A Quantitative Investigation of Mental Fatigue Elicited during Motor Imagery Practice: Selective Effects on Maximal Force Performance and Imagery Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
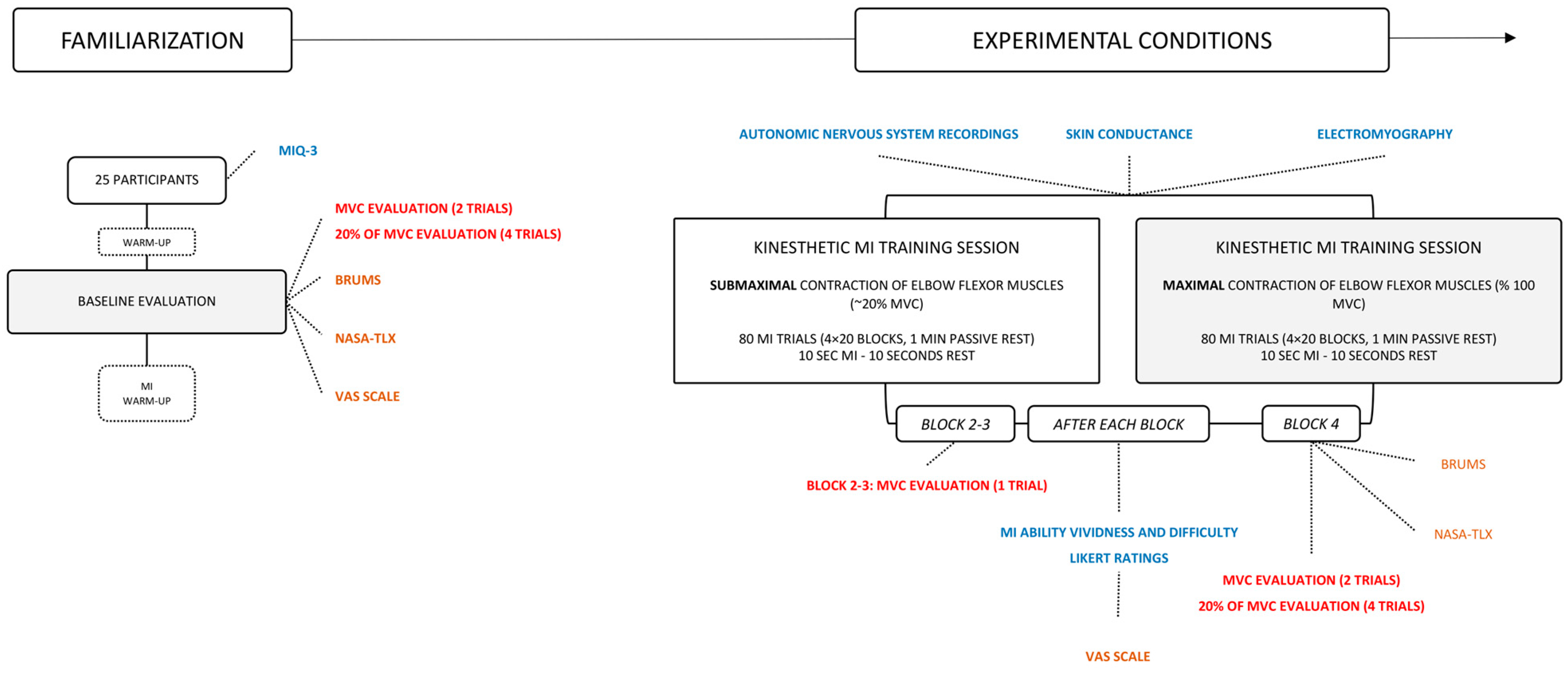
2.2. Experimental Design
2.2.1. Baseline Evaluations
2.2.2. Experimental Conditions
2.3. Data Processing and Extraction
2.3.1. Force Plate Recordings
2.3.2. Neurophysiological Recordings
Electromyographic and Electrocardiographic Recordings
Skin Conductance Recordings
2.4. Statistical Analysis
2.4.1. Power Considerations
2.4.2. Analysis of the Dependent Variables
3. Results
3.1. BRUMS and NASA-TLX Scores
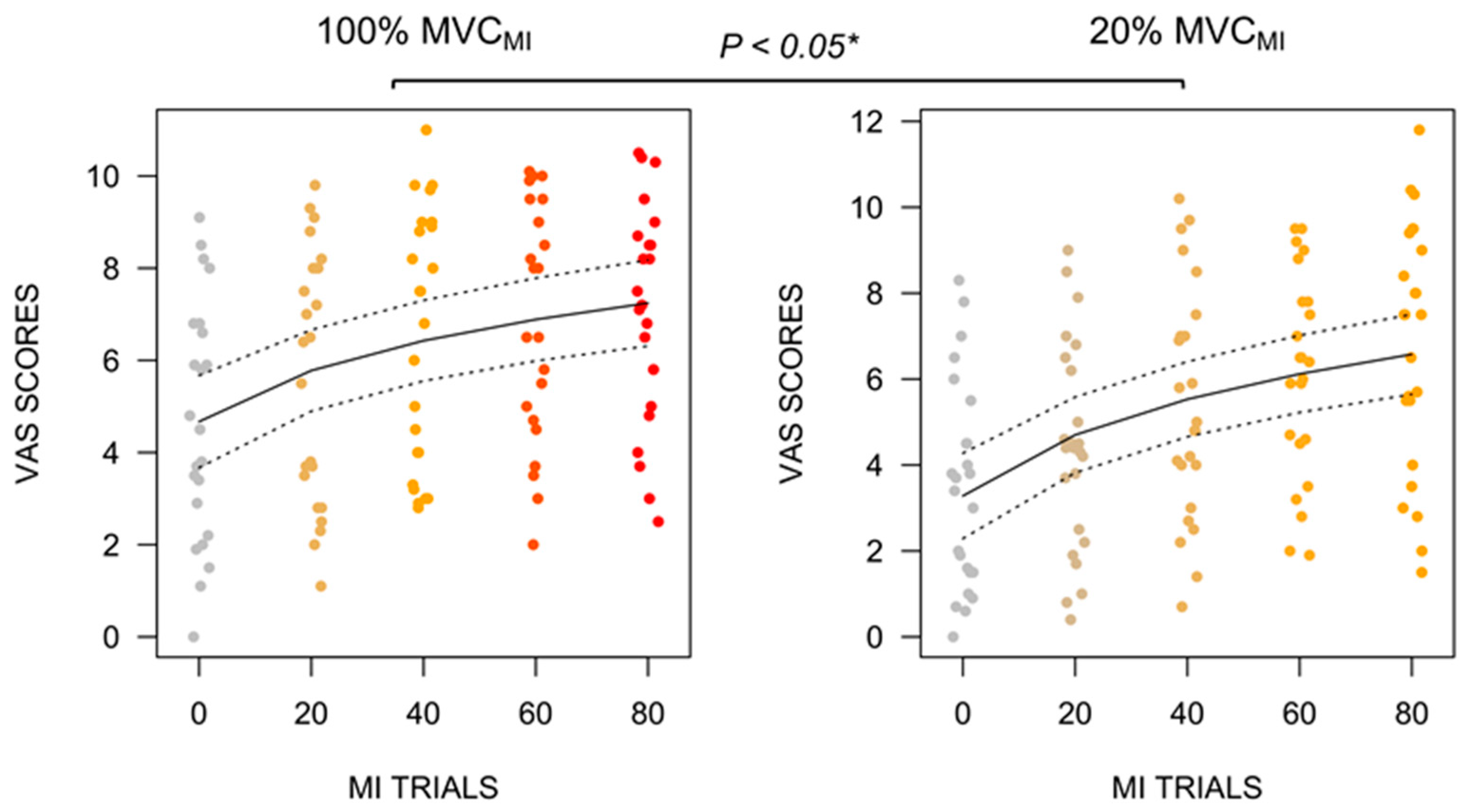
3.2. B.VAS Self-Report Ratings
3.3. Motor Performance Analysis
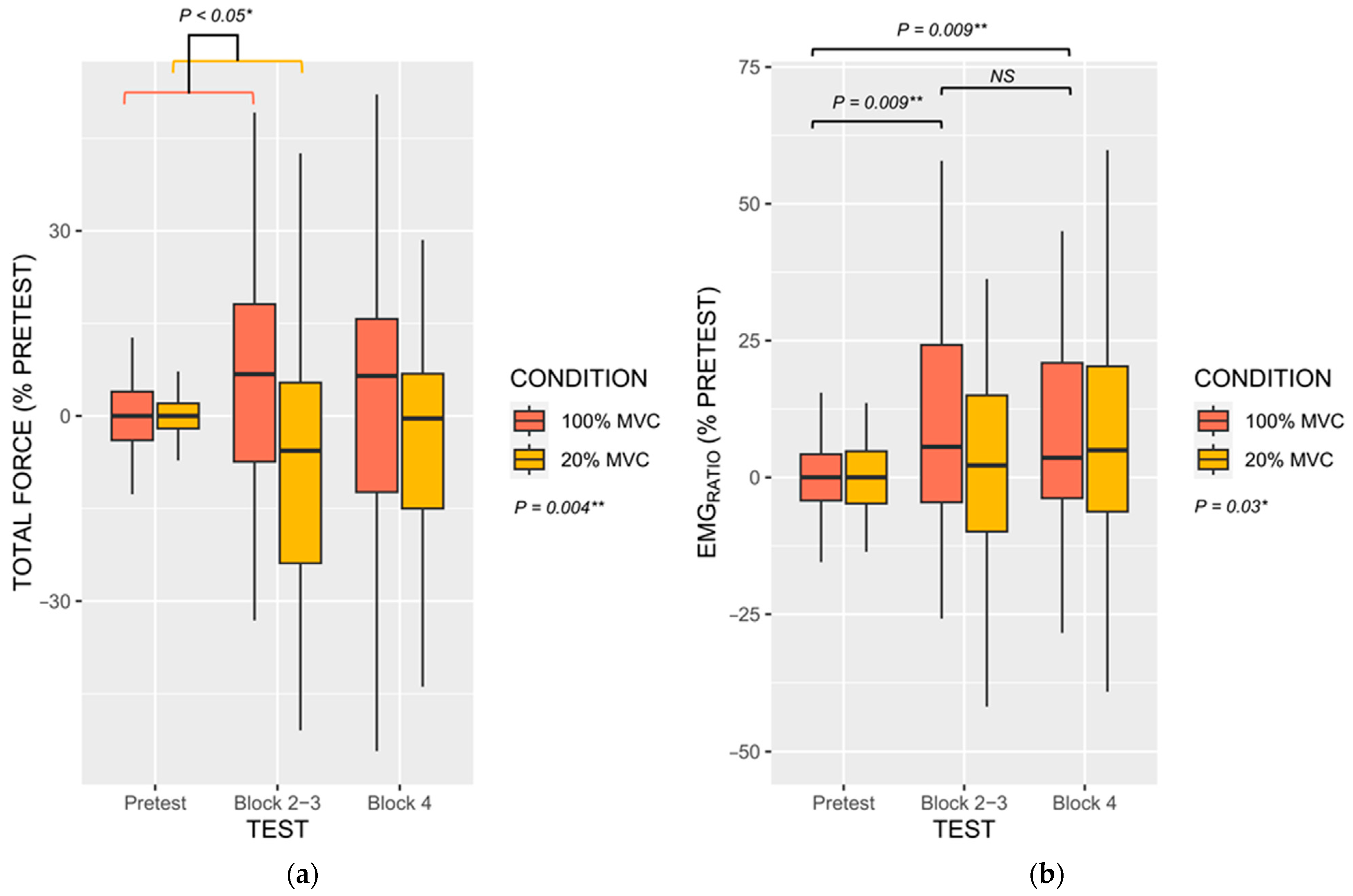
3.3.1. Force Performances
3.3.2. Electromyograophic Activity
3.4. Motor Imagery Ability
3.4.1. Subjective Measures
3.4.2. Physiological Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillot, A.; Rienzo, F.D.; Frank, C.; Debarnot, U.; MacIntyre, T.E. From simulation to motor execution: A review of the impact of dynamic motor imagery on performance. Int. Rev. Sport Exerc. Psychol. 2021, 1–20. [Google Scholar] [CrossRef]
- Moran, A.; Guillot, A.; MacIntyre, T.; Collet, C. Re-imagining motor imagery: Building bridges between cognitive neuroscience and sport psychology. Br. J. Psychol. 2012, 103, 224–247. [Google Scholar] [CrossRef]
- Guillot, A.; Collet, C. Construction of the Motor Imagery Integrative Model in Sport: A review and theoretical investigation of motor imagery use. Int. Rev. Sport Exerc. Psychol. 2008, 1, 31–44. [Google Scholar] [CrossRef]
- Ortiz-Echeverri, C.J.; Salazar-Colores, S.; Rodríguez-Reséndiz, J.; Gómez-Loenzo, R.A. A New Approach for Motor Imagery Classification Based on Sorted Blind Source Separation, Continuous Wavelet Transform, and Convolutional Neural Network. Sensors 2019, 19, 4541. [Google Scholar] [CrossRef] [Green Version]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.P.; Eugène, F.; Michon, P.E.; Jackson, P.L. The neural network of motor imagery: An ALE meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 930–949. [Google Scholar] [CrossRef]
- Pageaux, B.; Lepers, R. The effects of mental fatigue on sport-related performance. In Progress in Brain Research; Marcora, S., Sarkar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 291–315, Chapter 16. [Google Scholar] [CrossRef]
- Van Cutsem, J.; Marcora, S.; De Pauw, K.; Bailey, S.; Meeusen, R.; Roelands, B. The Effects of Mental Fatigue on Physical Performance: A Systematic Review. Sports Med. 2017, 47, 1569–1588. [Google Scholar] [CrossRef] [Green Version]
- Itoh, S. Effect of Imagery Dose Variables on Performance in Sport. Doctoral Dissertation, Institute for Health and Sport, Victoria University, Footscray, Australia, 2020. [Google Scholar]
- Morris, T.; Fazel, F.; Maher, R.; Azizuddin Khan, T.K.; Kuan, G.; Spittle, M. How Much Imagery is Enough? Developing a Research Protocol. In Proceedings of the 3rd Chinese Conference on Sport Psychology, Macau, China; 2012. [Google Scholar]
- Schuster, C.; Hilfiker, R.; Amft, O.; Scheidhauer, A.; Andrews, B.; Butler, J.; Kischka, U.; Ettlin, T. Best practice for motor imagery: A systematic literature review on motor imagery training elements in five different disciplines. BMC Med. 2011, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Rozand, V.; Lebon, F.; Papaxanthis, C.; Lepers, R. Does a Mental Training Session Induce Neuromuscular Fatigue? Med. Sci. Sports Exerc. 2014, 46, 1918–1989. [Google Scholar] [CrossRef] [Green Version]
- Rozand, V.; Pageaux, B.; Marcora, S.M.; Papaxanthis, C.; Lepers, R. Does mental exertion alter maximal muscle activation? Front. Hum. Neurosci. 2014, 8, 755. [Google Scholar] [CrossRef] [Green Version]
- Jacquet, T.; Lepers, R.; Poulin-Charronnat, B.; Bard, P.; Pfister, P.; Pageaux, B. Mental fatigue induced by prolonged motor imagery increases perception of effort and the activity of motor areas. Neuropsychologia 2021, 150, 107701. [Google Scholar] [CrossRef]
- Nakashima, A.; Moriuchi, T.; Matsuda, D.; Nakamura, J.; Fujiwara, K.; Ikio, Y.; Hasegawa, T.; Mitunaga, W.; Higashi, T. Continuous Repetition Motor Imagery Training and Physical Practice Training Exert the Growth of Fatigue and Its Effect on Performance. Brain Sci. 2022, 12, 1087. [Google Scholar] [CrossRef]
- Rozand, V.; Lebon, F.; Stapley, P.J.; Papaxanthis, C.; Lepers, R. A prolonged motor imagery session alter imagined and actual movement durations: Potential implications for neurorehabilitation. Behav. Brain Res. 2016, 297, 67–75. [Google Scholar] [CrossRef]
- Migliaccio, G.M.; Di Filippo, G.; Russo, L.; Orgiana, T.; Ardigò, L.P.; Casal, M.Z.; Peyré-Tartaruga, L.A.; Padulo, J. Effects of Mental Fatigue on Reaction Time in Sportsmen. Int. J. Env. Res. Pub. Health 2022, 19, 14360. [Google Scholar] [CrossRef]
- Collet, C.; Guillot, A.; Lebon, F.; MacIntyre, T.; Moran, A. Measuring Motor Imagery Using Psychometric, Behavioral, and Psychophysiological Tools. Exerc. Sport Sci. Rev. 2011, 39, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Guillot, A.; Collet, C. Contribution from neurophysiological and psychological methods to the study of motor imagery. Brain Res. Rev. 2005, 50, 387–397. [Google Scholar] [CrossRef]
- Di Rienzo, F.; Collet, C.; Hoyek, N.; Guillot, A. Impact of Neurologic Deficits on Motor Imagery: A Systematic Review of Clinical Evaluations. Neuropsychol. Rev. 2014, 24, 116–147. [Google Scholar] [CrossRef]
- Avanzino, L.; Gueugneau, N.; Bisio, A.; Ruggeri, P.; Papaxanthis, C.; Bove, M. Motor cortical plasticity induced by motor learning through mental practice. Front. Behav. Neurosci. 2015, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Reiser, M.; Büsch, D.; Munzert, J. Strength Gains by Motor Imagery with Different Ratios of Physical to Mental Practice. Front. Psychol. 2011, 2, 194. [Google Scholar] [CrossRef] [Green Version]
- Robin, N.; Dominique, L.; Toussaint, L.; Blandin, Y.; Guillot, A.; Her, M.L. Effects of motor imagery training on service return accuracy in tennis: The role of imagery ability. Int. J. Sport Exerc. Psychol. 2007, 5, 175–186. [Google Scholar] [CrossRef]
- Roure, R.; Collet, C.; Deschaumes-Molinaro, C.; Delhomme, G.; Dittmar, A.; Vernet-Maury, E. Imagery Quality Estimated by Autonomic Response Is Correlated to Sporting Performance Enhancement. Physiol. Behav. 1999, 66, 63–72. [Google Scholar] [CrossRef]
- Lorey, B.; Pilgramm, S.; Bischoff, M.; Stark, R.; Vaitl, D.; Kindermann, S.; Munzert, J.; Zentgraf, K. Activation of the Parieto-Premotor Network Is Associated with Vivid Motor Imagery—A Parametric fMRI Study. PLoS ONE 2011, 6, e20368. [Google Scholar] [CrossRef] [Green Version]
- van der Meulen, M.; Allali, G.; Rieger, S.W.; Assal, F.; Vuilleumier, P. The influence of individual motor imagery ability on cerebral recruitment during gait imagery. Hum. Brain Mapp. 2014, 35, 455–470. [Google Scholar] [CrossRef] [Green Version]
- Guillot, A.; Daligault, S.; Schwartz, D.; Di Rienzo, F. Timing-specific patterns of cerebral activations during motor imagery: A case study of the expert brain signature. Brain Cogn. 2023, 167, 105971. [Google Scholar] [CrossRef]
- Guillot, A.; Collet, C.; Nguyen, V.A.; Malouin, F.; Richards, C.; Doyon, J. Functional neuroanatomical networks associated with expertise in motor imagery. NeuroImage 2008, 41, 1471–1483. [Google Scholar] [CrossRef]
- Talukdar, U.; Hazarika, S.M.; Gan, J.Q. Motor imagery and mental fatigue: Inter-relationship and EEG based estimation. J. Comput. Neurosci. 2019, 46, 55–76. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Nakata, H.; Kanosue, K. Activity of right premotor-parietal regions dependent upon imagined force level: An fMRI study. Front. Hum. Neurosci. 2014, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Olsson, C.-J.; Hedlund, M.; Sojka, P.; Lundström, R.; Lindström, B. Increased prefrontal activity and reduced motor cortex activity during imagined eccentric compared to concentric muscle actions. Front. Hum. Neurosci. 2012, 6, 255. [Google Scholar] [CrossRef] [Green Version]
- Robin, N.; Coudevylle, G.R.; Guillot, A.; Toussaint, L. French translation and validation of the Movement Imagery Questionnaire-third version (MIQ-3f). Mov. Sport Sci. 2020, 108, 23–31. [Google Scholar] [CrossRef]
- Terry, P.C.; Lane, A.M.; Fogarty, G.J. Construct validity of the Profile of Mood States—Adolescents for use with adults. Psychol. Sport Exerc. 2003, 4, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.I.; Chau, M.; Lorenzetti, M.S.; Hill, L.D.; Fins, A.I.; Tartar, J.L. Acute sleep deprivation disrupts emotion, cognition, inflammation, and cortisol in young healthy adults. Front. Behav. Neurosci. 2022, 16, 945661. [Google Scholar] [CrossRef]
- Di Rienzo, F.; Blache, Y.; Kanthack, T.F.D.; Monteil, K.; Collet, C.; Guillot, A. Short-term effects of integrated motor imagery practice on muscle activation and force performance. Neuroscience 2015, 305, 146–156. [Google Scholar] [CrossRef]
- Dos Anjos, T.; Guillot, A.; Kerautret, Y.; Daligault, S.; Di Rienzo, F. Corticomotor Plasticity Underlying Priming Effects of Motor Imagery on Force Performance. Brain Sci. 2022, 12, 1537. [Google Scholar] [CrossRef]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of Empirical and Theoretical Research. Adv. Psychol. 1988, 52, 139–183. [Google Scholar] [CrossRef]
- Jiang, C.H.; Ranganathan, V.; Siemionow, V.; Yue, G. The level of effort, rather than muscle exercise intensity determines strength gain following a six-week training. Life Sci. 2017, 178, 30–34. [Google Scholar] [CrossRef]
- Liu, X.; Ge, S.; Cordova, A.; Yaghi, Z.; Jiang, B.; Yue, G.; Yao, W. Elderly may benefit more from motor imagery training in gaining muscle strength than young adults: A systematic review and meta-analysis. Front. Psychol. 2023, 13, 1052826. [Google Scholar] [CrossRef]
- Yao, W.; Ranganathan, V.; Allexandre, D.; Siemionow, V.; Yue, G. Kinesthetic imagery training of forceful muscle contractions increases brain signal and muscle strength. Front. Hum. Neurosci. 2013, 7, 561. [Google Scholar] [CrossRef] [Green Version]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Collet, C.; Di Rienzo, F.; Hoyek, N.; Guillot, A. Autonomic nervous system correlates in movement observation and motor imagery. Front. Hum. Neurosci. 2013, 7, 415. [Google Scholar] [CrossRef] [Green Version]
- Champely, S. pwr: Basic Functions for Power Analysis. R Package Version 1.3-0. 2020. Available online: https://CRAN.R-project.org/package=pwr (accessed on 16 March 2020).
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘nlme.’ Linear Nonlinear Mix. Eff. Models Version 3.1-162. 2023. Available online: https://rdrr.io/cran/nlme/ (accessed on 31 January 2023).
- Ben-Shachar, M.S.; Makowski, D.; Lüdecke, D.; Kelley, K.; Stanley, D. Package ‘Effectsize’; Version 0.8.3. 2023. Available online: https://cran.r-project.org/web/packages/effectsize/effectsize.pdf (accessed on 28 January 2023).
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S.; Hothorn, M.T. Package ‘Multcomp’. Simultaneous Inference in Generic Parametric Models Version 1.4-25. 2016. Available online: https://cran.r-project.org/web/packages/multcomp/multcomp.pdf (accessed on 20 June 2023).
- Bakker, F.C.; Boschker, M.S.; Chung, T. Changes in muscular activity while imagining weight lifting using stimulus or response propositions. J. Sport Exerc. Psychol. 1996, 18, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Cowley, P.M.; Clark, B.C.; Ploutz-Snyder, L.L. Kinesthetic motor imagery and spinal excitability: The effect of contraction intensity and spatial localization. Clin. Neurophysiol. 2008, 119, 1849–1856. [Google Scholar] [CrossRef]
- Decety, J.; Jeannerod, M.; Germain, M.; Pastene, J. Vegetative response during imagined movement is proportional to mental effort. Behav. Brain Res. 1991, 42, 1–5. [Google Scholar] [CrossRef]
- Martin, K.; Meeusen, R.; Thompson, K.G.; Keegan, R.; Rattray, B. Mental fatigue impairs endurance performance: A physiological explanation. Sports Med. 2018, 48, 2041–2051. [Google Scholar] [CrossRef]
- Meeusen, R.; Van Cutsem, J.; Roelands, B. Endurance exercise-induced and mental fatigue and the brain. Exp. Physiol. 2021, 106, 2294–2298. [Google Scholar] [CrossRef]
- Pageaux, B. The psychobiological model of endurance performance: An effort-based decision-making theory to explain self-paced endurance performance. Sports Med. 2014, 44, 1319–1320. [Google Scholar] [CrossRef]
- Grosprêtre, S.; Lebon, F.; Papaxanthis, C.; Martin, A. Spinal plasticity with motor imagery practice. J. Physiol. 2019, 597, 921–934. [Google Scholar] [CrossRef] [Green Version]
- Grosprêtre, S.; Jacquet, T.; Lebon, F.; Papaxanthis, C.; Martin, A. Neural mechanisms of strength increase after one-week motor imagery training. Eur. J. Sport Sci. 2018, 18, 209–218. [Google Scholar] [CrossRef]
- Grosprêtre, S.; Lebon, F.; Papaxanthis, C.; Martin, A. New evidence of corticospinal network modulation induced by motor imagery. J. Neurophysiol. 2016, 115, 1279–1288. [Google Scholar] [CrossRef]
- Li, G.; Huang, S.; Xu, W.; Jiao, W.; Jiang, Y.; Gao, Z.; Zhang, J. The impact of mental fatigue on brain activity: A comparative study both in resting state and task state using EEG. BMC Neurosci. 2020, 21, 20. [Google Scholar] [CrossRef]
- Soylu, Y.; Arslan, E.; Kilit, B. Psychophysiological responses and cognitive performance: A systematic review of mental fatigue on soccer performance. Int. J. Sport Stud. Health 2021, 4, e124244. [Google Scholar] [CrossRef]
- Wright, R.A.; Junious, T.R.; Neal, C.; Avello, A.; Graham, C.; Herrmann, L.; Junious, S.; Walton, N. Mental fatigue influence on effort-related cardiovascular response: Difficulty effects and extension across cognitive performance domains. Motiv. Emot. 2007, 31, 219–231. [Google Scholar] [CrossRef]
- Lebon, F.; Byblow, W.D.; Collet, C.; Guillot, A.; Stinear, C.M. The modulation of motor cortex excitability during motor imagery depends on imagery quality. Eur. J. Neurosci. 2012, 35, 323–331. [Google Scholar] [CrossRef]
- Williams, J.; Pearce, A.J.; Loporto, M.; Morris, T.; Holmes, P.S. The relationship between corticospinal excitability during motor imagery and motor imagery ability. Behav. Brain Res. 2012, 226, 369–375. [Google Scholar] [CrossRef]
- Kanthack, T.F.D.; Guillot, A.; Blache, Y.; Di Rienzo, F. Revisiting the acute effects of resistance exercise on motor imagery ability. Behav. Brain Res. 2021, 412, 113441. [Google Scholar] [CrossRef]
- McMorris, T.; Barwood, M.; Corbett, J. Central fatigue theory and endurance exercise: Toward an interoceptive model. Neurosci. Biobehav. Rev. 2018, 93, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Rejeski, W.J. Perceived exertion: An active or passive process? J. Sport Exerc. Psychol. 1985, 7, 371–378. [Google Scholar] [CrossRef]
- Demougeot, L.; Papaxanthis, C. Muscle fatigue affects mental simulation of action. J. Neurosci. 2011, 31, 10712–10720. [Google Scholar] [CrossRef]
- Rieger, M.; Boe, S.G.; Ingram, T.G.J.; Bart, V.K.E.; Dahm, S.F. A theoretical perspective on action consequences in action imagery: Internal prediction as an essential mechanism to detect errors. Psychol. Res. 2023, 1–10. [Google Scholar] [CrossRef]
- Louis, M.; Collet, C.; Guillot, A. Differences in motor imagery times during aroused and relaxed conditions. J. Cogn. Psychol. 2011, 23, 374–382. [Google Scholar] [CrossRef]
- Xia, L.; Wang, J.; Liang, F.; Li, W.; Guo, J.; Deng, Q. Mental fatigue assessment based on physiological signals. Nan Fang Yi Ke Da Xue Xue Bao 2012, 32, 870–873. [Google Scholar]
- Cheng, S.-Y.; Hsu, H.-T. Mental fatigue measurement using EEG. In Risk Management Trends; Nota, G., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Trejo, L.J.; Kubitz, K.; Rosipal, R.; Kochavi, R.L.; Montgomery, L.D. EEG-based estimation and classification of mental fatigue. Psychology 2015, 6, 572. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Rienzo, F.; Rozand, V.; Le Noac’h, M.; Guillot, A. A Quantitative Investigation of Mental Fatigue Elicited during Motor Imagery Practice: Selective Effects on Maximal Force Performance and Imagery Ability. Brain Sci. 2023, 13, 996. https://doi.org/10.3390/brainsci13070996
Di Rienzo F, Rozand V, Le Noac’h M, Guillot A. A Quantitative Investigation of Mental Fatigue Elicited during Motor Imagery Practice: Selective Effects on Maximal Force Performance and Imagery Ability. Brain Sciences. 2023; 13(7):996. https://doi.org/10.3390/brainsci13070996
Chicago/Turabian StyleDi Rienzo, Franck, Vianney Rozand, Marie Le Noac’h, and Aymeric Guillot. 2023. "A Quantitative Investigation of Mental Fatigue Elicited during Motor Imagery Practice: Selective Effects on Maximal Force Performance and Imagery Ability" Brain Sciences 13, no. 7: 996. https://doi.org/10.3390/brainsci13070996
APA StyleDi Rienzo, F., Rozand, V., Le Noac’h, M., & Guillot, A. (2023). A Quantitative Investigation of Mental Fatigue Elicited during Motor Imagery Practice: Selective Effects on Maximal Force Performance and Imagery Ability. Brain Sciences, 13(7), 996. https://doi.org/10.3390/brainsci13070996





