Detection of Alzheimer’s Disease Using Logistic Regression and Clock Drawing Errors
Abstract
1. Introduction
- All necessary data should be collected within a few minutes;
- The collection of the data should not require a medical professional;
- Necessary data should be simple and thus suitable for comprehension by the general population.
2. Materials and Methods
2.1. Study Design and Aims
2.2. Data Source
2.3. Data Selection
2.4. Data Description
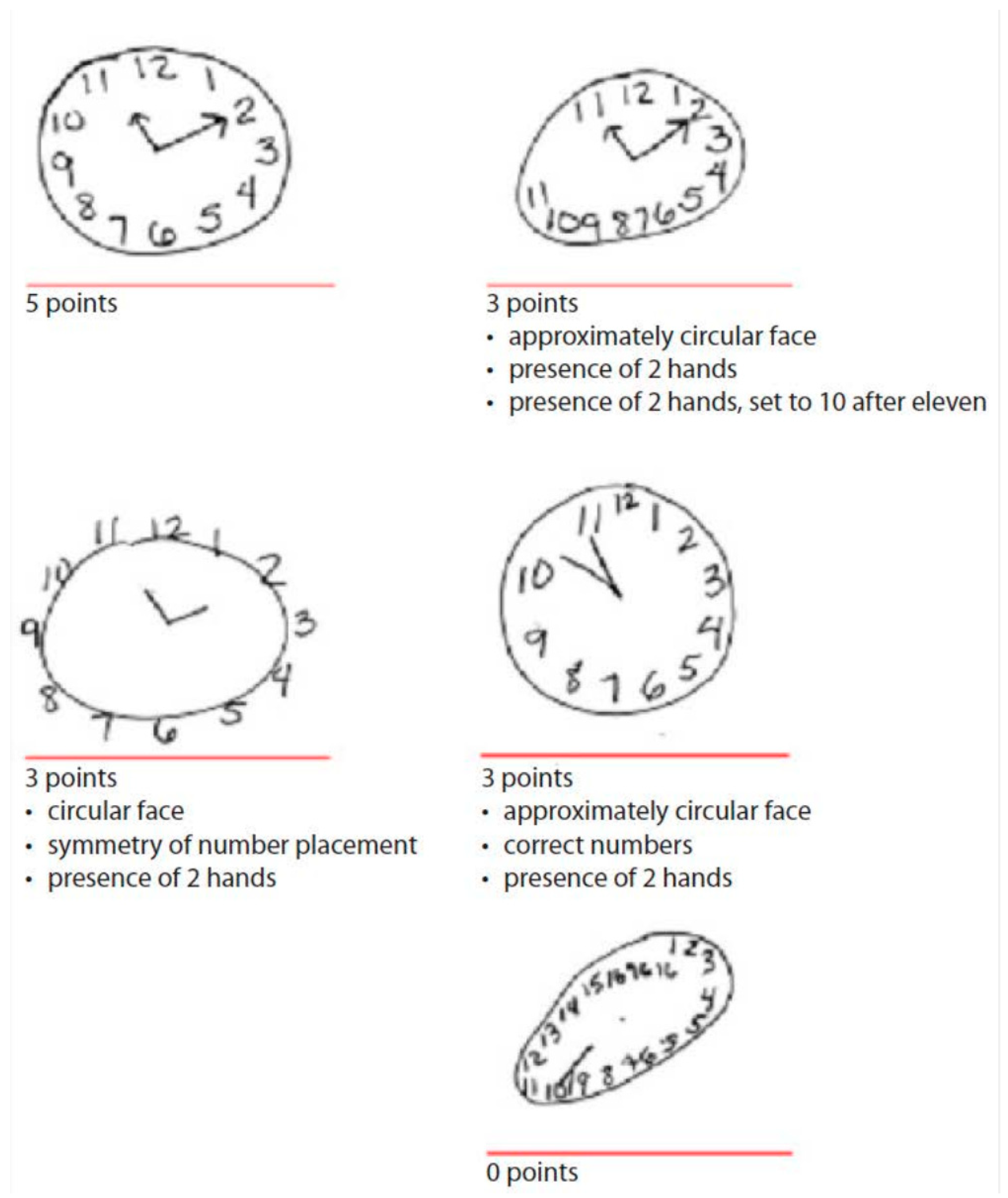
- Drawing approximately a circular face
- Placing clock numbers symmetrically
- Correct clock numbers (must have all numbers in the correct order, placed inside the circle)
- Presence of two hands
- Presence of two hands set to ten after eleven
2.5. Data Analysis
2.5.1. Model Development
2.5.2. Model Evaluation
3. Results
3.1. Sample Characteristics
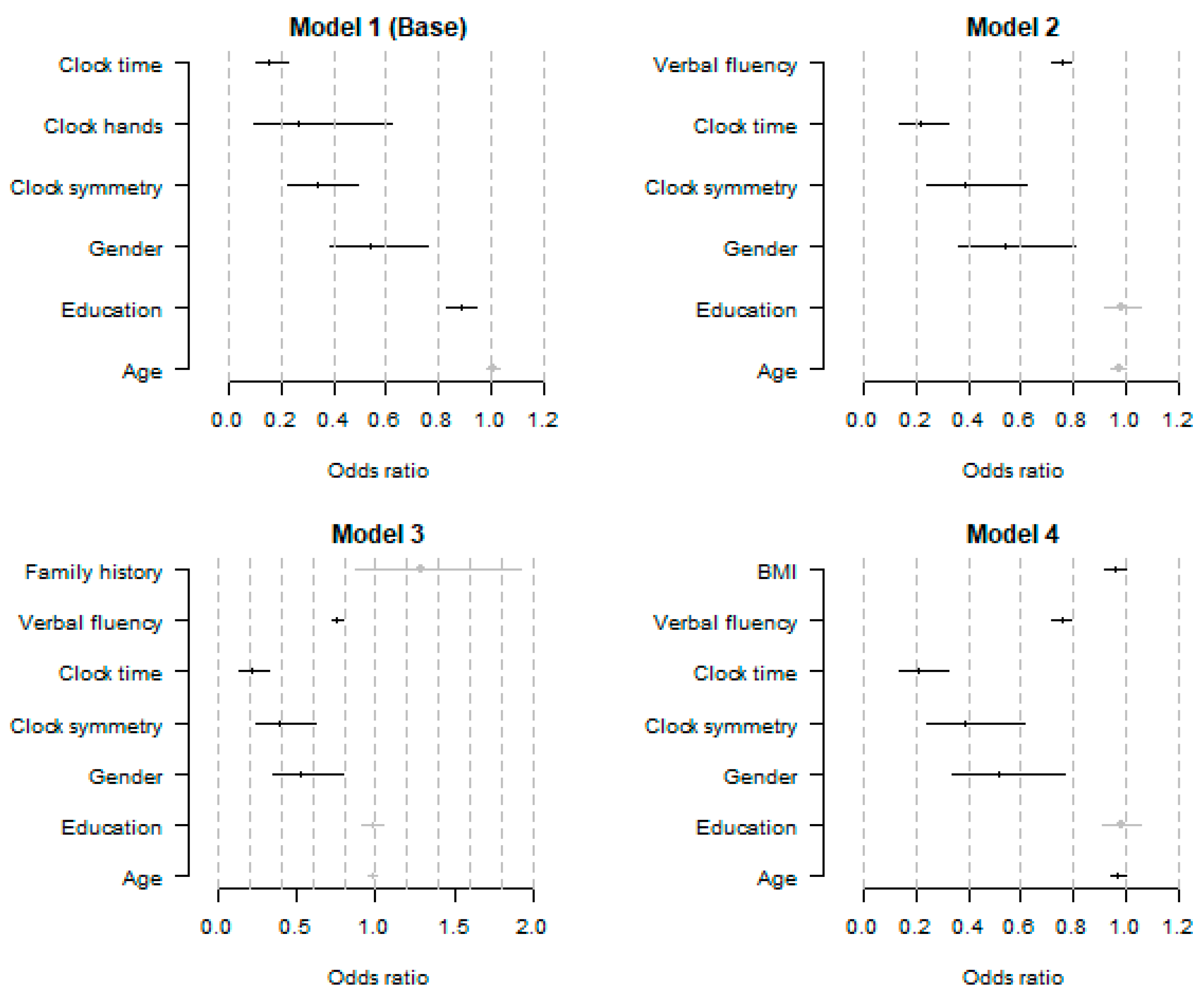
3.2. Logistic Regression Models for the Detection of AD
3.3. Discrimination of Logistic Regression Models for AD Detection
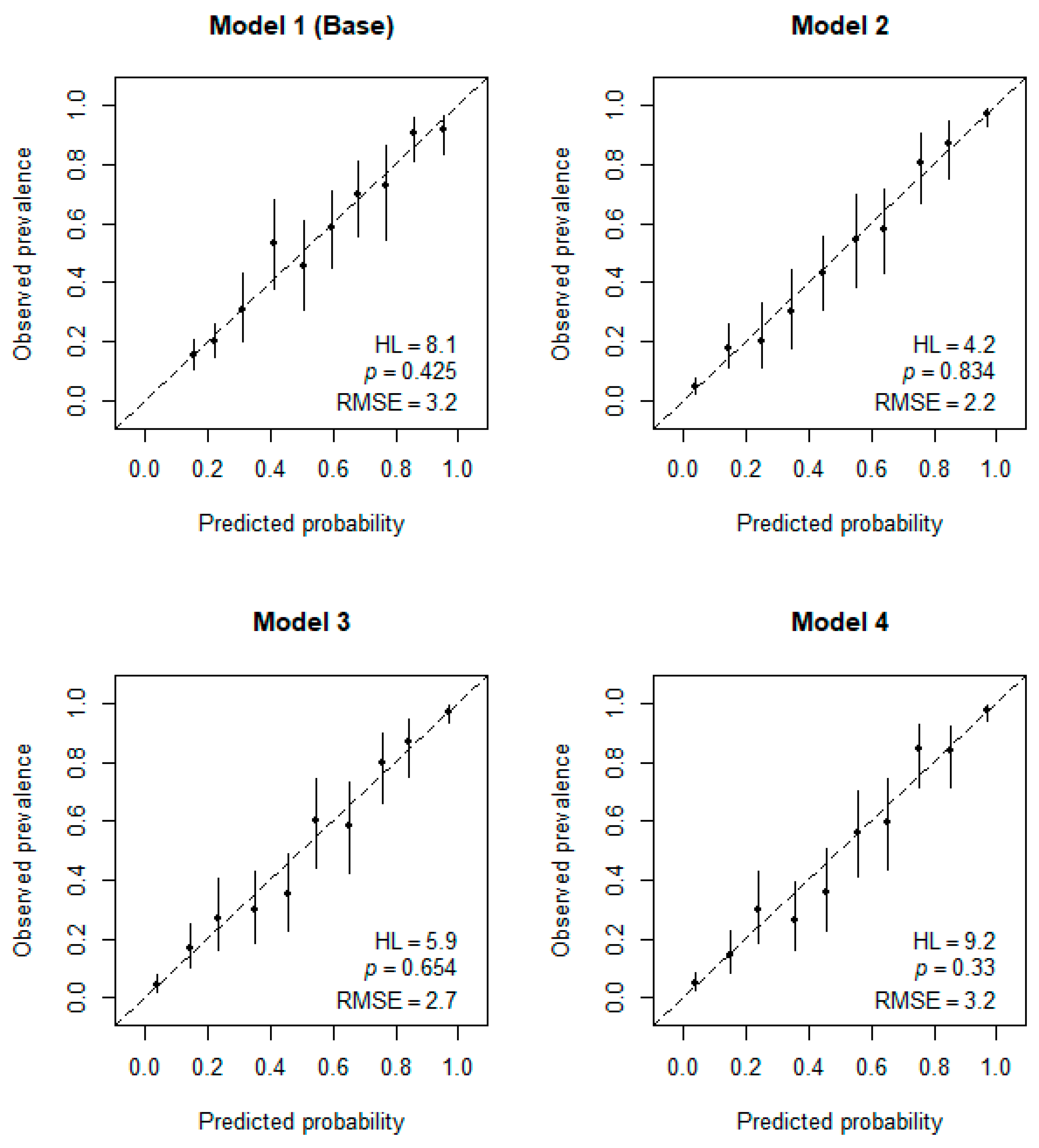
3.4. Calibration of Logistic Regression Models for AD Detection
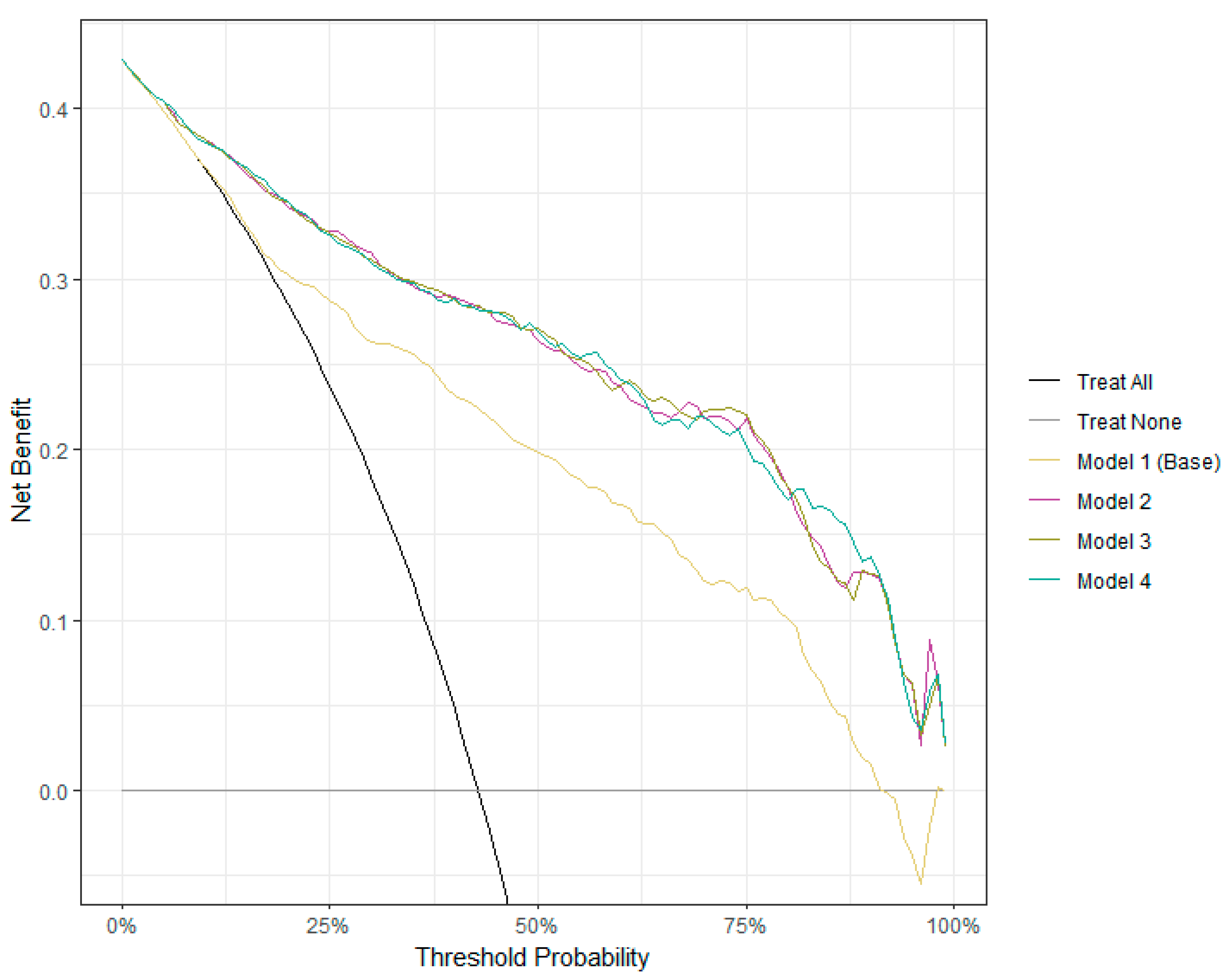
3.5. Clinical Utility of Logistic Regression Models for AD Detection
4. Discussion
5. Limitations & Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottesman, R.T.; Stern, Y. Behavioral and Psychiatric Symptoms of Dementia and Rate of Decline in Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 1062. [Google Scholar] [CrossRef] [PubMed]
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J. 2017, 150, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.F.; Loures, C.D.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan on the Public Health Response to Dementia 2017–2025. 2017. Available online: https://www.who.int/publications/i/item/9789241513487. (accessed on 13 April 2022).
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 13 April 2022).
- Li, X.; Feng, X.; Sun, X.; Hou, N.; Han, F.; Liu, Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Front. Aging Neurosci. 2022, 14, 937486. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Clifford, A.; Wei, L.; Zhang, D.; Leung, D.; Augustine, G.; Danat, I.M.; Zhou, W.; Copeland, J.R.; Anstey, K.J.; et al. Prevalence and determinants of undetected dementia in the community: A systematic literature review and a meta-analysis. BMJ Open 2017, 7, e011146. [Google Scholar] [CrossRef] [PubMed]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimer Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Valcour, V.G.; Masaki, K.H.; Curb, J.D.; Blanchette, P.L. The Detection of Dementia in the Primary Care Setting. Arch. Intern. Med. 2000, 160, 2964–2968. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.W. Frequency and characteristics of silent dementia among elderly Japanese-American men. The Honolulu-Asia Aging Study. JAMA 1997, 277, 800–805. [Google Scholar] [CrossRef]
- Werner, P. Beliefs About Memory Problems and Help Seeking in Elderly Persons. Clin. Gerontol. 2004, 27, 19–30. [Google Scholar] [CrossRef]
- Roberts, J.S.; Connell, C.M. Illness Representations Among First-Degree Relatives of People with Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2000, 14, 129–136. [Google Scholar] [CrossRef]
- Nagel, A.K.; Loetscher, T.; Smith, A.E.; Keage, H.A. What do the public really know about dementia and its risk factors? Dementia 2021, 20, 2424–2440. [Google Scholar] [CrossRef] [PubMed]
- Blay, S.L.; de Peluso, T.P. The Public’s Ability to Recognize Alzheimer Disease and Their Beliefs About Its Causes. Alzheimer Dis. Assoc. Disord. 2008, 22, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.; Son, M.; Lee, J.; Yeo, I.; Choi, K.Y.; Kim, H.; Kim, B.C.; Lee, K.H.; Kim, Y. Plasma protein biomarker model for screening Alzheimer disease using multiple reaction monitoring-mass spectrometry. Sci. Rep. 2022, 12, 1282. [Google Scholar] [CrossRef]
- Shen, X.-N.; Huang, Y.-Y.; Chen, S.-D.; Guo, Y.; Tan, L.; Dong, Q.; Yu, J.-T.; Weiner, M.W.; Aisen, P.; Petersen, R.; et al. Plasma phosphorylated-tau181 as a predictive biomarker for Alzheimer’s amyloid, tau and FDG PET status. Transl. Psychiatry 2021, 11, 585. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Stomrud, E.; Zetterberg, H.; Karl, J.; Zink, K.; Bittner, T.; Mattsson, N.; Eichenlaub, U.; Blennow, K.; et al. Performance of Fully Automated Plasma Assays as Screening Tests for Alzheimer Disease–Related β-Amyloid Status. JAMA Neurol. 2019, 76, 1060–1069. [Google Scholar] [CrossRef]
- Palmqvist, S.; Stomrud, E.; Cullen, N.; Janelidze, S.; Manuilova, E.; Jethwa, A.; Bittner, T.; Eichenlaub, U.; Suridjan, I.; Kollmorgen, G.; et al. An accurate fully automated panel of plasma biomarkers for Alzheimer’s disease. Alzheimer’s Dement. 2022, 19, 1204–1215. [Google Scholar] [CrossRef]
- Opwonya, J.; Doan, D.N.T.; Kim, S.G.; Kim, J.I.; Ku, B.; Kim, S.; Park, S.; Kim, J.U. Saccadic Eye Movement in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2022, 32, 193–227. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Silva, G.; Duque, C.; Januário, C.; Santana, I.; Ambrósio, A.F.; Castelo-Branco, M.; Bernardes, R. Retinal texture biomarkers may help to discriminate between Alzheimer’s, Parkinson’s, and healthy controls. PLoS ONE 2019, 14, e0218826. [Google Scholar] [CrossRef]
- Sharafi, S.M.; Sylvestre, J.; Chevrefils, C.; Soucy, J.; Beaulieu, S.; Pascoal, T.A.; Arbour, J.D.; Rhéaume, M.; Robillard, A.; Chayer, C.; et al. Vascular retinal biomarkers improves the detection of the likely cerebral amyloid status from hyperspectral retinal images. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2019, 5, 610–617. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Xu, S.; Jin, Y.; Jin, X.; Qiao, S.; Chen, Q.; Xing, W.; Zhao, Z.; Yan, J.; et al. Develop a diagnostic tool for dementia using machine learning and non-imaging features. Front. Aging Neurosci. 2022, 14, 945274. [Google Scholar] [CrossRef]
- Kleiman, M.J.; Barenholtz, E.; Galvin, J.E.; Initiative, F.T.A.D.N. Screening for Early-Stage Alzheimer’s Disease Using Optimized Feature Sets and Machine Learning. J. Alzheimer’s Dis. 2021, 81, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.C.; Choi, S.H.; Shin, H.-W.; Kim, K.W.; Jang, J.-W.; Jung, J.J.; Hsiung, G.-Y.R.; Kim, S. Detection of cognitive impairment using a machine-learning algorithm. Neuropsychiatr. Dis. Treat. 2018, 14, 2939–2945. [Google Scholar] [CrossRef]
- Zhao, Z.; Chuah, J.H.; Lai, K.W.; Chow, C.-O.; Gochoo, M.; Dhanalakshmi, S.; Wang, N.; Bao, W.; Wu, X. Conventional machine learning and deep learning in Alzheimer’s disease diagnosis using neuroimaging: A review. Front. Comput. Neurosci. 2023, 17, 1038636. [Google Scholar] [CrossRef] [PubMed]
- Fristed, E.; Skirrow, C.; Meszaros, M.; Lenain, R.; Meepegama, U.; Papp, K.V.; Ropacki, M.; Weston, J. Leveraging speech and artificial intelligence to screen for early Alzheimer’s disease and amyloid beta positivity. Brain Commun. 2022, 4, fcac231. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Mallick, E.; Schwed, L.; König, A.; Zhao, J.; Linz, N.; Bodin, T.H.; Skoog, J.; Possemis, N.; ter Huurne, D.; et al. Screening for Mild Cognitive Impairment Using a Machine Learning Classifier and the Remote Speech Biomarker for Cognition: Evidence from Two Clinically Relevant Cohorts. J. Alzheimer’s Dis. 2023, 91, 1165–1171. [Google Scholar] [CrossRef]
- Thabtah, F.; Peebles, D.; Retzler, J.; Hathurusingha, C. Dementia medical screening using mobile applications: A systematic review with a new mapping model. J. Biomed. Informatics 2020, 111, 103573. [Google Scholar] [CrossRef]
- Kwon, S.J.; Kim, H.S.; Han, J.H.; Bin Bae, J.; Kim, K.W. Reliability and Validity of Alzheimer’s Disease Screening With a Semi-automated Smartphone Application Using Verbal Fluency. Front. Neurol. 2021, 12, 684902. [Google Scholar] [CrossRef]
- Aprahamian, I.; Martinelli, J.E.; Neri, A.L.; Yassuda, M.S. TTeste do Desenho do Relógio: Revisão da acurácia no rastreamento de demência. Dement. Neuropsychol. 2009, 3, 74–81. [Google Scholar] [CrossRef]
- Wolf-Klein, G.P.; Silverstone, F.A.; Levy, A.P.; Brod, M.S.; Breuer, J. Screening for Alzheimer’s Disease by Clock Drawing. J. Am. Geriatr. Soc. 1989, 37, 730–734. [Google Scholar] [CrossRef]
- Cahn, D.A.; Salmon, D.P.; Monsch, A.U.; Butters, N.; Wiederholt, W.; Corey-Bloom, J.; Barrett-Connor, E. Screening for dementia of the alzheimer type in the community: The utility of the clock drawing test. Arch. Clin. Neuropsychol. 1996, 11, 529–539. [Google Scholar] [CrossRef]
- Müller, S.; Preische, O.; Heymann, P.; Elbing, U.; Laske, C. Increased Diagnostic Accuracy of Digital vs. Conventional Clock Drawing Test for Discrimination of Patients in the Early Course of Alzheimer’s Disease from Cognitively Healthy Individuals. Front. Aging Neurosci. 2017, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Liao, M. An Intelligent Screening Mobile Application for Alzheimer’s Disease Using Clock Drawing Test. In Proceedings of the 2021 4th International Conference on Signal Processing and Machine Learning, Beijing, China, 18–20 August 2021; pp. 112–116. [Google Scholar] [CrossRef]
- Harbi, Z.; Hicks, Y.; Setchi, R.; Bayer, A. Segmentation of Clock Drawings Based on Spatial and Temporal Features. Procedia Comput. Sci. 2015, 60, 1640–1648. [Google Scholar] [CrossRef]
- Rentz, D.M.; Papp, K.V.; Mayblyum, D.V.; Sanchez, J.S.; Klein, H.; Souillard-Mandar, W.; Sperling, R.A.; Johnson, K.A. Association of Digital Clock Drawing with PET Amyloid and Tau Pathology in Normal Older Adults. Neurology 2021, 96, e1844–e1854. [Google Scholar] [CrossRef]
- Lessig, M.C.; Scanlan, J.M.; Nazemi, H.; Borson, S. Time that tells: Critical clock-drawing errors for dementia screening. Int. Psychogeriatrics 2008, 20, 459–470. [Google Scholar] [CrossRef]
- Rouleau, I.; Salmon, D.P.; Butters, N.; Kennedy, C.; McGuire, K. Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn. 1992, 18, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, Y.; Ueda, H.; Narumoto, J.; Nakamura, K.; Kita, H.; Fukui, K. Qualitative analyses of clock drawings in Alzheimer’s disease and vascular dementia. Psychiatry Clin. Neurosci. 2001, 55, 485–491. [Google Scholar] [CrossRef]
- Duro, D.; Tábuas-Pereira, M.; Freitas, S.; Santiago, B.; Botelho, M.A.; Santana, I. Validity and Clinical Utility of Different Clock Drawing Test Scoring Systems in Multiple Forms of Dementia. J. Geriatr. Psychiatry Neurol. 2018, 31, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Mochizuki, H.; Oki, M.; Matsumoto, M.; Fukushima, M.; Yoshikawa, Y.; Nagasawa, A.; Takakura, T.; Shimoda, N. Quantitative and Qualitative Analyses of the Clock Drawing Test in Fall and Non-Fall Patients with Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. Extra 2019, 9, 381–388. [Google Scholar] [CrossRef]
- Alzheimer’s Disease Neuroimaging Initiative (ADNI). Available online: http://adni.loni.usc.edu/ (accessed on 13 March 2023).
- Alzheimer’s Disease Neuroimaging Initiative. ADNI Procedures Manual. Available online: https://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf. (accessed on 28 February 2023).
- Weakley, A.; Schmitter-Edgecombe, M. Analysis of Verbal Fluency Ability in Alzheimer’s Disease: The Role of Clustering, Switching and Semantic Proximities. Arch. Clin. Neuropsychol. 2014, 29, 256–268. [Google Scholar] [CrossRef]
- Sutin, A.R.; Stephan, Y.; Terracciano, A. Verbal fluency and risk of dementia. Int. J. Geriatr. Psychiatry 2019, 34, 863–867. [Google Scholar] [CrossRef]
- Kivimäki, M.; Luukkonen, R.; Batty, G.D.; Ferrie, J.E.; Pentti, J.; Nyberg, S.T.; Shipley, M.J.; Alfredsson, L.; Fransson, E.I.; Goldberg, M.; et al. Body mass index and risk of dementia: Analysis of individual-level data from 1.3 million individuals. Alzheimer’s Dement. 2018, 14, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Yi, D.; Byun, M.S.; Ahn, H.; Jung, J.H.; Kong, N.; Kim, M.J.; Jung, G.; Lee, J.-Y.; Lee, Y.-S.; et al. Synergistic interaction of high blood pressure and cerebral beta-amyloid on tau pathology. Alzheimer’s Res. Ther. 2022, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- R Foundation for Statistical Computing, Vienna, Austria. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 10 May 2023).
- Wu, T.; Wei, Y.; Wu, J.; Yi, B.; Li, H. Logistic regression technique is comparable to complex machine learning algorithms in predicting cognitive impairment related to post intensive care syndrome. Sci. Rep. 2023, 13, 2485. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Indrayan, A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011, 48, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Brown, J.A.; Jimenez-Valverde, A.; Real, R. The ‘modEva’ package. Available online: https://cran.r-project.org/web/packages/modEvA/modEvA.pdf. (accessed on 12 April 2023).
- Van Calster, B.; McLernon, D.J.; van Smeden, M.; Wynants, L.; Steyerberg, E.W.; On behalf of Topic Group ‘Evaluating diagnostic tests and prediction models’ of the STRATOS initiative. Calibration: The Achilles heel of predictive analytics. BMC Med. 2019, 17, 230. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
- Vickers, A.J.; Van Calster, B.; Steyerberg, E.W. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ 2016, 352, i6. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression. In Wiley Series in Probability and Statistics, 1st ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 89–151. [Google Scholar]
- Tuokko, H.; Hadjistavropoulos, T.; Rae, S.; O’Rourke, N. A Comparison of Alternative Approaches to the Scoring of Clock Drawing. Arch. Clin. Neuropsychol. 2000, 15, 137–148. [Google Scholar] [CrossRef]

| ADNI Group Inclusion Criteria | |
|---|---|
| CN Group | AD Group |
| No memory complaints aside from those common to other normal subjects of that age range. | Reported memory complaint by patient or study partner |
| Normal memory function score on the Wechsler Memory Scale (adjusted for education) | Abnormal memory function score on the Wechsler Memory Scale (adjusted for education) |
| Mini-Mental State Exam score between 24 and 30 (inclusive) | Mini-Mental State Exam score between 20 and 26 |
| Clinical Dementia Rating = 0; Memory Box score must be 0 | Clinical Dementia Rating = 0.5; Memory Box score at least 1.0 |
| Cognitively normal, based on the absence of significant impairment in cognitive functions or activities of daily living. | NINCDS/ADRDA criteria for probable AD |
| Variable | AD | CN | p-Value (ANOVA F Test) |
|---|---|---|---|
| Age | 74.78 ± 7.94 | 73.44 ± 6.28 | 0.0049 ** |
| Education (in years) | 15.17 ± 2.92 | 16.42 ± 2.59 | <0.0001 *** |
| BMI | 25.96 ± 4.75 | 27.32 ± 8.27 | 0.0040 ** |
| Systolic BP | 133.80 ± 17.45 | 132.80 ± 16.27 | 0.3913 |
| Diastolic BP | 73.80 ± 9.65 | 73.90 ± 9.80 | 0.8835 |
| Verbal fluency | 12.21 ± 5.07 | 21.15 ± 5.62 | <0.0001 *** |
| Variable | AD | CN | p-Value (Chi-Square Test) | ||
|---|---|---|---|---|---|
| Gender | Male | Female | Male | Female | 0.0060 ** |
| 218 (56.77%) | 166 (43.23%) | 242 (47.27%) | 270 (52.73%) | ||
| Family history | No | Yes | No | Yes | 0.3493 |
| 205 (53.39%) | 179 (46.61%) | 256 | 256 | ||
| (50%) | (50%) | ||||
| Clock Component | AD | CN | p-Value (Chi-Square Test) | ||
| Failure (0) | Success (1) | Failure (0) | Success (1) | ||
| Clock circle | 13 | 371 | 4 | 508 (99.22%) | 0.0099 *** |
| (3.39%) | (96.61%) | (0.78%) | |||
| Clock symmetry | 177 (46.09%) | 207 | 61 | 451 (88.09%) | <0.0001 *** |
| (53.91%) | (11.91%) | ||||
| Clock numbers | 114 (29.69%) | 270 | 36 | 476 (92.97%) | <0.0001 *** |
| (70.31%) | (7.03%) | ||||
| Clock hands | 79 | 305 | 6 | 506 (98.83%) | <0.0001 *** |
| (20.57%) | (79.43%) | (1.17%) | |||
| Clock time | 240 | 144 | 68 | 444 (86.72%) | <0.0001 *** |
| (62.5%) | (37.5%) | (13.28%) | |||
| Copy circle | 7 | 377 | 1 | 511 | 0.0275 * |
| (1.82%) | (98.18%) | (0.2%) | (99.8%) | ||
| Copy symmetry | 95 | 289 | 33 | 479 (93.55%) | <0.0001 *** |
| (24.74%) | (75.26%) | (6.45%) | |||
| Copy numbers | 42 | 342 | 6 | 506 (98.83%) | <0.0001 *** |
| (10.94%) | (89.06%) | (1.17%) | |||
| Copy hands | 30 | 354 | 0 | 512 | <0.0001 *** |
| (7.81%) | (92.19%) | (0%) | (100%) | ||
| Copy time | 107 (27.86%) | 277 | 30 | 482 (94.14%) | <0.0001 *** |
| (72.14%) | (5.86%) | ||||
| Predictor | Estimate | Standard Error | Z-Test | p-Value |
|---|---|---|---|---|
| Model 1 (Base model) | ||||
| Intercept | 4.580 | 1.149 | 3.99 | <0.0001 *** |
| Age | 0.008 | 0.012 | 0.69 | 0.492 |
| Education | −0.121 | 0.031 | −3.85 | 0.0001 *** |
| Gender (female) | −0.613 | 0.174 | −3.53 | 0.0004 *** |
| Clock symmetry (success) | −1.094 | 0.199 | −5.49 | <0.0001 *** |
| Clock hands (success) | −1.328 | 0.470 | −2.83 | 0.0047 ** |
| Clock time (success) | −1.868 | 0.186 | −10.02 | <0.0001 *** |
| Model 2 | ||||
| Intercept | 8.558 | 1.342 | 6.38 | <0.0001 *** |
| Age | −0.026 | 0.014 | −1.80 | 0.072 |
| Education | −0.015 | 0.036 | −0.42 | 0.673 |
| Gender (female) | −0.613 | 0.205 | −2.99 | 0.0028 ** |
| Clock symmetry (success) | −0.947 | 0.239 | −3.97 | <0.0001 *** |
| Clock time (success) | −1.541 | 0.214 | −7.19 | <0.0001 *** |
| Verbal fluency (success) | −0.282 | 0.023 | −12.34 | <0.0001 *** |
| Model 3 | ||||
| Intercept | 8.303 | 1.355 | 6.13 | <0.0001 *** |
| Age | −0.022 | 0.014 | −1.54 | 0.123 |
| Education | −0.020 | 0.037 | −0.54 | 0.586 |
| Gender (female) | −0.638 | 0.207 | −3.09 | 0.002 ** |
| Clock symmetry (success) | −0.959 | 0.239 | −4.01 | <0.0001 *** |
| Clock time (success) | −1.549 | 0.215 | −7.20 | <0.0001 *** |
| Verbal fluency (success) | −0.283 | 0.023 | −12.33 | <0.0001 *** |
| Family history (yes) | 0.255 | 0.202 | 1.26 | 0.206 |
| Model 4 | ||||
| Intercept | 10.050 | 1.542 | 6.52 | <0.0001 *** |
| Age | −0.029 | 0.014 | −2.01 | 0.0448 * |
| Education | −0.020 | 0.037 | −0.53 | 0.593 |
| Gender (female) | −0.665 | 0.207 | −3.21 | 0.0013 ** |
| Clock symmetry (success) | −0.950 | 0.240 | −3.97 | <0.0001 *** |
| Clock time (success) | −1.548 | 0.215 | −7.20 | <0.0001 *** |
| Verbal fluency (success) | −0.280 | 0.023 | −12.26 | <0.0001 *** |
| BMI | −0.044 | 0.022 | −2.06 | 0.039 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarova, S.; Grigorova, D.; Petrova-Antonova, D.; for the Alzheimer’s Disease Neuroimaging Initiative. Detection of Alzheimer’s Disease Using Logistic Regression and Clock Drawing Errors. Brain Sci. 2023, 13, 1139. https://doi.org/10.3390/brainsci13081139
Lazarova S, Grigorova D, Petrova-Antonova D, for the Alzheimer’s Disease Neuroimaging Initiative. Detection of Alzheimer’s Disease Using Logistic Regression and Clock Drawing Errors. Brain Sciences. 2023; 13(8):1139. https://doi.org/10.3390/brainsci13081139
Chicago/Turabian StyleLazarova, Sophia, Denitsa Grigorova, Dessislava Petrova-Antonova, and for the Alzheimer’s Disease Neuroimaging Initiative. 2023. "Detection of Alzheimer’s Disease Using Logistic Regression and Clock Drawing Errors" Brain Sciences 13, no. 8: 1139. https://doi.org/10.3390/brainsci13081139
APA StyleLazarova, S., Grigorova, D., Petrova-Antonova, D., & for the Alzheimer’s Disease Neuroimaging Initiative. (2023). Detection of Alzheimer’s Disease Using Logistic Regression and Clock Drawing Errors. Brain Sciences, 13(8), 1139. https://doi.org/10.3390/brainsci13081139






