Multiple Sclerosis—A Demyelinating Disorder and Its Dental Considerations—A Literature Review with Own Case Report
Abstract
1. Introduction
2. Multiple Sclerosis (MS)
2.1. Etiology
2.2. Pathogenesis
2.3. Epidemiology
2.4. Clinical Types
2.5. Clinical Features
2.6. Diagnosis
2.7. Differential Diagnosis
2.8. Medical Management
3. Dental Considerations
3.1. Role of Dentist in Multiple Sclerosis
3.2. Dental Treatment Considerations
3.3. Oral Manifestations
4. Database Search and Review of Literature
5. Management of Oral and Craniofacial Manifestations
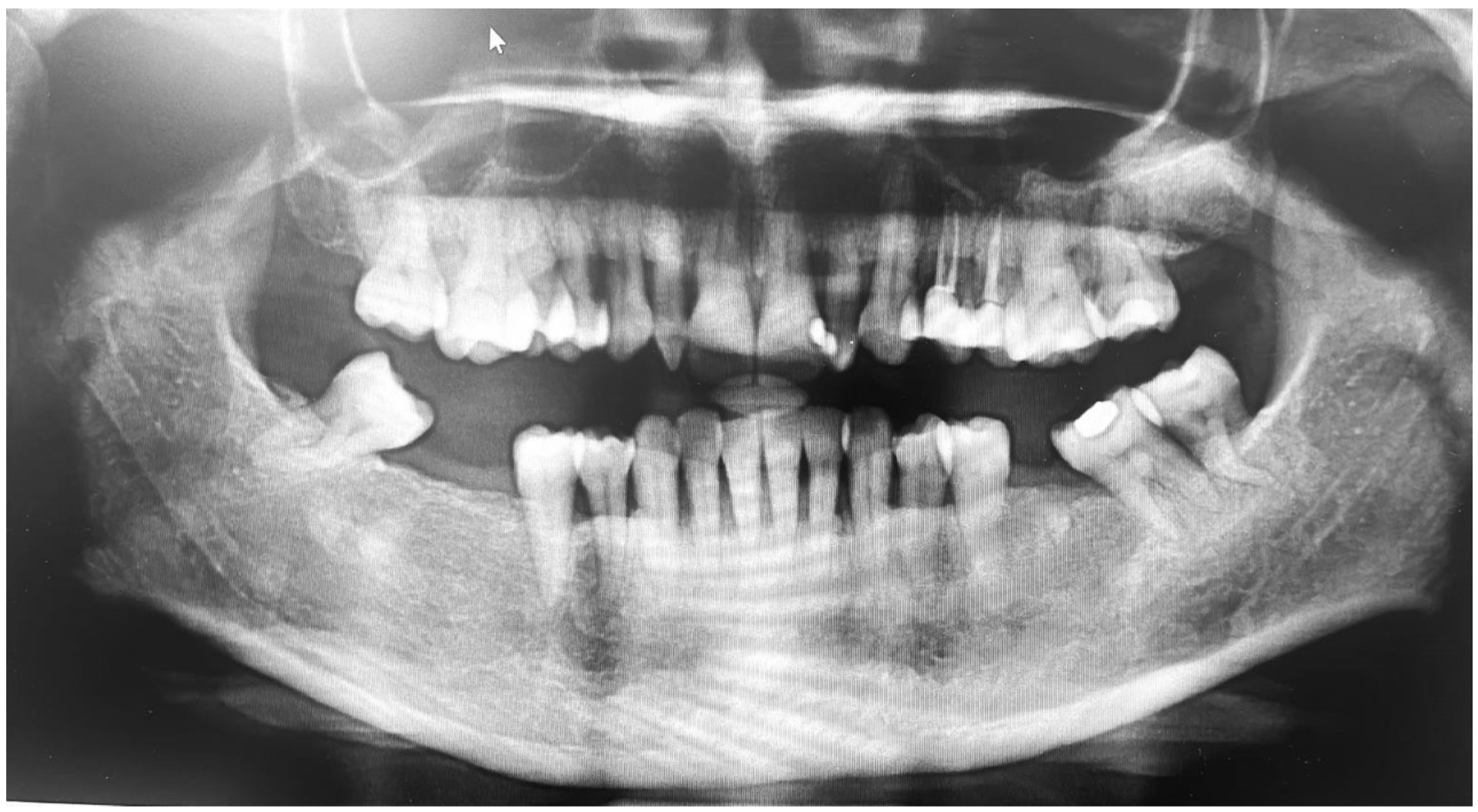
6. Case Report
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Multiple Sclerosis |
| TN | Trigeminal Neuralgia |
| RRMS | Relapsing–Remitting Multiple Sclerosis |
| SPMS | Secondary Progressive Multiple Sclerosis |
| PPMS | Primary Progressive Multiple Sclerosis |
| PRMS | Progressive–Relapsing Multiple Sclerosis |
| EBV | Epstein–Barr Virus |
| HHV-6 | Human Herpesvirus 6 |
| HSR | Hypersensitivity Reaction |
| BBB | Blood–Brain Barrier |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| INF-γ | Interferon gamma |
| TNF-α | Tumor Necrosis Factor Alpha |
| TGF-β | Transforming Growth Factor Beta |
| CNS | Central Nervous System |
| WBCs | White Blood Cells |
| CSF | Cerebrospinal Fluid |
| MRI | Magnetic Resonance Imaging |
| VEP | Visual Evoked Potential |
| QoL | Quality of Life |
References
- Goldenberg, M.M. Multiple Sclerosis Review. PTA Peer-Rev. J. Formul. Manag. 2012, 37, 175–184. [Google Scholar]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple Sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Celius, E.G.; Thompson, H.; Pontaga, M.; Langdon, D.; Laroni, A.; Potra, S.; Bharadia, T.; Yeandle, D.; Shanahan, J.; van Galen, P.; et al. Disease Progression in Multiple Sclerosis: A Literature Review Exploring Patient Perspectives. Patient Prefer. Adherence 2021, 15, 15–27. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Ascherio, A. Environmental Factors in Multiple Sclerosis. Expert Rev. Neurother. 2013, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between Genetic, Lifestyle and Environmental Risk Factors for Multiple Sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef]
- Ciccarelli, O.; Barkhof, F.; Bodini, B.; De Stefano, N.; Golay, X.; Nicolay, K.; Pelletier, D.; Pouwels, P.J.W.; Smith, S.A.; Wheeler-Kingshott, C.A.M.; et al. Pathogenesis of Multiple Sclerosis: Insights from Molecular and Metabolic Imaging. Lancet Neurol. 2014, 13, 807–822. [Google Scholar] [CrossRef]
- Loma, I.; Heyman, R. Multiple Sclerosis: Pathogenesis and Treatment. Curr. Neuropharmacol. 2011, 9, 409–416. [Google Scholar] [CrossRef]
- Shah, A.; Panchal, V.; Patel, K.; Alimohamed, Z.; Kaka, N.; Sethi, Y.; Patel, N. Pathogenesis and Management of Multiple Sclerosis Revisited. Dis. Mon. DM 2022, 101497. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive Multiple Sclerosis: Pathology and Pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS, Third Edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, Regional, and National Burden of Neurological Disorders during 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Bohlega, S.; Inshasi, J.; Tahan, A.R.; Madani, A.B.; Qahtani, H.; Rieckmann, P. Multiple Sclerosis in the Arabian Gulf Countries: A Consensus Statement. J. Neurol. 2013, 260, 2959–2963. [Google Scholar] [CrossRef]
- Etemadifar, M.; Nikanpour, Y.; Neshatfar, A.; Mansourian, M.; Fitzgerald, S. Incidence and Prevalence of Multiple Sclerosis in Persian Gulf Area: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2020, 40, 101959. [Google Scholar] [CrossRef]
- AlJumah, M.; Bunyan, R.; Al Otaibi, H.; Al Towaijri, G.; Karim, A.; Al Malik, Y.; Kalakatawi, M.; Alrajeh, S.; Al Mejally, M.; Algahtani, H.; et al. Rising Prevalence of Multiple Sclerosis in Saudi Arabia, a Descriptive Study. BMC Neurol. 2020, 20, 49. [Google Scholar] [CrossRef]
- AlJumah, M.; Otaibi, H.A.; Al Towaijri, G.; Hassan, A.; Kareem, A.; Kalakatawi, M.; Alrajeh, S.; Al Mejally, M.; Algahtani, H.; Almubarak, A.; et al. Familial Aggregation of Multiple Sclerosis: Results from the National Registry of the Disease in Saudi Arabia. Mult. Scler. J.-Exp. Transl. Clin. 2020, 6, 2055217320960499. [Google Scholar] [CrossRef]
- Bunyan, R.; Al Towaijri, G.; Al Otaibi, H.; Kareem, A.; Algahtani, H.; Al Mejally, M.; Almubarak, A.; Alrajeh, S.; Cupler, E.; Qureshi, S.; et al. Prevalence of Pediatric Onset Multiple Sclerosis in Saudi Arabia. Mult. Scler. Int. 2021, 2021, 4226141. [Google Scholar] [CrossRef]
- Patsopoulos, N.A. Genetics of Multiple Sclerosis: An Overview and New Directions. Cold Spring Harb. Perspect. Med. 2018, 8, a028951. [Google Scholar] [CrossRef]
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sorensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the Clinical Course of Multiple Sclerosis: The 2013 Revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Ford, H. Clinical Presentation and Diagnosis of Multiple Sclerosis. Clin. Med. 2020, 20, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Kimoff, R.J.; Kaminska, M.; Trojan, D. Multiple Sclerosis and Related Disorders. Handb. Clin. Neurol. 2022, 189, 177–200. [Google Scholar] [CrossRef]
- Yamout, B.; Sahraian, M.; Bohlega, S.; Al-Jumah, M.; Goueider, R.; Dahdaleh, M.; Inshasi, J.; Hashem, S.; Alsharoqi, I.; Khoury, S.; et al. Consensus Recommendations for the Diagnosis and Treatment of Multiple Sclerosis: 2019 Revisions to the MENACTRIMS Guidelines. Mult. Scler. Relat. Disord. 2020, 37, 101459. [Google Scholar] [CrossRef]
- Srivastava, D.; Shrivastava, D.; Austin, D. Journey towards the 3D dental imaging-the milestones in the advancement of dental imaging. Int. J. Adv. Res. 2016, 4, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Rocca, M.A.; Ciccarelli, O.; De Stefano, N.; Evangelou, N.; Kappos, L.; Rovira, A.; Sastre-Garriga, J.; Tintorè, M.; Frederiksen, J.L.; et al. MRI Criteria for the Diagnosis of Multiple Sclerosis: MAGNIMS Consensus Guidelines. Lancet. Neurol. 2016, 15, 292–303. [Google Scholar] [CrossRef]
- Calugaru, L.; Calugaru, G.T.; Calugaru, O.M. Evoked Potentials in Multiple Sclerosis Diagnosis and Management. Curr. Health Sci. J. 2016, 42, 385–389. [Google Scholar] [CrossRef]
- Rammohan, K.W. Cerebrospinal Fluid in Multiple Sclerosis. Ann. Indian Acad. Neurol. 2009, 12, 246–253. [Google Scholar] [CrossRef]
- Ömerhoca, S.; Akkaş, S.Y.; İçen, N.K. Multiple Sclerosis: Diagnosis and Differential Diagnosis. Arch. Neuropsychiatry 2018, 55, S1–S9. [Google Scholar] [CrossRef]
- Wildner, P.; Stasiołek, M.; Matysiak, M. Differential Diagnosis of Multiple Sclerosis and Other Inflammatory CNS Diseases. Mult. Scler. Relat. Disord. 2020, 37, 101452. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, H.A.; Shirah, B.H.; Alzahrani, F.A.; Abobaker, H.A.; Alghanaim, N.A.; Manlangit, J.S. Quality of Life among Multiple Sclerosis Patients in Saudi Arabia. Neurosciences 2017, 22, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Ontaneda, D.; Rae-Grant, A.D. Management of Acute Exacerbations in Multiple Sclerosis. Ann. Indian Acad. Neurol. 2009, 12, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.M.; Porter, B.; Thompson, A.J. Multiple Sclerosis: Diagnosis and the Management of Acute Relapses. Postgrad. Med. J. 2005, 81, 302–308. [Google Scholar] [CrossRef]
- Polman, C.; Uitdehaag, B. Drug Treatment of Multiple Sclerosis. West. J. Med. 2000, 173, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L. Immunosuppressive Treatment in Multiple Sclerosis. J. Neurol. Sci. 2004, 223, 1–11. [Google Scholar] [CrossRef]
- D’Amico, E.; Leone, C.; Graziano, G.; Amato, M.P.; Bergamaschi, R.; Cavalla, P.; Coniglio, G.; Di Battista, G.; Ferrò, M.T.; Granella, F.; et al. The Use of Immunosuppressant Therapy for Multiple Sclerosis in Italy: A Multicenter Retroprospective Study. PLoS ONE 2016, 11, e0157721. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Spagnuolo, G.; Bramanti, E.; Laino, L.; Lauritano, F.; Cicciù, M. Interface between MTA and Dental Bonding Agents: Scanning Electron Microscope Evaluation. J. Int. Soc. Prev. Community Dent. 2017, 7, 64–68. [Google Scholar] [CrossRef]
- Covello, F.; Ruoppolo, G.; Carissimo, C.; Zumbo, G.; Ferrara, C.; Polimeni, A.; Vozza, I. Multiple Sclerosis: Impact on Oral Hygiene, Dysphagia, and Quality of Life. Int. J. Environ. Res. Public Health 2020, 17, 3979. [Google Scholar] [CrossRef]
- Chemaly, D.; Lefrançois, A.; Pérusse, R. Oral and Maxillofacial Manifestations of Multiple Sclerosis. J. Can Dent. Assoc. 2000, 66, 600–605. [Google Scholar]
- Danesh-Sani, S.A.; Rahimdoost, A.; Soltani, M.; Ghiyasi, M.; Haghdoost, N.; Sabzali-Zanjankhah, S. Clinical Assessment of Orofacial Manifestations in 500 Patients With Multiple Sclerosis. J. Oral Maxillofac. Surg. 2013, 71, 290–294. [Google Scholar] [CrossRef]
- Patel, J.; Prasad, R.; Bryant, C.; Connolly, H.; Teasdale, B.; Moosajee, S. Multiple Sclerosis and Its Impact on Dental Care. Br. Dent. J. 2021, 231, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.J.; Epstein, J.B.; Klasser, G. Multiple Sclerosis: An Update for Oral Health Care Providers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, N.; Gamoh, S.; Kanazumi, M.; Nakajima, M.; Momota, Y.; Tsutsumi, Y.M. Anesthetic Management of a Patient With Multiple Sclerosis. Anesth. Prog. 2017, 64, 97–101. [Google Scholar] [CrossRef]
- Bin Rubaia’an, M.A.; Alotaibi, M.K.; Alotaibi, N.M.; Alqhtani, N.R. Cortisol in Oral and Maxillofacial Surgery: A Double-Edged Sword. Int. J. Dent. 2021, 2021, 7642875. [Google Scholar] [CrossRef]
- Nimbulkar, G.; Garacha, V.; Shetty, V.; Bhor, K.; Srivastava, K.; Shrivastava, D.; Sghaireen, M. Microbiological and Clinical Evaluation of Neem Gel and Chlorhexidine Gel on Dental Plaque and Gingivitis in 20-30 Years Old Adults: A Randomized Parallel-Armed, Double-Blinded Controlled Trial. J. Pharm. Bioall. Sci. 2020, 12, 345. [Google Scholar] [CrossRef]
- Shrivastava, D.; Srivastava, K.C.; Dayakara, J.K.; Sghaireen, M.G.; Gudipaneni, R.K.; Al-Johani, K.; Baig, M.N.; Khurshid, Z. BactericidalActivity of Crevicular Polymorphonuclear Neutrophils in Chronic Periodontitis Patients and Healthy Subjects under the Influence of Areca Nut Extract: An In Vitro Study. Appl. Sci. 2020, 10, 5008. [Google Scholar] [CrossRef]
- Shrivastava, D.; Natoli, V.; Srivastava, K.C.; Alzoubi, I.A.; Nagy, A.I.; Hamza, M.O.; Al-Johani, K.; Alam, M.K.; Khurshid, Z. Novel Approach to Dental Biofilm Management through Guided Biofilm Therapy (GBT): A Review. Microorganisms 2021, 9, 1966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-Q.; Meng, Y. Oral and Craniofacial Manifestations of Multiple Sclerosis: Implications for the Oral Health Care Provider. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4610–4620. [Google Scholar]
- Di Stefano, G.; Maarbjerg, S.; Truini, A. Trigeminal Neuralgia Secondary to Multiple Sclerosis: From the Clinical Picture to the Treatment Options. J. Headache Pain 2019, 20, 20. [Google Scholar] [CrossRef]
- Costa, C.; Santiago, H.; Pereira, S.; Castro, A.R.; Soares, S.C. Oral Health Status and Multiple Sclerosis: Classic and Non-Classic Manifestations—Case Report. Diseases 2022, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.C.; Shrivastava, D.; Khan, Z.A.; Nagarajappa, A.K.; Mousa, M.A.; Hamza, M.O.; Al-Johani, K.; Alam, M.K. Evaluation of Temporomandibular Disorders among Dental Students of Saudi Arabia Using Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): A Cross-Sectional Study. BMC Oral Health 2021, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Prevalence of Temporomandibular Disorders in Children and Adolescents Evaluated with Diagnostic Criteria for Temporomandibular Disorders: A Systematic Review with Meta-analysis. J. Oral Rehabil. 2023, 50, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Economic Inequalities and Temporomandibular Disorders: A Systematic Review with Meta-analysis. J. Oral Rehabil. 2023, joor.13491. [Google Scholar] [CrossRef]
- Qamar, Z.; Alghamdi, A.M.S.; Haydarah, N.K.B.; Balateef, A.A.; Alamoudi, A.A.; Abumismar, M.A.; Shivakumar, S.; Cicciù, M.; Minervini, G. Impact of Temporomandibular Disorders on Oral Health-related Quality of Life: A Systematic Review and Meta-analysis. J. Oral Rehabil. 2023, joor.13472. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Ronsivalle, V.; Shapira, I.; Cicciù, M. Prevalence of Temporomandibular Disorders in Subjects Affected by Parkinson Disease: A Systematic Review and Metanalysis. J. Oral Rehabil. 2023, joor.13496. [Google Scholar] [CrossRef]
- Gupta, K.; Burchiel, K.J. Atypical Facial Pain in Multiple Sclerosis Caused by Spinal Cord Seizures: A Case Report and Review of the Literature. J. Med. Case Rep. 2016, 10, 101. [Google Scholar] [CrossRef]
- Craner, M.; Al Malik, Y.; Babtain, F.A.; Alshamrani, F.; Alkhawajah, M.M.; Alfugham, N.; Al-Yafeai, R.H.; Aljarallah, S.; Makkawi, S.; Qureshi, S.; et al. Unmet Needs and Treatment of Relapsing-Remitting Multiple Sclerosis in Saudi Arabia: Focus on the Role of Ofatumumab. Neurol. Ther. 2022, 11, 1457–1473. [Google Scholar] [CrossRef]
- Symons, A.L.; Bortolanza, M.; Godden, S.; Seymour, G. A Preliminary Study into the Dental Health Status of Multiple Sclerosis Patients. Spec. Care Dent. 1993, 13, 96–101. [Google Scholar] [CrossRef]
- Cruccu, G.; Biasiotta, A.; Di Rezze, S.; Fiorelli, M.; Galeotti, F.; Innocenti, P.; Mameli, S.; Millefiorini, E.; Truini, A. Trigeminal Neuralgia and Pain Related to Multiple Sclerosis. Pain 2009, 143, 186–191. [Google Scholar] [CrossRef]
- Huggins, H.A.; Levy, T.E. Cerebrospinal Fluid Protein Changes in Multiple Sclerosis after Dental Amalgam Removal. Altern Med. Rev. 1998, 3, 295–300. [Google Scholar]
- Santa Eulalia-Troisfontaines, E.; Martinez-Perez, E.; Miegimolle-Herrero, M.; Planells Del Pozo, P. Oral Health Status of a Population with Multiple Sclerosis. Med. Oral 2012, 17, e223–e227. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-J.; Lin, H.-C. Association between Multiple Sclerosis and Chronic Periodontitis: A Population-Based Pilot Study. Eur. J. Neurol. 2013, 20, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Stockl, K.M.; Shin, J.S.; Gong, S.; Harada, A.S.M.; Solow, B.K.; Lew, H.C. Improving Patient Self-Management of Multiple Sclerosis through a Disease Therapy Management Program. Am. J. Manag. Care 2010, 16, 139–144. [Google Scholar]
- Rafiee Zadeh, A.; Ghadimi, K.; Ataei, A.; Askari, M.; Sheikhinia, N.; Tavoosi, N.; Falahatian, M. Mechanism and Adverse Effects of Multiple Sclerosis Drugs: A Review Article. Part 2. Int. J. Physiol. Pathophysiol. Pharm. 2019, 11, 105–114. [Google Scholar]
- Tavazzi, E.; Rovaris, M.; La Mantia, L. Drug Therapy for Multiple Sclerosis. CMAJ 2014, 186, 833–840. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-Term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef] [PubMed]
- Voge, N.; Alvarez, E. Monoclonal Antibodies in Multiple Sclerosis: Present and Future. Biomedicines 2019, 7, 20. [Google Scholar] [CrossRef]
- Casetta, I.; Iuliano, G.; Filippini, G. Azathioprine for Multiple Sclerosis. Cochrane Database Syst. Rev. 2007, 4. [Google Scholar] [CrossRef]
- Ashtari, F.; Savoj, M.R. Effects of Low Dose Methotrexate on Relapsing-Remitting Multiple Sclerosis in Comparison to Interferon β-1α: A Randomized Controlled Trial. J. Res. Med. Sci. 2011, 16, 457–462. [Google Scholar]
- Gómez-Figueroa, E.; Gutierrez-Lanz, E.; Alvarado-Bolaños, A.; Casallas-Vanegas, A.; Garcia-Estrada, C.; Zabala-Angeles, I.; Cadena-Fernandez, A.; Veronica, R.-A.; Irene, T.-F.; Flores-Rivera, J. Cyclophosphamide Treatment in Active Multiple Sclerosis. Neurol. Sci. 2021, 42, 3775–3780. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Malhotra, R.; Grover, V.; Grover, D. Systemic Antibiotic Therapy in Periodontics. Dent. Res. J. 2012, 9, 505–515. [Google Scholar] [CrossRef] [PubMed]



| Database | Search Strategy |
|---|---|
| PubMed | “Multiple Sclerosis” [Mesh] OR “Demyelinating Autoimmune Diseases, CNS” [Mesh] OR “relapsing–remitting” [tw] AND “Oral Manifestations” [Mesh] OR “Oral Health” [Mesh] OR Mouth [tw] OR “oral health care” [tw] OR “oral mucous irritation” [tw] |
| SCOPUS | (TITLE-ABS-KEY (multiple AND sclerosis) AND TITLE-ABS-KEY (oral AND mucous AND irritation) OR TITLE-ABS-KEY (trismus) OR TITLE-ABS-KEY (xerostomia) OR TITLE-ABS-KEY (gingivitis)) |
| Cochrane | multiple sclerosis in Title Abstract Keyword AND trismus in Title Abstract Keyword OR oral mucosal irritation in Title Abstract Keyword OR gingivitis in Title Abstract Keyword OR xerostomia in Title Abstract Keyword—(Word variations have been searched) |
| Author | Study Design | Sample Size and Intervention | Outcome and Key Findings of the Study |
|---|---|---|---|
| Covello F et al. [39] | Cross-sectional study | A total of 101 patients with MS were interviewed with three different questionnaires to evaluate the different aspects of MS, including status of oral health, level of functional disability, dysphagia, and quality of life. | The responses from three questionnaires, namely, oral hygiene test, dysphagia in MS (DYMUS), and oral health impact on quality of life (IOHIP), showed non-significant differences between gender, except for one question—“difficulty in relaxing”. Majority of participants were reported to have paraesthesia of soft tissues, such as lips and gums. Dysphagia and dysarthria were a common reported symptom among the participants. The quality of life of participants was reported to be compromised due to symptoms, including gingivitis, dry mouth, numbness, and difficulty swallowing. |
| Symons AL et al. [59] | Cross-sectional study | A total of 22 patients were interviewed regarding their past medical and dental records. Both extra-oral and intra-oral clinical examinations were carried out. | Based on dental caries and periodontal indices, the MS patients were not reported to have any significantly higher risk. However, the symptoms of TN and TMJ disorders were found to be more prevalent, and need attention and care. |
| Cruccu G et al. [60] | Multicenter, cross-sectional study | A total of 130 patients of MS were recruited. Based on the diagnosis, they were categorized into three groups—MS with TN (n = 50), MS with sensory disturbance other than TN (n = 30), and control subjects (n = 50). All subjects underwent pain assessment and trigeminal reflex testing. The retrieved MRI scans were incorporated into brainstem models. | The results suggest that the development of TN in predisposed MS patients is probably due to the lesion at intra-pontine trigeminal primary afferents. The ages for the onset of disease and the symptoms of TN were found to be significantly greater in the subjects with TN. |
| Huggins HA et al. [61] | Cross-sectional study | The biochemical changes in the CSF were labeled and monitored after the removal of amalgam and other dental materials. | With the process of photolabeling of CSF proteins, marked changes were identified, which suggests that these can be potentially used as markers to monitor MS. |
| Santa Eulalia-Troisfontaines E et al. [62] | Cross-sectional study | A total of 64 MS patients in three different age groups were recruited. Complete oral examination, including various caries and periodontal indices, were measured. | The caries prevalence was found to be comparable with the general population. However, periodontal, especially the gingival, health was compromised, and needs more attention and care. |
| Sheu JJ et al. [63] | Population-based, case–control study | A total of 316 MS patients, along with 1580 control patients, were recruited in the study. The study attempted to investigate the association between MS and chronic periodontitis. | The statistical analysis revealed that female MS patients have a 1.86 times higher chance of having CP compared to the control counterpart, whereas no such association was established for male MS patients. |
| Danesh-Sani SA et al. [41] | Cross-sectional study | A total of 500 MS patients were included in the study. Their past medical records, along with sociodemographic data, were gathered. All patients underwent standard neurological examination to assess MS-associated conditions, especially TN, dysphagia, and associated difficulty in speech. | The study found that TMJ disorders, difficulty in speech, and disturbance of vision to be the most commonly reported disorders. It was recommended that the awareness of dental practitioners should be raised regarding the medications routinely prescribed to MS patients, so as to avoid any drug-related interactions. |
| Drug | Adverse Side-Effects | Monitoring | Drug Interactions |
|---|---|---|---|
| Corticosteroids | Adrenocortical deficiency Delayed wound healing Postoperative infections Osteoporosis | Fasting blood sugar Bone density | Aspirin Ciprofloxacin Fluconazole Warfarin |
| Monoclonal antibody | Oral lichenoid drug-induced eruptions MRONJ | Virus infections Full blood count Liver function test | Tramadol Zidovudine Acetaminophen |
| Azathioprine | Risk of infections Gastric irritation Hepatotoxicity Nephritis Malignancies on long-term use | Thiopurine S-methyltransferase (TPMT) | Aspirin Co-trimoxazole Warfarin |
| Methotrexate | Allergic reactions Hepatotoxicity Nephrotoxicity | Complete blood count, serum creatinine, transaminases | Amoxicillin Aspirin Omeprazole |
| Cyclophosphamide | Infections Malignancies Infertility | Fasting blood sugar Liver function test Renal function test | Lidocaine Metronidazole Zidovudine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Johani, K.; Fudah, M.; Al-Zahrani, M.; Abed, H.; Srivastava, K.C.; Shrivastava, D.; Cicciù, M.; Minervini, G. Multiple Sclerosis—A Demyelinating Disorder and Its Dental Considerations—A Literature Review with Own Case Report. Brain Sci. 2023, 13, 1009. https://doi.org/10.3390/brainsci13071009
Al Johani K, Fudah M, Al-Zahrani M, Abed H, Srivastava KC, Shrivastava D, Cicciù M, Minervini G. Multiple Sclerosis—A Demyelinating Disorder and Its Dental Considerations—A Literature Review with Own Case Report. Brain Sciences. 2023; 13(7):1009. https://doi.org/10.3390/brainsci13071009
Chicago/Turabian StyleAl Johani, Khalid, Mashael Fudah, Mohammad Al-Zahrani, Hassan Abed, Kumar Chandan Srivastava, Deepti Shrivastava, Marco Cicciù, and Giuseppe Minervini. 2023. "Multiple Sclerosis—A Demyelinating Disorder and Its Dental Considerations—A Literature Review with Own Case Report" Brain Sciences 13, no. 7: 1009. https://doi.org/10.3390/brainsci13071009
APA StyleAl Johani, K., Fudah, M., Al-Zahrani, M., Abed, H., Srivastava, K. C., Shrivastava, D., Cicciù, M., & Minervini, G. (2023). Multiple Sclerosis—A Demyelinating Disorder and Its Dental Considerations—A Literature Review with Own Case Report. Brain Sciences, 13(7), 1009. https://doi.org/10.3390/brainsci13071009









