Excitatory Dorsal Lateral Prefrontal Cortex Transcranial Magnetic Stimulation Increases Social Anxiety
Abstract
1. Introduction
1.1. The Right Dorsal Lateral Prefrontal Cortex
1.2. TMS and rTMS
1.3. Cyberball: Creating Exclusion
1.4. Gap in Literature and Hypothesis
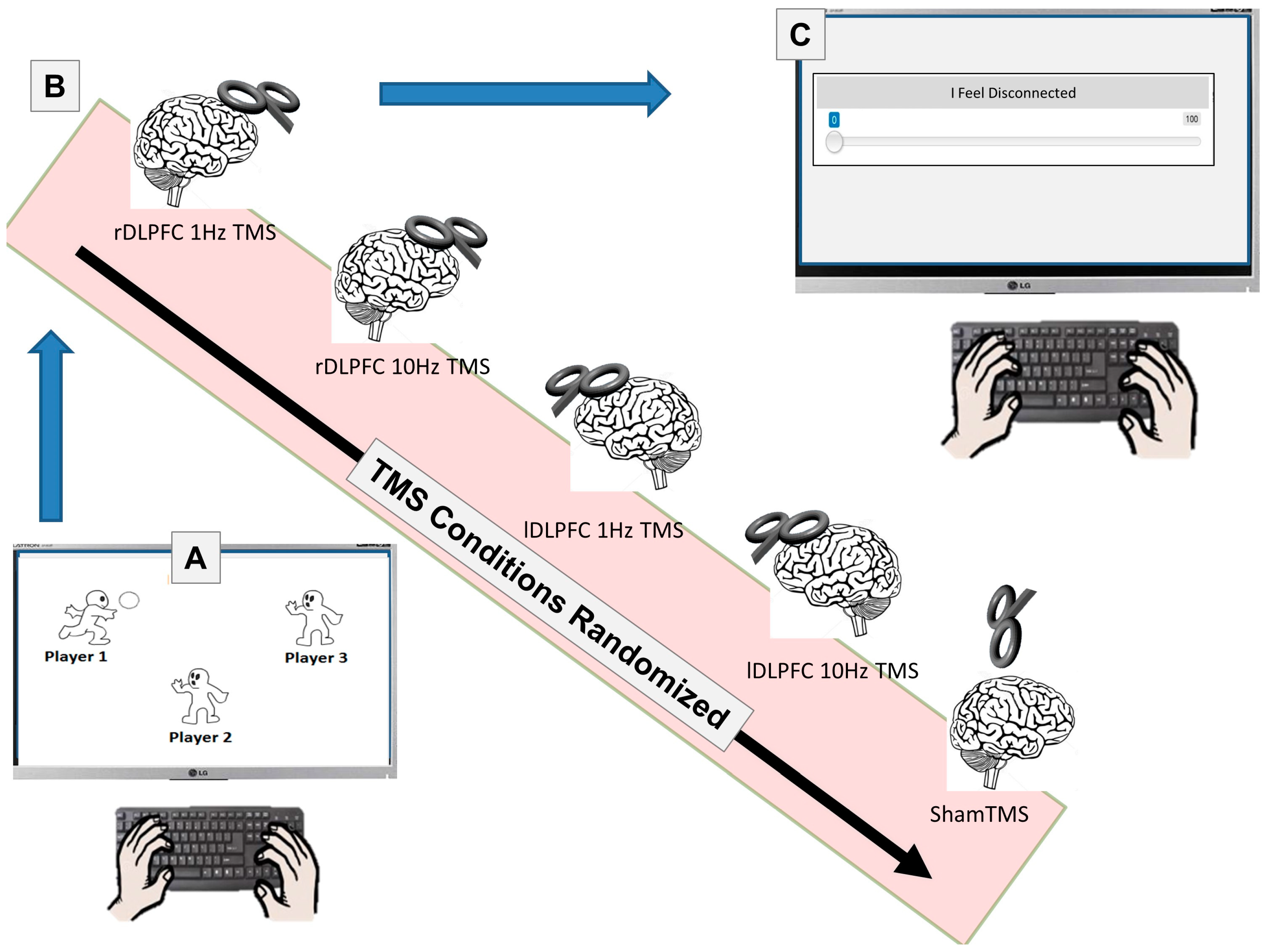
2. Materials and Methods
2.1. Participants
2.2. Materials
2.3. Stimuli
2.4. Procedure
2.5. Statistical Analyses
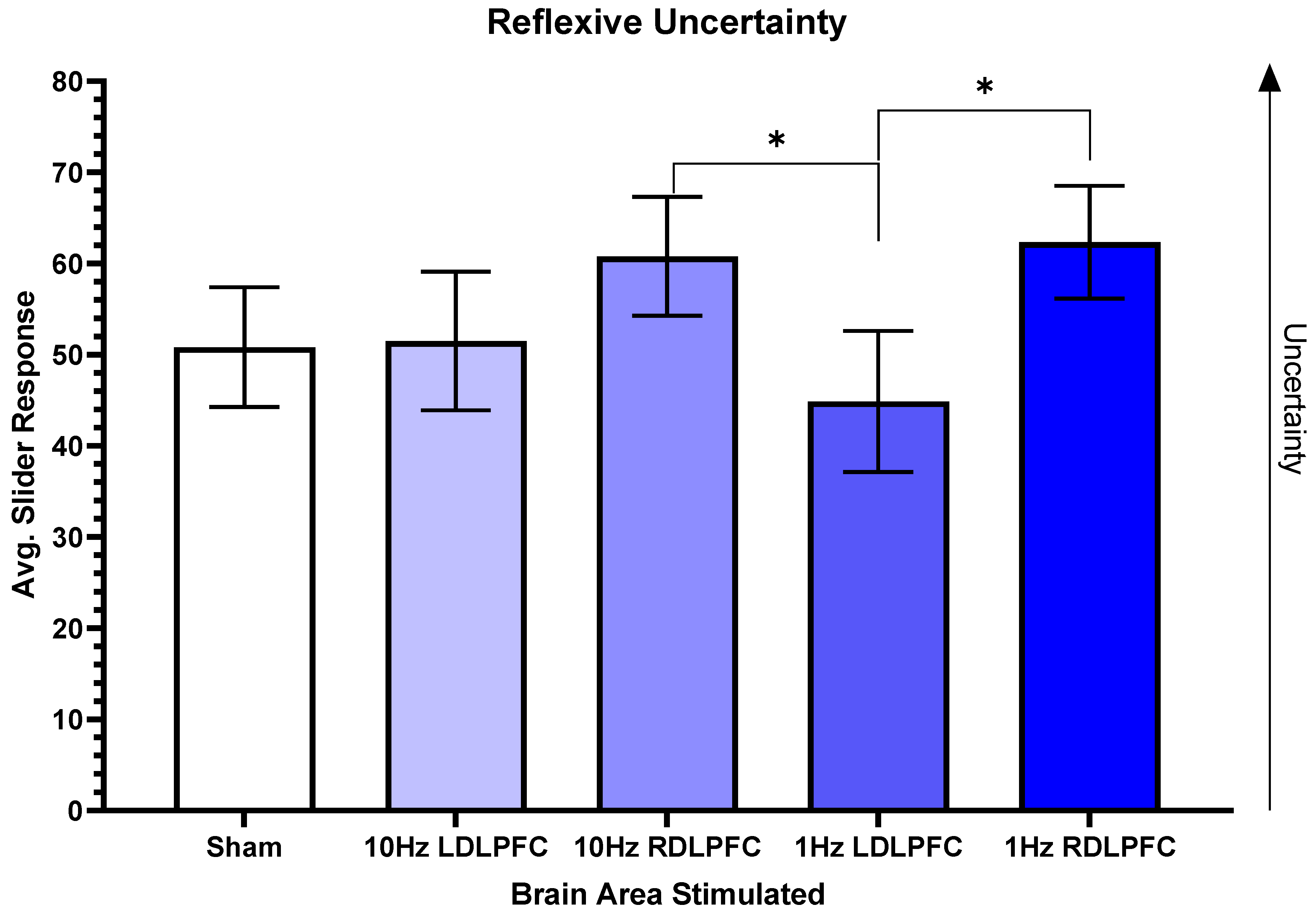
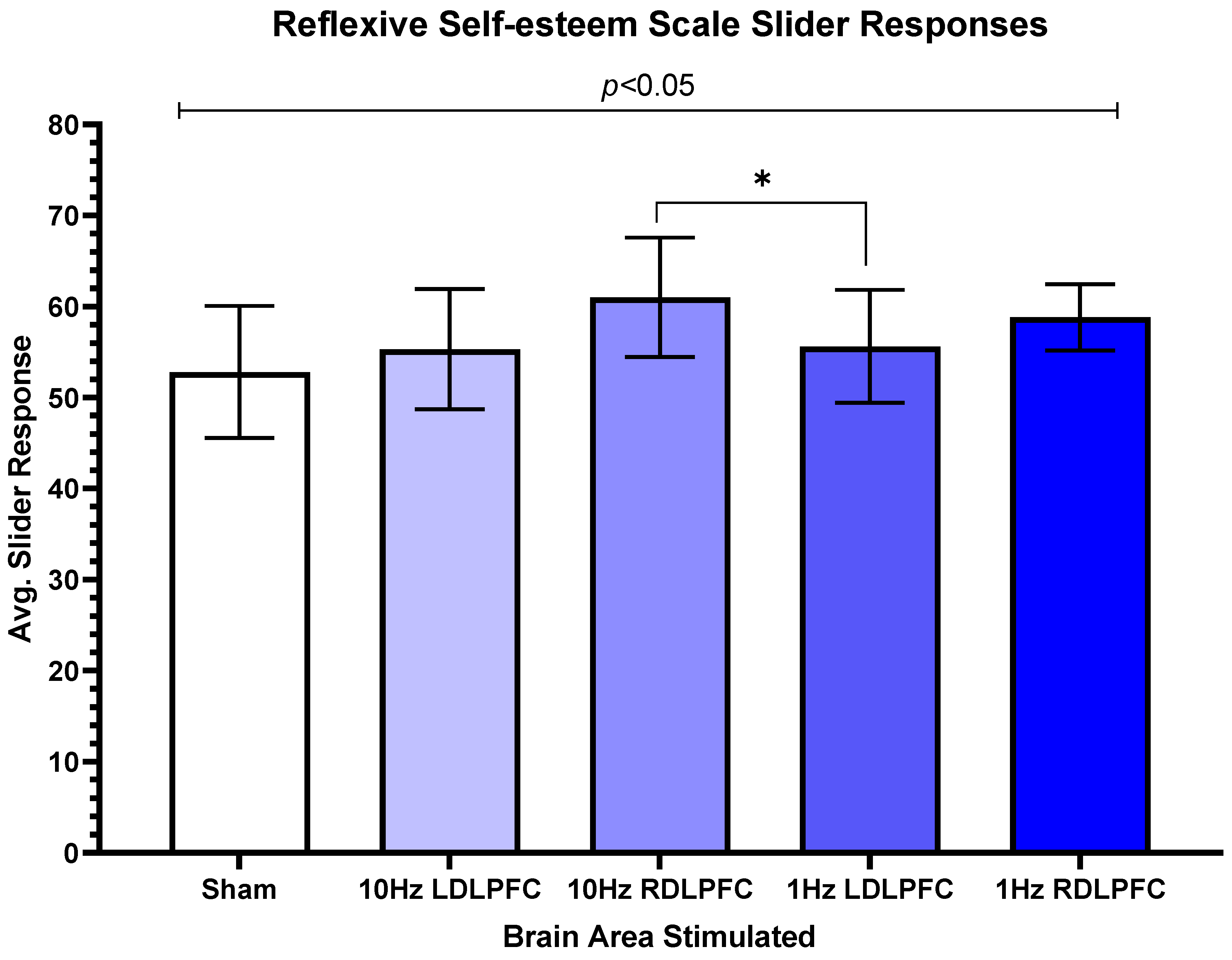
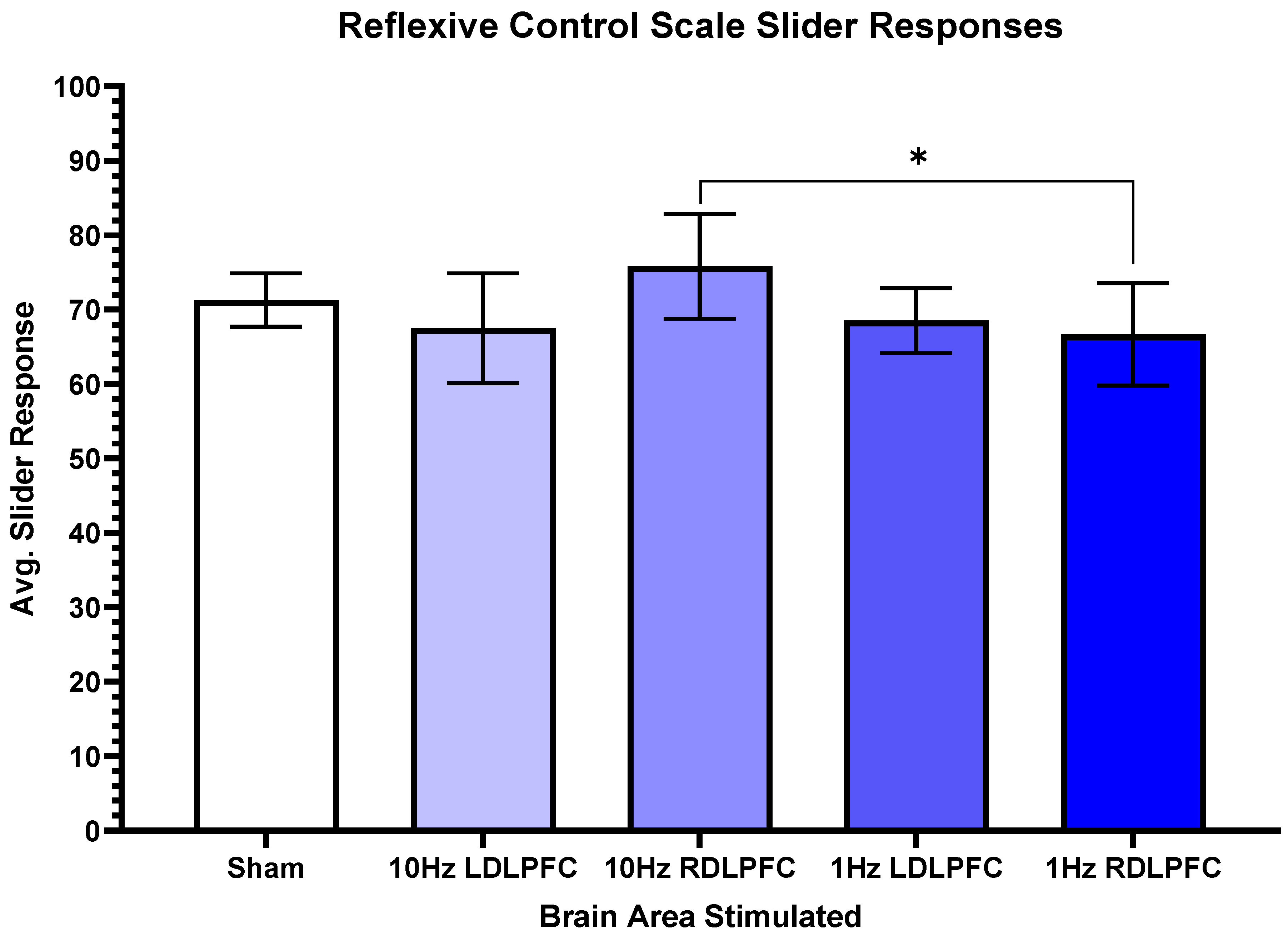
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumeister, R.F.; Leary, M.R. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 1995, 117, 497–529. [Google Scholar] [CrossRef]
- Snyder-Mackler, N.; Burger, J.R.; Gaydosh, L.; Belsky, D.W.; Noppert, G.A.; Campos, F.A.; Bartolomucci, A.; Yang, Y.C.; Aiello, A.E.; O’Rand, A.; et al. Social determinants of health and survival in humans and other animals. Science 2020, 368, eaax9553. [Google Scholar] [CrossRef]
- Macdonald, G.; Leary, M.R. Why does social exclusion hurt? The relationship between social and physical pain. Psychol. Bull. 2005, 131, 202–223. [Google Scholar] [CrossRef]
- Riva, P.; Eck, J. The many faces of social exclusion. In Social Exclusion: Psychological Approaches to Understanding and Reducing Its Impact; Springer: Cham, Switzerland, 2016; Volume 26. [Google Scholar]
- Twenge, J.M.; Baumeister, R.F.; Tice, D.M.; Stucke, T.S. If you can’t join them, beat them: Effects of social exclusion on aggressive behavior. J. Pers. Soc. Psychol. 2001, 81, 1058–1069. [Google Scholar] [CrossRef]
- Wang, H.; Braun, C.; Enck, P. How the brain reacts to social stress (exclusion)—A scoping review. Neurosci. Biobehav. Rev. 2017, 80, 80–88. [Google Scholar] [CrossRef]
- DeWall, C.N.; Deckman, T.; Pond, R.S., Jr.; Bonser, I. Belongingness as a core personality trait: How social exclusion influences social functioning and personality expression. J. Pers. 2011, 79, 1281–1314. [Google Scholar] [CrossRef]
- Williams, K.D.; Cheung, C.K.T.; Choi, W. CyberOstracism: Effects of being ignored over the Internet. J. Personal. Soc. Psychol. 2000, 79, 748. [Google Scholar] [CrossRef]
- Hartgerink, C.H.; van Beest, I.; Wicherts, J.M.; Williams, K.D. The ordinal effects of ostracism: A meta-analysis of 120 Cyberball studies. PLoS ONE 2015, 10, e0127002. [Google Scholar] [CrossRef]
- Jamieson, J.P.; Harkins, S.G.; Williams, K.D. Need threat can motivate performance after ostracism. Pers. Soc. Psychol. Bull. 2010, 36, 690–702. [Google Scholar] [CrossRef]
- Schoel, C.; Eck, J.; Greifeneder, R. A matter of vertical position: Consequences of ostracism differ for those above versus below its perpetrators. Soc. Psychol. Personal. Sci. 2014, 5, 149–157. [Google Scholar] [CrossRef]
- Zadro, L.; Williams, K.D.; Richardson, R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. J. Exp. Soc. Psychol. 2004, 40, 560–567. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Berntson, G.G. Social psychological contributions to the decade of the brain. Doctrine of multilevel analysis. Am. Psychol. 1992, 47, 1019–1028. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Cacioppo, S.; Capitanio, J.P.; Cole, S.W. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 2015, 66, 733–767. [Google Scholar] [CrossRef]
- Caspi, A.; Harrington, H.; Moffitt, T.E.; Milne, B.J.; Poulton, R. Socially isolated children 20 years later: Risk of cardiovascular disease. Arch. Pediatr. Adolesc. Med. 2006, 160, 805–811. [Google Scholar] [CrossRef]
- Patterson, A.C.; Veenstra, G. Loneliness and risk of mortality: A longitudinal investigation in Alameda County, California. Soc. Sci. Med. 2010, 71, 181–186. [Google Scholar] [CrossRef]
- Danese, A.; Moffitt, T.E.; Harrington, H.; Milne, B.J.; Polanczyk, G.; Pariante, C.M.; Poulton, R.; Caspi, A. Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009, 163, 1135–1143. [Google Scholar] [CrossRef]
- Chen, X.F.; He, P.; Xu, K.H.; Jin, Y.H.; Chen, Y.; Wang, B.; Hu, X.; Qi, L.; Wang, M.W.; Li, J. Disrupted Spontaneous Neural Activity and Its Interaction with Pain and Emotion in Temporomandibular Disorders. Front. Neurosci. 2022, 16, 941244. [Google Scholar] [CrossRef]
- Ducasse, D.; Courtet, P.; Olié, E. Physical and social pains in borderline disorder and neuroanatomical correlates: A systematic review. Curr. Psychiatry Rep. 2014, 16, 443. [Google Scholar] [CrossRef]
- Eisenberger; Lieberman, M.; Williams, K. Does rejection hurt? An FMRI study of social exclusion. Science 2003, 302, 290–292. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, Y.; Chen, J.; Wang, C.; Tang, Y.; Liu, J.; Lan, X.; Yu, H.; Lai, Y.; Hu, Y.; et al. Altered Functional Connectivity in Pain-Related Brain Regions and Its Correlation with Pain Duration in Bone Metastasis with Cancer Pain. Dis. Markers 2022, 2022, 3044186. [Google Scholar] [CrossRef]
- Eisenberger, N.I. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci. 2012, 13, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Eisenberger, N.I.; Jarcho, J.M.; Lieberman, M.D.; Naliboff, B.D. An experimental study of shared sensitivity to physical pain and social rejection. Pain 2006, 126, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Qiao, M.; Lu, Y.; Jiang, Y. Neuroimaging Mechanism of Cognitive Behavioral Therapy in Pain Management. Pain Res. Manag. 2022, 2022, 6266619. [Google Scholar] [CrossRef]
- Kross, E.; Berman, M.G.; Mischel, W.; Smith, E.E.; Wager, T.D. Social rejection shares somatosensory representations with physical pain. Proc. Natl. Acad. Sci. USA 2011, 108, 6270–6275. [Google Scholar] [CrossRef]
- Hatzitaskos, P.; Soldatos, C.R.; Kokkevi, A.; Stefanis, C.N. Substance abuse patterns and their association with psychopathology and type of hostility in male patients with borderline and antisocial personality disorder. Compr. Psychiatry 1999, 40, 278–282. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. Chronic pain syndromes and borderline personality. Innov. Clin. Neurosci. 2012, 9, 10–14. [Google Scholar]
- Schmahl, C.; Ludäscher, P.; Greffrath, W.; Kraus, A.; Valerius, G.; Schulze, T.G.; Treutlein, J.; Rietschel, M.; Smolka, M.N.; Bohus, M. COMT val158met polymorphism and neural pain processing. PLoS ONE 2012, 7, e23658. [Google Scholar] [CrossRef]
- Goldin, P.R.; McRae, K.; Ramel, W.; Gross, J.J. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol. Psychiatry 2008, 63, 577–586. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.W., 3rd; Cohen, J.D.; Stenger, V.A.; Carter, C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000, 288, 1835–1838. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Gross, J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005, 9, 242–249. [Google Scholar] [CrossRef]
- Schmitz, T.W.; Kawahara-Baccus, T.N.; Johnson, S.C. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage 2004, 22, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.B.; Gerson, S.A.; Vanderwert, R.E.; Cannon, E.N.; Fox, N.A. Hypervigilance to Rejecting Stimuli in Rejection Sensitive Individuals: Behavioral and Neurocognitive Evidence. Pers. Individ. Differ. 2015, 85, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto; Nittono, H.; Ura, M. Trait rejection sensitivity is associated with vigilance and defensive response rather than detection of social rejection cues. Front. Psychol. 2015, 6, 1516. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto; Ura, M.; Nittono, H. Intrapersonal and interpersonal processes of social exclusion. Front. Neurosci. 2015, 9, 62. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Masui, K.; Furutani, K.; Nomura, M.; Yoshida, H.; Ura, M. Temporal distance insulates against immediate social pain: An NIRS study of social exclusion. Soc. Neurosci. 2011, 6, 377–387. [Google Scholar] [CrossRef]
- Dörfel, D.; Lamke, J.P.; Hummel, F.; Wagner, U.; Erk, S.; Walter, H. Common and differential neural networks of emotion regulation by Detachment, Reinterpretation, Distraction, and Expressive Suppression: A comparative fMRI investigation. Neuroimage 2014, 101, 298–309. [Google Scholar] [CrossRef]
- Kohn, N.; Eickhoff, S.B.; Scheller, M.; Laird, A.R.; Fox, P.T.; Habel, U. Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. Neuroimage 2014, 87, 345–355. [Google Scholar] [CrossRef]
- Morawetz, C.; Bode, S.; Derntl, B.; Heekeren, H.R. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2017, 72, 111–128. [Google Scholar] [CrossRef]
- Christian, P.; Soutschek, A. Causal role of right dorsolateral prefrontal cortex for norm-guided social decision making: A meta-analysis of TMS studies. Neuropsychologia 2022, 176, 108393. [Google Scholar] [CrossRef]
- Li, X.; Lu, T.; Yu, H.; Shen, J.; Chen, Z.; Yang, X.; Huang, Z.; Yang, Y.; Feng, Y.; Zhou, X.; et al. Repetitive Transcranial Magnetic Stimulation for Neuropathic Pain and Neuropsychiatric Symptoms in Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Neural Plast. 2022, 2022, 2036736. [Google Scholar] [CrossRef] [PubMed]
- Wiech, K.; Eippert, F.; Vandekerckhove, J.; Zaman, J.; Placek, K.; Tuerlinckx, F.; Vlaeyen, J.W.S.; Tracey, I. Cortico-Brainstem Mechanisms of Biased Perceptual Decision-Making in the Context of Pain. J. Pain 2022, 23, 680–692. [Google Scholar] [CrossRef]
- Baumgartner, T.; Knoch, D.; Hotz, P.; Eisenegger, C.; Fehr, E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nat. Neurosci. 2011, 14, 1468–1474. [Google Scholar] [CrossRef]
- Knoch, D.; Pascual-Leone, A.; Meyer, K.; Treyer, V.; Fehr, E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science 2006, 314, 829–832. [Google Scholar] [CrossRef]
- Strang, S.; Gross, J.; Schuhmann, T.; Riedl, A.; Weber, B.; Sack, A.T. Be nice if you have to—The neurobiological roots of strategic fairness. Soc. Cogn. Affect. Neurosci. 2014, 10, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Spielberg, J.M.; Miller, G.A.; Engels, A.S.; Herrington, J.D.; Sutton, B.P.; Banich, M.T.; Heller, W. Trait approach and avoidance motivation: Lateralized neural activity associated with executive function. NeuroImage 2011, 54, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Cappon, D.; den Boer, T.; Jordan, C.; Yu, W.; Metzger, E.; Pascual-Leone, A. Transcranial magnetic stimulation (TMS) for geriatric depression. Ageing Res. Rev. 2022, 74, 101531. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.; George, M.S.; Grammer, G.; Janicak, P.G.; Pascual-Leone, A.; Wirecki, T.S. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef]
- Janicak, P.G.; Nahas, Z.; Lisanby, S.H.; Solvason, H.B.; Sampson, S.M.; McDonald, W.M.; Marangell, L.B.; Rosenquist, P.; McCall, W.V.; Kimball, J.; et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: Assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010, 3, 187–199. [Google Scholar] [CrossRef]
- McDonald, W.M.; Durkalski, V.; Ball, E.R.; Holtzheimer, P.E.; Pavlicova, M.; Lisanby, S.H.; Avery, D.; Anderson, B.S.; Nahas, Z.; Zarkowski, P.; et al. Improving the antidepressant efficacy of transcranial magnetic stimulation: Maximizing the number of stimulations and treatment location in treatment-resistant depression. Depress. Anxiety 2011, 28, 973–980. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef]
- Mishra, B.R.; Sarkar, S.; Praharaj, S.K.; Mehta, V.S.; Diwedi, S.; Nizamie, S.H. Repetitive transcranial magnetic stimulation in psychiatry. Ann. Indian Acad. Neurol. 2011, 14, 245–251. [Google Scholar]
- Huerta, P.T.; Volpe, B.T. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J. Neuroeng. Rehabil. 2009, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.; Gold, A.K.; Nardi, A.E.; Ornelas, A.C.; Nierenberg, A.A.; Camprodon, J.; Kinrys, G. Transcranial magnetic stimulation in anxiety and trauma-related disorders: A systematic review and meta-analysis. Brain Behav. 2019, 9, e01284. [Google Scholar] [CrossRef]
- Diefenbach, G.J.; Bragdon, L.B.; Zertuche, L.; Hyatt, C.J.; Hallion, L.S.; Tolin, D.F.; Goethe, J.W.; Assaf, M. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: A pilot randomised, double-blind, sham-controlled trial. Br. J. Psychiatry 2016, 209, 222–228. [Google Scholar] [CrossRef]
- Dilkov, D.; Hawken, E.R.; Kaludiev, E.; Milev, R. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: A randomized, double-blind sham controlled clinical trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 78, 61–65. [Google Scholar] [CrossRef]
- Bystritsky, A.; Kaplan, J.T.; Feusner, J.D.; Kerwin, L.E.; Wadekar, M.; Burock, M.; Wu, A.D.; Iacoboni, M. A preliminary study of fMRI-guided rTMS in the treatment of generalized anxiety disorder. J. Clin. Psychiatry 2008, 69, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Bystritsky, A.; Kerwin, L.E.; Feusner, J.D. A preliminary study of fMRI-guided rTMS in the treatment of generalized anxiety disorder: 6-month follow-up. J. Clin. Psychiatry 2009, 70, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J.; Chapman, C.A.; Trepel, C.; Teskey, G.C.; Milgram, N.W. Post-activation potentiation in the neocortex. IV. Multiple sessions required for induction of long-term potentiation in the chronic preparation. Brain Res. 1995, 702, 87–93. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhao, J.; Shen, J.; Muhlert, N.; Elliott, R.; Zhang, D. The right VLPFC and downregulation of social pain: A TMS study. Hum. Brain Mapp. 2020, 41, 1362–1371. [Google Scholar] [CrossRef]
- He, Z.; Lin, Y.; Xia, L.; Liu, Z.; Zhang, D.; Elliott, R. Critical role of the right VLPFC in emotional regulation of social exclusion: A tDCS study. Soc. Cogn. Affect. Neurosci. 2018, 13, 357–366. [Google Scholar] [CrossRef]
- He, Z.; Liu, Z.; Zhao, J.; Elliott, R.; Zhang, D. Improving emotion regulation of social exclusion in depression-prone individuals: A tDCS study targeting right VLPFC. Psychol. Med. 2020, 50, 2768–2779. [Google Scholar] [CrossRef]
- Zhao, J.; Mo, L.; Bi, R.; He, Z.; Chen, Y.; Xu, F.; Xie, H.; Zhang, D. The VLPFC versus the DLPFC in Downregulating Social Pain Using Reappraisal and Distraction Strategies. J. Neurosci. 2021, 41, 1331–1339. [Google Scholar] [CrossRef]
- Sliwa, J.; Freiwald, W.A. A dedicated network for social interaction processing in the primate brain. Science 2017, 356, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Schrag, A.; Trimble, M.R. Dissociation, childhood interpersonal trauma, and family functioning in patients with somatization disorder. Am. J. Psychiatry 2005, 162, 899–905. [Google Scholar] [CrossRef]
- Levine, F.M.; Krass, S.M.; Padawer, W.J. Failure hurts: The effects of stress due to difficult tasks and failure feedback on pain report. Pain 1993, 54, 335–340. [Google Scholar] [CrossRef]
- van den Hout, J.H.; Vlaeyen, J.W.; Peters, M.L.; Engelhard, I.M.; van den Hout, M.A. Does failure hurt? The effects of failure feedback on pain report, pain tolerance and pain avoidance. Eur. J. Pain. 2000, 4, 335–346. [Google Scholar] [CrossRef]
- Wilson, J.M.; Colebaugh, C.A.; Flowers, K.M.; Meints, S.M.; Edwards, R.R.; Schreiber, K.L. Social Support and Psychological Distress among Chronic Pain Patients: The Mediating Role of Mindfulness. Pers. Individ. Differ. 2022, 190. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, M.; van Duijvenvoorde, A.C.K.; van, I.M.H.; Bakermans-Kranenburg, M.J.; Crone, E.A. Longitudinal changes in DLPFC activation during childhood are related to decreased aggression following social rejection. Proc. Natl. Acad. Sci. USA 2020, 117, 8602–8610. [Google Scholar] [CrossRef] [PubMed]
- Chester, D.S.; Eisenberger, N.I.; Pond, R.S., Jr.; Richman, S.B.; Bushman, B.J.; Dewall, C.N. The interactive effect of social pain and executive functioning on aggression: An fMRI experiment. Soc. Cogn. Affect. Neurosci. 2014, 9, 699–704. [Google Scholar] [CrossRef]
- Cacioppo, S.; Frum, C.; Asp, E.; Weiss, R.M.; Lewis, J.W.; Cacioppo, J.T. A quantitative meta-analysis of functional imaging studies of social rejection. Sci. Rep. 2013, 3, 2027. [Google Scholar] [CrossRef]
- Holte, A.J.; Fisher, W.N.; Ferraro, F.R. Afraid of Social Exclusion: Fear of Missing Out Predicts Cyberball-Induced Ostracism. J. Technol. Behav. Sci. 2022, 7, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.A.; Flynn, C.; Garber, J. Prospective relations between rejection and depression in young adolescents. J. Pers. Soc. Psychol. 2003, 85, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Rudert, S.C.; Janke, S.; Greifeneder, R. Ostracism breeds depression: Longitudinal associations between ostracism and depression over a three-year-period. J. Affect. Disord. Rep. 2021, 4, 100118. [Google Scholar] [CrossRef]
- Taylor, H.O.; Taylor, R.J.; Nguyen, A.W.; Chatters, L. Social Isolation, Depression, and Psychological Distress Among Older Adults. J. Aging Health 2018, 30, 229–246. [Google Scholar] [CrossRef]
- Cash, R.F.H.; Cocchi, L.; Lv, J.; Wu, Y.; Fitzgerald, P.B.; Zalesky, A. Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility. Hum. Brain Mapp. 2021, 42, 4155–4172. [Google Scholar] [CrossRef]
- Cheng, C.M.; Li, C.T.; Tsai, S.J. Current Updates on Newer Forms of Transcranial Magnetic Stimulation in Major Depression. Adv. Exp. Med. Biol. 2021, 1305, 333–349. [Google Scholar]
- Sonmez, A.I.; Camsari, D.D.; Nandakumar, A.L.; Voort, J.L.V.; Kung, S.; Lewis, C.P.; Croarkin, P.E. Accelerated TMS for Depression: A systematic review and meta-analysis. Psychiatry Res. 2019, 273, 770–781. [Google Scholar] [CrossRef]
- Crone, E.A.; Steinbeis, N. Neural Perspectives on Cognitive Control Development during Childhood and Adolescence. Trends Cogn. Sci. 2017, 21, 205–215. [Google Scholar] [CrossRef]
- Zwanzger, P.; Steinberg, C.; Rehbein, M.A.; Bröckelmann, A.K.; Dobel, C.; Zavorotnyy, M.; Domschke, K.; Junghöfer, M. Inhibitory repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex modulates early affective processing. Neuroimage 2014, 101, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Lillquist, B.; Kanpa, V.; Crawford, M.; El Filali, M.; Oakes, J.; Jonasz, A.; Disney, A.; Keenan, J.P. Preliminary Evidence of the Role of Medial Prefrontal Cortex in Self-Enhancement: A Transcranial Magnetic Stimulation Study. Brain Sci. 2020, 10, 535. [Google Scholar] [CrossRef]
- Shelansky, T.; Chavarria, K.; Pagano, K.; Sierra, S.; Martinez, V.; Ahmad, N.; Brenya, J.; Janowska, A.; Zorns, S.; Straus, A.; et al. Employing Transcranial Magnetic Stimulation in a Resource Limited Environment to Establish Brain-Behavior Relationships. J. Vis. Exp. 2022, 182, e62773. [Google Scholar]
- Duran, K.A.; O’Halloran, H.; Soder, H.; Yasin, S.; Kramer, R.; Rosen, S.; Brenya, J.; Chavarria, K.; Savitska, L.; Keenan, J.P. The medial prefrontal cortex: A potential link between self-deception and affect. Int. J. Neurosci. 2021, 131, 701–707. [Google Scholar] [CrossRef]
- Kramer, R.; Jordan, K.; Soder, H.; Applegate, L.; Youssef, A.; Criscione, M.; Keenan, J.P. The Special Brain: Subclinical Grandiose Narcissism and Self-Face Recognition in the Right Prefrontal Cortex. Am. J. Psychol. 2020, 133, 487–500. [Google Scholar] [CrossRef]
- Williams, K.D. Ostracism: A temporal need-threat model. Adv. Exp. Soc. Psychol. 2009, 41, 279–314. [Google Scholar]
- Hales, A.H.; Williams, K.D. Marginalized individuals and extremism: The role of ostracism in openness to extreme groups. J. Soc. Issues 2018, 74, 75–92. [Google Scholar] [CrossRef]
- Wassermann, E.M. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr. Clin. Neurophysiol. 1998, 108, 1–16. [Google Scholar] [CrossRef]
- Keenan, J.P. An Examination of Right Dorsolateral Prefrontal Lobe Function in Self-Directed Attention by Use of Repetitive Transcranial Magnetic Stimulation, Electroencephalograph, and Visual Evoked Potentials. Ph.D. Thesis, State University of New York at Albany, New York, NY, USA, 1998. [Google Scholar]
- Pascual-Leone, A.; Bartres-Faz, D.; Keenan, J.P. Transcranial magnetic stimulation: Studying the brain-behaviour relationship by induction of ‘virtual lesions’. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 2000, 133, 425–430. [Google Scholar] [CrossRef]
- Burke, M.J.; Fried, P.J.; Pascual-Leone, A. Transcranial magnetic stimulation: Neurophysiological and clinical applications. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 163, pp. 73–92. [Google Scholar]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Bicks, L.K.; Yamamuro, K.; Flanigan, M.E.; Kim, J.M.; Kato, D.; Lucas, E.K.; Koike, H.; Peng, M.S.; Brady, D.M.; Chandrasekaran, S.; et al. Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior. Nat. Commun. 2020, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- McCambridge, J.; de Bruin, M.; Witton, J. The effects of demand characteristics on research participant behaviours in non-laboratory settings: A systematic review. PLoS ONE 2012, 7, e39116. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Ryu, C.; Kim, S.; Jeong, S.J.; Koo, J.W.; Lee, Y.S.; Kim, S.J. Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice. Cell Rep. 2021, 35, 109104. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, A.C.; Medaglia, J.D.; Tinker, J.R.; Ayaz, H.; Forman, E.M.; Newman, C.F.; Williams, J.M.; Hillary, F.G.; Platek, S.M.; Onaral, B.; et al. Medial prefrontal cortex hyperactivation during social exclusion in borderline personality disorder. Psychiatry Res. 2010, 181, 233–236. [Google Scholar] [CrossRef]
- Elliott, R.; Lythe, K.; Lee, R.; McKie, S.; Juhasz, G.; Thomas, E.J.; Downey, D.; Deakin, J.F.; Anderson, I.M. Reduced medial prefrontal responses to social interaction images in remitted depression. Arch. Gen. Psychiatry 2012, 69, 37–45. [Google Scholar] [CrossRef]
- Makinodan, M.; Rosen, K.M.; Ito, S.; Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012, 337, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.S.; Johnstone, T.; Peterson, M.J.; Kolden, G.G.; Kalin, N.H.; Davidson, R.J. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry 2013, 70, 1181–1189. [Google Scholar] [CrossRef]
- Chrysikou, E.G.; Gorey, C.; Aupperle, R.L. Anodal transcranial direct current stimulation over right dorsolateral prefrontal cortex alters decision making during approach-avoidance conflict. Soc. Cogn. Affect. Neurosci. 2017, 12, 468–475. [Google Scholar] [CrossRef]
- Longe, O.; Maratos, F.A.; Gilbert, P.; Evans, G.; Volker, F.; Rockliff, H.; Rippon, G. Having a word with yourself: Neural correlates of self-criticism and self-reassurance. Neuroimage 2010, 49, 1849–1856. [Google Scholar] [CrossRef]
- Lord, C.; Steiner, M.; Soares, C.N.; Carew, C.L.; Hall, G.B. Stress response in postpartum women with and without obsessive-compulsive symptoms: An fMRI study. J. Psychiatry Neurosci. 2012, 37, 78–86. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stein, M.B. Interoception in anxiety and depression. Brain Struct. Funct. 2010, 214, 451–463. [Google Scholar] [CrossRef]
- Heine, S.J.; Lehman, D.R.; Markus, H.R.; Kitayama, S. Is there a universal need for positive self-regard? Psychol. Rev. 1999, 106, 766–794. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Wu, S.; Shi, Z.; Liu, M.; Peng, M.; Shen, Y.; Yang, J. Activations of the dorsolateral prefrontal cortex and thalamus during agentic self-evaluation are negatively associated with trait self-esteem. Brain Res. 2018, 1692, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Gross, J.J. Thinking makes it so: A social cognitive neuroscience approach to emotion regulation. In Handbook of Self-Regulation: Research, Theory, and Application; The Guilford Press: New York, NY, USA, 2004. [Google Scholar]
- Fuster, J.M. The prefrontal cortex--an update: Time is of the essence. Neuron 2001, 30, 319–333. [Google Scholar] [CrossRef]
- Petrides, M. Lateral prefrontal cortex: Architectonic and functional organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Rakic, P.S. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 1445–1453. [Google Scholar] [PubMed]


| Questions | Category |
|---|---|
| I felt “disconnected” during the game. | Belonging |
| I felt “rejected” during the game. | Belonging |
| I felt like an outsider during the game. | Belonging |
| I felt good about myself during the game. | Self-esteem |
| My self-esteem was high during the game. | Self-esteem |
| I felt insecure during the game. | Self-esteem |
| I felt like I had control during the game. | Control |
| I felt the other players decided everything during the game. | Control |
| I felt powerful during the game. | Control |
| I felt uncertain about myself during the game. | Certainty |
| I did not know what I should be doing during the game. | Certainty |
| I felt unsure of what makes me who I am during the game. | Certainty |
| I felt friendly during the game. | Mood |
| I felt angry during the game. | Mood |
| I felt happy during the game. | Mood |
| I was ignored during the game. | Manipulation check |
| I was excluded during the game. | Manipulation check |
| I felt like I wanted to escape the game. | Anxiety |
| I felt like I wanted to leave the game. | Anxiety |
| I felt uneasy during the game. | Anxiety |
| I liked the other players. | Perception of others |
| I enjoyed playing with the others. | Perception of others |
| I was angry at the other players. | Perception of others |
| Questions | Category |
|---|---|
| I feel ‘disconnected’. | Belonging |
| I feel ‘rejected’. | Belonging |
| I feel like an outsider. | Belonging |
| I feel good about myself. | Self-esteem |
| My self-esteem is high. | Self-esteem |
| I feel insecure. | Self-esteem |
| I feel like I have control. | Control |
| I feel the other players decided everything. | Control |
| I feel powerful. | Control |
| I feel uncertain about myself. | Certainty |
| I do not know what I should be doing. | Certainty |
| I feel unsure of what makes me who I am. | Certainty |
| I feel friendly. | Mood |
| I feel angry. | Mood |
| I feel happy. | Mood |
| I would join another game. | Anxiety |
| I would sign up for another game. | Anxiety |
| I look forward to my next social event. | Anxiety |
| I like the other players. | Perception of others |
| I enjoy playing with the other players. | Perception of others |
| I am angry at the other players. | Perception of others |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minervini, A.; LaVarco, A.; Zorns, S.; Propper, R.; Suriano, C.; Keenan, J.P. Excitatory Dorsal Lateral Prefrontal Cortex Transcranial Magnetic Stimulation Increases Social Anxiety. Brain Sci. 2023, 13, 989. https://doi.org/10.3390/brainsci13070989
Minervini A, LaVarco A, Zorns S, Propper R, Suriano C, Keenan JP. Excitatory Dorsal Lateral Prefrontal Cortex Transcranial Magnetic Stimulation Increases Social Anxiety. Brain Sciences. 2023; 13(7):989. https://doi.org/10.3390/brainsci13070989
Chicago/Turabian StyleMinervini, Anthony, Adriana LaVarco, Samantha Zorns, Ruth Propper, Christos Suriano, and Julian Paul Keenan. 2023. "Excitatory Dorsal Lateral Prefrontal Cortex Transcranial Magnetic Stimulation Increases Social Anxiety" Brain Sciences 13, no. 7: 989. https://doi.org/10.3390/brainsci13070989
APA StyleMinervini, A., LaVarco, A., Zorns, S., Propper, R., Suriano, C., & Keenan, J. P. (2023). Excitatory Dorsal Lateral Prefrontal Cortex Transcranial Magnetic Stimulation Increases Social Anxiety. Brain Sciences, 13(7), 989. https://doi.org/10.3390/brainsci13070989





