Organization of the Subdiaphragmatic Vagus Nerve and Its Connection with the Celiac Plexus and the Ovaries in the Female Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Gross Anatomy
2.3. Acetylcholinesterase Histochemistry (AChE)
2.4. Histological Analysis
2.5. Immunofluorescence Technique
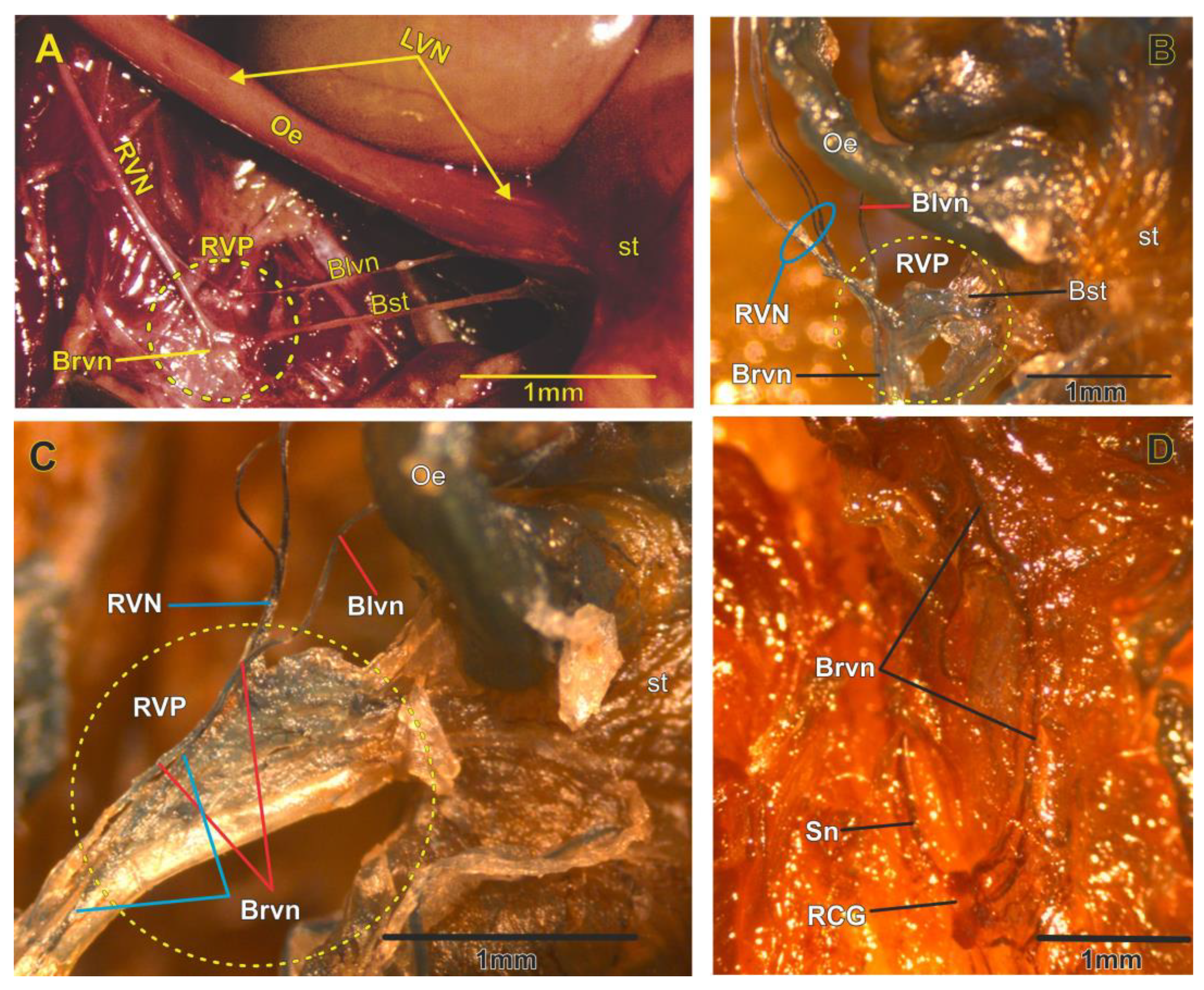
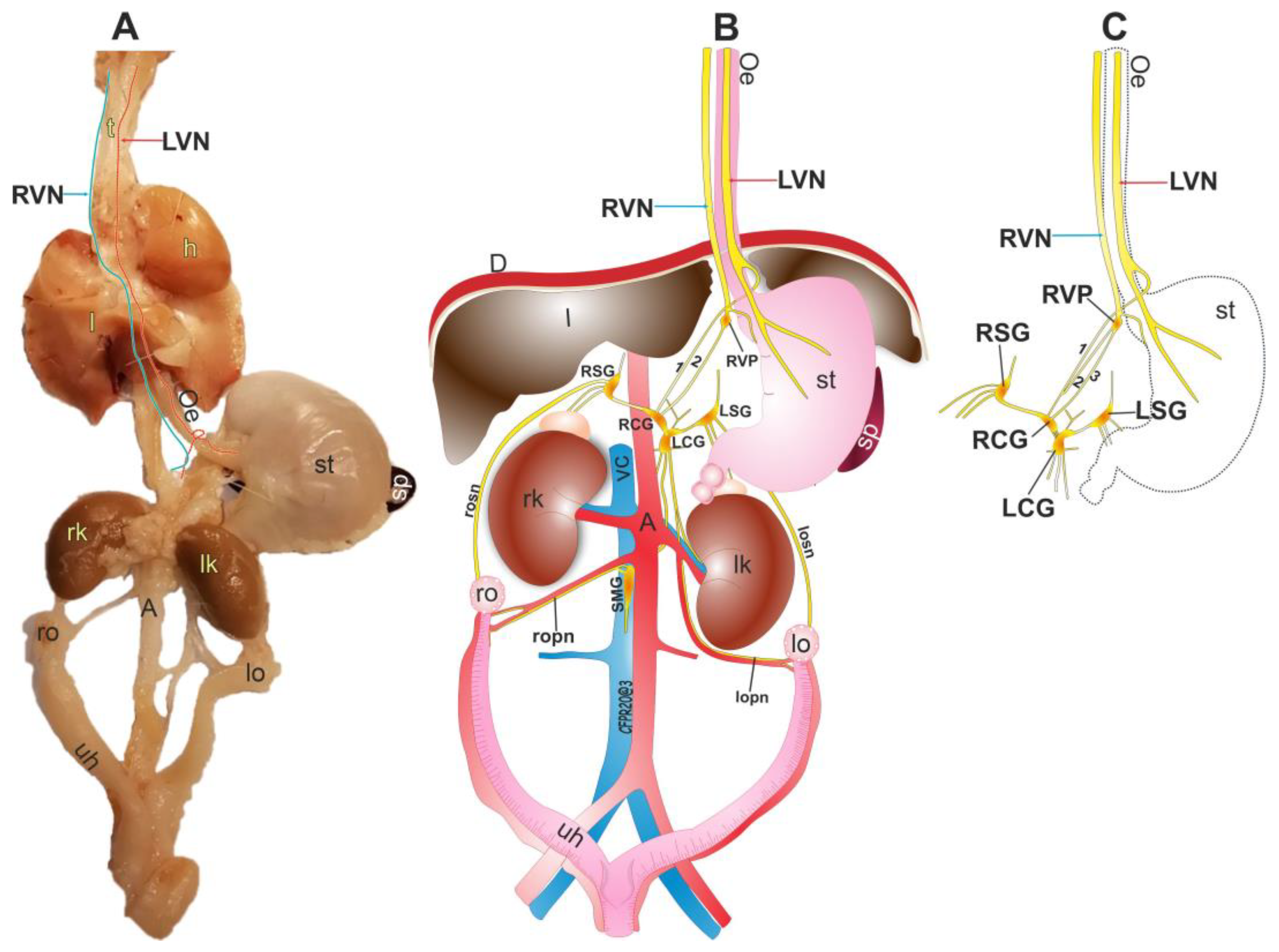
3. Results
3.1. Anatomy of the Right Vagus Nerve
3.2. Anatomy of the Left Vagus Nerve
3.3. Right Vagal Plexus as a Center of Synapsis of the RVN
3.4. The Vagal Nerve Plexus as a Communicating Network in the Abdominal Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baljet, B.; Drukker, J. The extrinsic innervation of the abdominal organs in the female rat. Cells Tissues Organs 1979, 104, 243–267. [Google Scholar] [CrossRef]
- Ojeda, S.R.; White, S.S.; Aguado, L.I.; Advis, J.P.; Andersen, J.M. Abdominal Vagotomy Delays the Onset of Puberty and Inhibits Ovarian Function in the Female Rat. Neuroendocrinology 1983, 36, 261–267. [Google Scholar] [CrossRef]
- Burden, H.W.; Capps, M.L.; Smith, C.P.; Harris, P.; Lawrence, I.E. Extrinsic sensory innervation of the rat ovary. In Federation Proceedings; Federation American Societies for Experimental Biology: Bethesda, MD, USA, 1982; p. 1492. [Google Scholar]
- Lawrence, I.E., Jr.; Burden, H.W. The origin of the extrinsic adrenergic innervation to the rat ovary. Anat. Rec. 1980, 196, 51–59. [Google Scholar] [CrossRef]
- Pastelín, C.F.; Rosas, N.H.; Morales-Ledesma, L.; Linares, R.; Domínguez, R.; Morán, C. Anatomical organization and neural pathways of the ovarian plexus nerve in rats. J. Ovarian Res. 2017, 10, 18. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Walker, H.K. Cranial Nerves IX and X: The Glossopharyngeal and Vagus Nerves. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. Available online: http://www.ncbi.nlm.nih.gov/books/NBK386/ (accessed on 25 October 2022).
- Principios de Neurociencia Haines 4a Ed|PDF|Sistema Nervioso|Neurona, Scribd. Available online: https://es.scribd.com/document/531766985/Principios-de-Neurociencia-Haines-4a-Ed (accessed on 25 October 2022).
- Paxinos, G. The Rat Nervous System—4th Edition. Available online: https://www.elsevier.com/books/the-rat-nervous-system/paxinos/978-0-12-374245-2 (accessed on 25 October 2022).
- Wilson-Pauwels, L.; Akesson, E.J.; Stewart, P.A.; Spacey, S.D. Cranial Nerves in Health and Disease; PMPH USA (BC Decker): Shelton, CT, USA, 2002. [Google Scholar]
- Ellis, H. Clinical Anatomy: A Revision and Applied Anatomy for Clinical Students, 11th ed.; Blackwell Pub.: Malden, MA, USA, 2006. [Google Scholar]
- Neuroanatomy—Draw.it—To.know—It.2nd—Ed Parsamed—Ir|PDF|Cerebrospinal Fluid|Spinal Cord, Scribd. Available online: https://www.scribd.com/document/229323526/Neuroanatomy-draw-It-to-Know-it-2nd-ed-Parsamed-ir (accessed on 25 October 2022).
- Browning, K.N.; Verheijden, S.; Boeckxstaens, G.E. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology 2017, 152, 730–744. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Powley, T.L. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J. Auton. Nerv. Syst. 1993, 42, 153–169. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Powley, T.L. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: Morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc. Res. Tech. 1996, 35, 80–86. [Google Scholar] [CrossRef]
- de Lartigue, G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J. Physiol. 2016, 594, 5791–5815. [Google Scholar] [CrossRef]
- Gerendai, I.; Tóth, I.E.; Boldogköi, Z.; Medveczky, I.; Halász, B. CNS structures presumably involved in vagal control of ovarian function. J. Auton. Nerv. Syst. 2000, 80, 40–45. [Google Scholar] [CrossRef]
- Chávez, R.; Sánchez, S.; Ulloa-Aguirre, A.; Domínguez, R. Effects on oestrous cyclicity and ovulation of unilateral section of the vagus nerve performed on different days of the oestrous cycle in the rat. J. Endocrinol. 1989, 123, 441–444. [Google Scholar] [CrossRef]
- Morales, L.; Chávez, R.; Ayala, M.; Dominguez, R. Effects of unilateral or bilateral superior ovarian nerve section in pre-pubertal rats on the ovulatory response to gonadotrophin administration. Rev. De Endocrinol. 1998, 158, 213–220. [Google Scholar]
- Morales-Ledesma, L.; Linares, R.; Rosas, G.; Morán, C.; Chavira, R.; Cárdenas, M.; Domínguez, R. Unilateral sectioning of the superior ovarian nerve of rats with polycystic ovarian syndrome restores ovulation in the innervated ovary. Reprod. Biol. Endocrinol. 2010, 8, 99. [Google Scholar] [CrossRef]
- Lara, H.E.; Dissen, G.A.; Leyton, V.; Paredes, A.; Fuenzalida, H.; Fiedler, J.L.; Ojeda, S.R. An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology 2000, 141, 1059–1072. [Google Scholar] [CrossRef]
- Linares, R.; Rosas, G.; Vieyra, E.; Ramírez, D.A.; Velázquez, D.R.; Espinoza, J.A.; Morán, C.; Domínguez, R.; Morales-Ledesma, L. In Adult Rats with Polycystic Ovarian Syndrome, Unilateral or Bilateral Vagotomy Modifies the Noradrenergic Concentration in the Ovaries and the Celiac Superior Mesenteric Ganglia in Different Ways. Front. Physiol. 2019, 10, 1309. [Google Scholar] [CrossRef]
- Jiron, J.M.; Calle, J.L.M.; Castillo, E.J.; Abraham, A.M.; Messer, J.G.; Malphurs, W.L.; Malinowski, C.; Grove, K.; Reznikov, L.R.; Zubcevic, J.; et al. Comparison of Isoflurane, Ketamine–Dexmedetomidine, and Ketamine–Xylazine for General Anesthesia during Oral Procedures in Rice Rats (Oryzomys palustris). J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 40–49. [Google Scholar] [CrossRef]
- Agrawal, R.K.; Kakkar, N.; Vasishta, R.K.; Kumari, V.; Samujh, R.; Rao, K.L.N. Acetylcholinesterase histochemistry (AChE)—A helpful technique in the diagnosis and in aiding the operative procedures of Hirschsprung disease. Diagn. Pathol. 2015, 10, 208. [Google Scholar] [CrossRef]
- Pastelin, C.F.; Rivera-Castro, M.E.; Mirto-Aguilar, N.; Moran, C. Structural organization of the neuronal pathways of the superior ovarian nerve in the rat. J. Ovarian Res. 2023, 16, 25. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, Q.; Straubinger, R.M. Signaling mechanisms between the brain and gut in glycemic control. 2014; 239, 335–341. [Google Scholar]
- Deitch, A.D.; Moses, M.J. The Nissl substance of living and fixed spinal ganglion cells. II. An ultraviolet absorption study. J. Cell Biol. 1957, 3, 449–456. [Google Scholar] [CrossRef]
- Humason, G.L.; Animal tissue techniques. Anim. Tissue Tech. 1962. Available online: https://www.cabdirect.org/cabdirect/abstract/19622204447 (accessed on 4 April 2023).
- Bravo-Benitez, J.M.; Cruz, Y.; Lucio, R.A.; Venegas, B.; Díaz, A.; Morales-Ledesma, L.; Domínguez, R.; Morán, C. Intrinsic innervation of the ovary and its variations in the rat senescence process. Histochem. J. 2022, 53, 347–356. [Google Scholar] [CrossRef]
- Abercrombie, M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946, 94, 239–247. [Google Scholar] [CrossRef]
- Burden, H.W.; Leonard, M.; Smith, C.P.; Lawrence, I.E., Jr. The sensory innervation of the ovary: A horseradish peroxidase study in the rat. Anat. Rec. 1983, 207, 623–627. [Google Scholar] [CrossRef]
- Linares, R.; Hernández, D.; Morán, C.; Chavira, R.; Cárdenas, M.; Domínguez, R.; Morales-Ledesma, L. Unilateral or bilateral vagotomy induces ovulation in both ovaries of rats with polycystic ovarian syndrome. Reprod. Biol. Endocrinol. 2013, 11, 68. [Google Scholar] [CrossRef]
- Murray, K.; Rude, K.M.; Sladek, J.; Reardon, C. Divergence of neuroimmune circuits activated by afferent and efferent vagal nerve stimulation in the regulation of inflammation. J. Physiol. 2021, 599, 2075–2084. [Google Scholar] [CrossRef]
- Fox, E.A. Vagal afferent controls of feeding: A possible role for gastrointestinal BDNF. Clin. Auton. Res. 2013, 23, 15–31. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K. Role of efferent and afferent vagal nerve activity during reproduction: Integrating function of oxytocin on metabolism and behaviour. Psychoneuroendocrinology 1994, 19, 687–695. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Erman, A.; Kejner, A.; Hogikyan, N.; Feldman, E. Disorders of Cranial Nerves IX and X. Semin. Neurol. 2009, 29, 085–092. [Google Scholar] [CrossRef]
- Jackson, R.G. Anatomy of the vagus nerves in the region of the lower esophagus and the stomach. Anat. Rec. 1949, 103, 1–18. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, X.; Miselis, R.R. The origin of catecholaminergic nerve fibers in the subdiaphragmatic vagus nerve of rat. J. Auton. Nerv. Syst. 1999, 76, 108–117. [Google Scholar] [CrossRef]
- Ottaviani, M.M.; Macefield, V.G. Structure and Functions of the Vagus Nerve in Mammals. Compr. Physiol. 2022, 12, 3989–4037. [Google Scholar] [CrossRef]
- Morales, L.; Ricardo, B.; Bolaños, A.; Chavira, R.; Domínguez, R. Ipsilateral vagotomy to unilaterally ovariectomized pre-pubertal rats modifies compensatory ovarian responses. Reprod. Biol. Endocrinol. 2007, 5, 24. [Google Scholar] [CrossRef]
- Burden, H.W.; Leonard, M.J.; Hodson, C.A.; Louis, T.M.; Lawrence, I.E., Jr. Effect of Abdominal Vagotomy at Proestrus on Ovarian Weight, Ovarian Antral Follicles, and Serum Levels of Gonadotropins, Estradiol, and Testosterone in the Rat. Neuroendocrinology 1986, 42, 449–455. [Google Scholar] [CrossRef]
- Vieyra, E.; García, J.C.; Zarco, H.A.; Linares, R.; Rosas, G.; Ramírez, D.A.; Chaparro, A.; Espinoza, J.A.; Domínguez, R.; Morales-Ledesma, L. Suprachiasmatic nucleus and vagus nerve trigger preovulatory LH and ovulation. Reproduction 2023, 165, 147–157. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Castro, M.E.; Pastelín, C.F.; Bravo-Benítez, J.; Morán, C. Organization of the Subdiaphragmatic Vagus Nerve and Its Connection with the Celiac Plexus and the Ovaries in the Female Rat. Brain Sci. 2023, 13, 1032. https://doi.org/10.3390/brainsci13071032
Rivera-Castro ME, Pastelín CF, Bravo-Benítez J, Morán C. Organization of the Subdiaphragmatic Vagus Nerve and Its Connection with the Celiac Plexus and the Ovaries in the Female Rat. Brain Sciences. 2023; 13(7):1032. https://doi.org/10.3390/brainsci13071032
Chicago/Turabian StyleRivera-Castro, María E., César F. Pastelín, Juan Bravo-Benítez, and Carolina Morán. 2023. "Organization of the Subdiaphragmatic Vagus Nerve and Its Connection with the Celiac Plexus and the Ovaries in the Female Rat" Brain Sciences 13, no. 7: 1032. https://doi.org/10.3390/brainsci13071032
APA StyleRivera-Castro, M. E., Pastelín, C. F., Bravo-Benítez, J., & Morán, C. (2023). Organization of the Subdiaphragmatic Vagus Nerve and Its Connection with the Celiac Plexus and the Ovaries in the Female Rat. Brain Sciences, 13(7), 1032. https://doi.org/10.3390/brainsci13071032





