplay of Reconfiguration and Interference Control. Psychol. Bull. 2010, 136, 601. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Liefooghe, B.; Szmalec, A.; Vandierendonck, A. Inhibiting Responses When Switching: Does It Matter? Exp. Psychol. 2005, 52, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Robbins, T.W. Inhibition and Impulsivity: Behavioral and Neural Basis of Response Control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T. Catecholamine Influences on Dorsolateral Prefrontal Cortical Networks. Biol. Psychiatry 2011, 69, e89–e99. [Google Scholar] [CrossRef]
- Robbins, T.W.; Arnsten, A.F.T. The Neuropsychopharmacology of Fronto-Executive Function: Monoaminergic Modulation. Annu. Rev. Neurosci. 2009, 32, 267–287. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Surmeier, D.J. Modulation of Striatal Projection Systems by Dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Carrillo-Reid, L.; Bargas, J. Dopaminergic Modulation of Striatal Neurons, Circuits, and Assemblies. Neuroscience 2011, 198, 3–18. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Zhang, J.; Wang, Z.; Xu, J.; Li, Y.; Yang, Z.; Liu, D. Abnormal Concentration of GABA and Glutamate in The Prefrontal Cortex in Schizophrenia—An in Vivo 1H-MRS Study. Shanghai Arch. Psychiatry 2017, 29, 277–286. [Google Scholar] [CrossRef]
- Satoh, H.; Suzuki, H.; Saitow, F. Downregulation of Dopamine D1-like Receptor Pathways of GABAergic Interneurons in the Anterior Cingulate Cortex of Spontaneously Hypertensive Rats. Neuroscience 2018, 394, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Lalchandani, R.R.; van der Goes, M.-S.; Partridge, J.G.; Vicini, S. Dopamine D2 Receptors Regulate Collateral Inhibition between Striatal Medium Spiny Neurons. J. Neurosci. 2013, 33, 14075–14086. [Google Scholar] [CrossRef]
- Ghisleni, C.; Bollmann, S.; Poil, S.-S.; Brandeis, D.; Martin, E.; Michels, L.; O’Gorman, R.L.; Klaver, P. Subcortical Glutamate Mediates the Reduction of Short-Range Functional Connectivity with Age in a Developmental Cohort. J. Neurosci. 2015, 35, 8433–8441. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.; Glass, Ä.; Kropp, P.; Müller-Hilke, B. Semantic Fluency Including Task Switching Predicts Academic Success in Medical School. PLoS ONE 2020, 15, e0244456. [Google Scholar] [CrossRef]
- Marjańska, M.; Lehéricy, S.; Valabrègue, R.; Popa, T.; Worbe, Y.; Russo, M.; Auerbach, E.J.; Grabli, D.; Bonnet, C.; Gallea, C.; et al. Brain Dynamic Neurochemical Changes in Dystonic Patients: A Magnetic Resonance Spectroscopy Study. Mov. Disord. 2013, 28, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Beaulé, V.; Proulx, S.; Lafleur, L.-P.; Doyon, J.; Marjańska, M.; Théoret, H. The Use of Magnetic Resonance Spectroscopy as a Tool for the Measurement of Bi-Hemispheric Transcranial Electric Stimulation Effects on Primary Motor Cortex Metabolism. J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Provencher, S.W. Estimation of Metabolite Concentrations from Localized in Vivo Proton NMR Spectra. Magn. Reason. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef]
- Kreis, R.; Bolliger, C.S. The Need for Updates of Spin System Parameters, Illustrated for the Case of γ-Aminobutyric Acid. NMR Biomed. 2012, 25, 1401–1403. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stock, A.-K.; Werner, A.; Kuntke, P.; Petasch, M.-S.; Bensmann, W.; Zink, N.; Koyun, A.H.; Quednow, B.B.; Beste, C. Gamma-Aminobutyric Acid and Glutamate Concentrations in the Striatum and Anterior Cingulate Cortex Not Found to Be Associated with Cognitive Flexibility. Brain Sci. 2023, 13, 1192. https://doi.org/10.3390/brainsci13081192
Stock A-K, Werner A, Kuntke P, Petasch M-S, Bensmann W, Zink N, Koyun AH, Quednow BB, Beste C. Gamma-Aminobutyric Acid and Glutamate Concentrations in the Striatum and Anterior Cingulate Cortex Not Found to Be Associated with Cognitive Flexibility. Brain Sciences. 2023; 13(8):1192. https://doi.org/10.3390/brainsci13081192
Chicago/Turabian StyleStock, Ann-Kathrin, Annett Werner, Paul Kuntke, Miriam-Sophie Petasch, Wiebke Bensmann, Nicolas Zink, Anna Helin Koyun, Boris B. Quednow, and Christian Beste. 2023. "Gamma-Aminobutyric Acid and Glutamate Concentrations in the Striatum and Anterior Cingulate Cortex Not Found to Be Associated with Cognitive Flexibility" Brain Sciences 13, no. 8: 1192. https://doi.org/10.3390/brainsci13081192
APA StyleStock, A.-K., Werner, A., Kuntke, P., Petasch, M.-S., Bensmann, W., Zink, N., Koyun, A. H., Quednow, B. B., & Beste, C. (2023). Gamma-Aminobutyric Acid and Glutamate Concentrations in the Striatum and Anterior Cingulate Cortex Not Found to Be Associated with Cognitive Flexibility. Brain Sciences, 13(8), 1192. https://doi.org/10.3390/brainsci13081192






