Antitumoral Activity of Molecular Hydrogen and Proton in the Treatment of Glioblastoma: An Atypical Pharmacology?
Abstract
1. Introduction
2. Hydrogen Homeostasis
3. Hydrogen and Protons as Therapeutic Approaches in Cancer
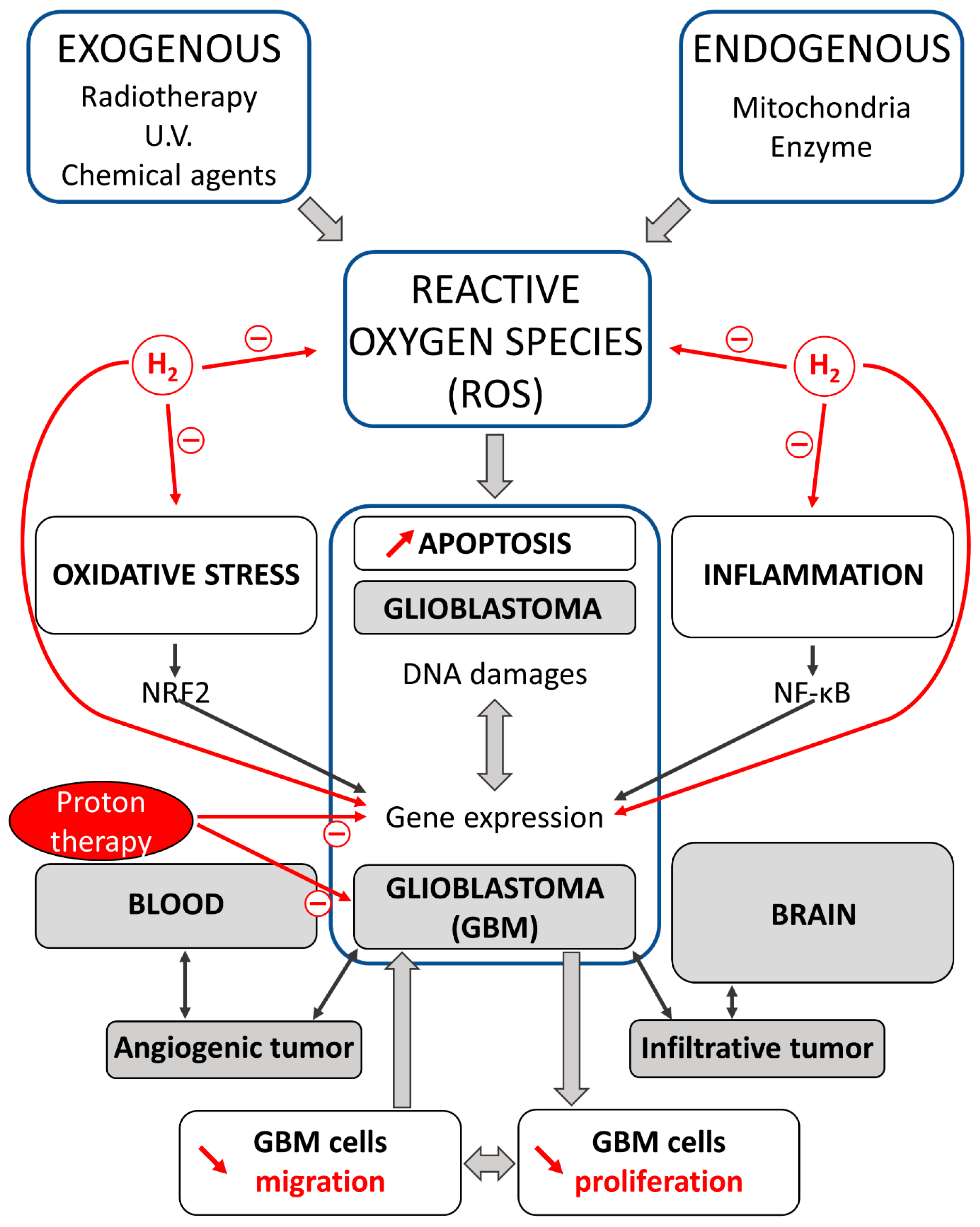
4. Hydrogen and Protons: Antitumor Agents in the Treatment of Glioblastoma (GBM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rochette, L.; Guenancia, C.; Gudjoncik, A.; Hachet, O.; Zeller, M.; Cottin, Y.; Vergely, C. Anthracyclines/trastuzumab: New aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol. Sci. 2015, 36, 326–348. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef] [PubMed]
- Domenicotti, C.; Marengo, B. Paradox Role of Oxidative Stress in Cancer: State of the Art. Antioxidants 2022, 11, 1027. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuca, K.; Musilek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Yang, H. Hydrogen: An Endogenous Regulator of Liver Homeostasis. Front. Pharmacol. 2020, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Antitumor Activity of Protons and Molecular Hydrogen: Underlying Mechanisms. Cancers 2021, 13, 893. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, M.; Chen, Z.; Wu, G.; Fujino, M.; Zhang, C.; Zhou, W.; Zhao, M.; Hirano, S.I.; Li, X.K.; et al. Hydrogen Gas Attenuates Hypoxic-Ischemic Brain Injury via Regulation of the MAPK/HO-1/PGC-1a Pathway in Neonatal Rats. Oxid. Med. Cell. Longev. 2020, 2020, 6978784. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Cardone, R.A.; Alfarouk, K.O.; Elliott, R.L.; Alqahtani, S.S.; Ahmed, S.B.M.; Aljarbou, A.N.; Greco, M.R.; Cannone, S.; Reshkin, S.J. The Role of Sodium Hydrogen Exchanger 1 in Dysregulation of Proton Dynamics and Reprogramming of Cancer Metabolism as a Sequela. Int. J. Mol. Sci. 2019, 20, 3694. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Park, R.; Nojima, H.; Kim, H.C.; Kim, Y.K.; Chung, J.H. Atomic hydrogen surrounded by water molecules, H(H2O)m, modulates basal and UV-induced gene expressions in human skin in vivo. PLoS ONE 2013, 8, e61696. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, J. Saturated hydrogen saline attenuates endotoxin-induced lung dysfunction. J. Surg. Res. 2015, 198, 41–49. [Google Scholar] [CrossRef]
- Sosa, V.; Moline, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; ME, L.L. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Ostrom, Q.T.; Cote, D. Epidemiology of Brain Tumors. Neurol. Clin. 2018, 36, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Xie, F.; Zhang, Y.; Wang, T.T.; Ma, S.N.; Zhao, P.X.; Zhang, X.; Lebaron, T.W.; Yan, X.L.; Ma, X.M. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem. Cell. Res. Ther. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Okumura, T.; Ishikawa, E.; Yamamoto, T.; Takano, S.; Matsumura, A.; Oshiro, Y.; Ishikawa, H.; Sakurai, H.; Tsuboi, K. Reirradiation for recurrent malignant brain tumor with radiotherapy or proton beam therapy. Technical considerations based on experience at a single institution. Strahlenther. Onkol. 2013, 189, 656–663. [Google Scholar] [CrossRef]
- Goff, K.M.; Zheng, C.; Alonso-Basanta, M. Proton radiotherapy for glioma and glioblastoma. Chin. Clin. Oncol. 2022, 11, 46. [Google Scholar] [CrossRef]
- Park, H.; Nam, K.S.; Lee, H.J.; Kim, K.S. Ionizing Radiation-Induced GDF15 Promotes Angiogenesis in Human Glioblastoma Models by Promoting VEGFA Expression Through p-MAPK1/SP1 Signaling. Front. Oncol. 2022, 12, 801230. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Insights Into Mechanisms of GDF15 and Receptor GFRAL: Therapeutic Targets. Trends Endocrinol. Metab 2020, 31, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Tian, W.; Jiang, R.; Lu, Y.; Sun, Q.; Fu, R.; He, Q.; Wang, J.; Liu, Y.; et al. ARRB1 inhibits non-alcoholic steatohepatitis progression by promoting GDF15 maturation. J. Hepatol. 2020, 72, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Harguindey, S.; Polo Orozco, J.; Alfarouk, K.O.; Devesa, J. Hydrogen Ion Dynamics of Cancer and a New Molecular, Biochemical and Metabolic Approach to the Etiopathogenesis and Treatment of Brain Malignancies. Int. J. Mol. Sci. 2019, 20, 4278. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, P.Y.; Bao, W.; Chen, S.J.; Wu, F.S.; Zhu, P.Y. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer 2020, 20, 28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochette, L.; Dogon, G.; Zeller, M.; Cottin, Y.; Vergely, C. Antitumoral Activity of Molecular Hydrogen and Proton in the Treatment of Glioblastoma: An Atypical Pharmacology? Brain Sci. 2023, 13, 1168. https://doi.org/10.3390/brainsci13081168
Rochette L, Dogon G, Zeller M, Cottin Y, Vergely C. Antitumoral Activity of Molecular Hydrogen and Proton in the Treatment of Glioblastoma: An Atypical Pharmacology? Brain Sciences. 2023; 13(8):1168. https://doi.org/10.3390/brainsci13081168
Chicago/Turabian StyleRochette, Luc, Geoffrey Dogon, Marianne Zeller, Yves Cottin, and Catherine Vergely. 2023. "Antitumoral Activity of Molecular Hydrogen and Proton in the Treatment of Glioblastoma: An Atypical Pharmacology?" Brain Sciences 13, no. 8: 1168. https://doi.org/10.3390/brainsci13081168
APA StyleRochette, L., Dogon, G., Zeller, M., Cottin, Y., & Vergely, C. (2023). Antitumoral Activity of Molecular Hydrogen and Proton in the Treatment of Glioblastoma: An Atypical Pharmacology? Brain Sciences, 13(8), 1168. https://doi.org/10.3390/brainsci13081168






