Modulating Individual Alpha Frequency through Short-Term Neurofeedback for Cognitive Enhancement in Healthy Young Adults
Abstract
1. Introduction
2. Methods
2.1. Participants
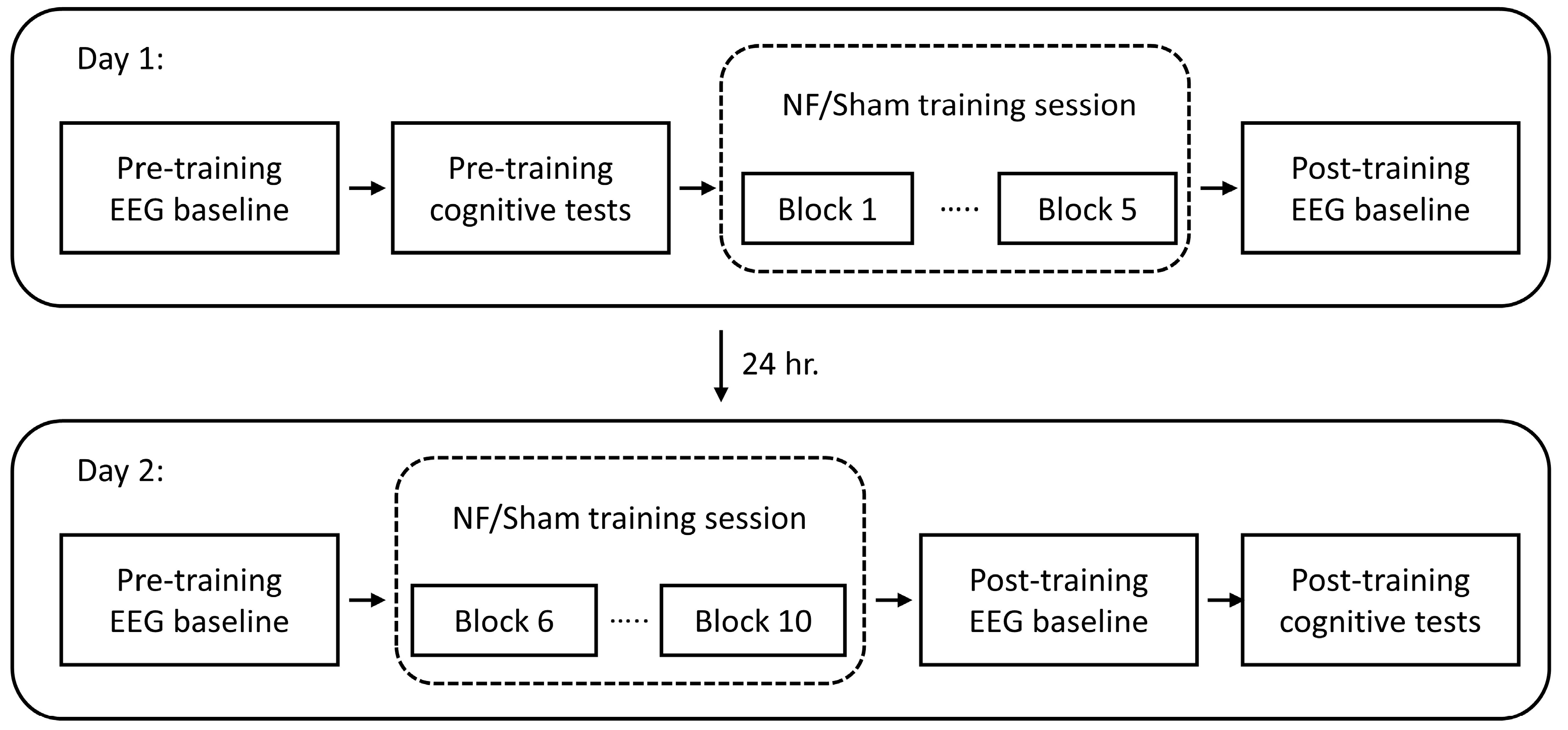
2.2. Experimental Design
2.3. Data Acquisition
2.4. Training Protocol
2.5. Cognitive Tests
2.6. Data Processing and Statistical Analysis
3. Results
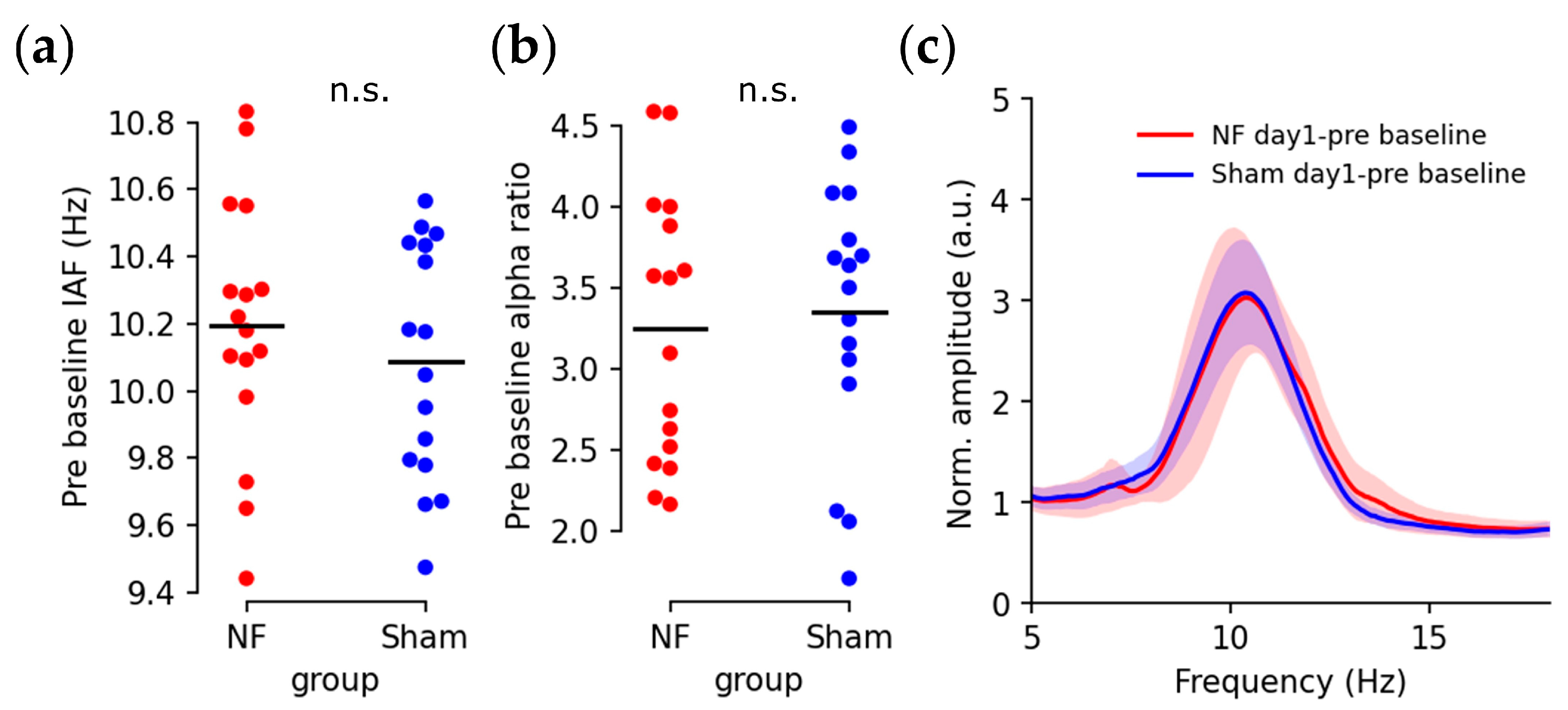
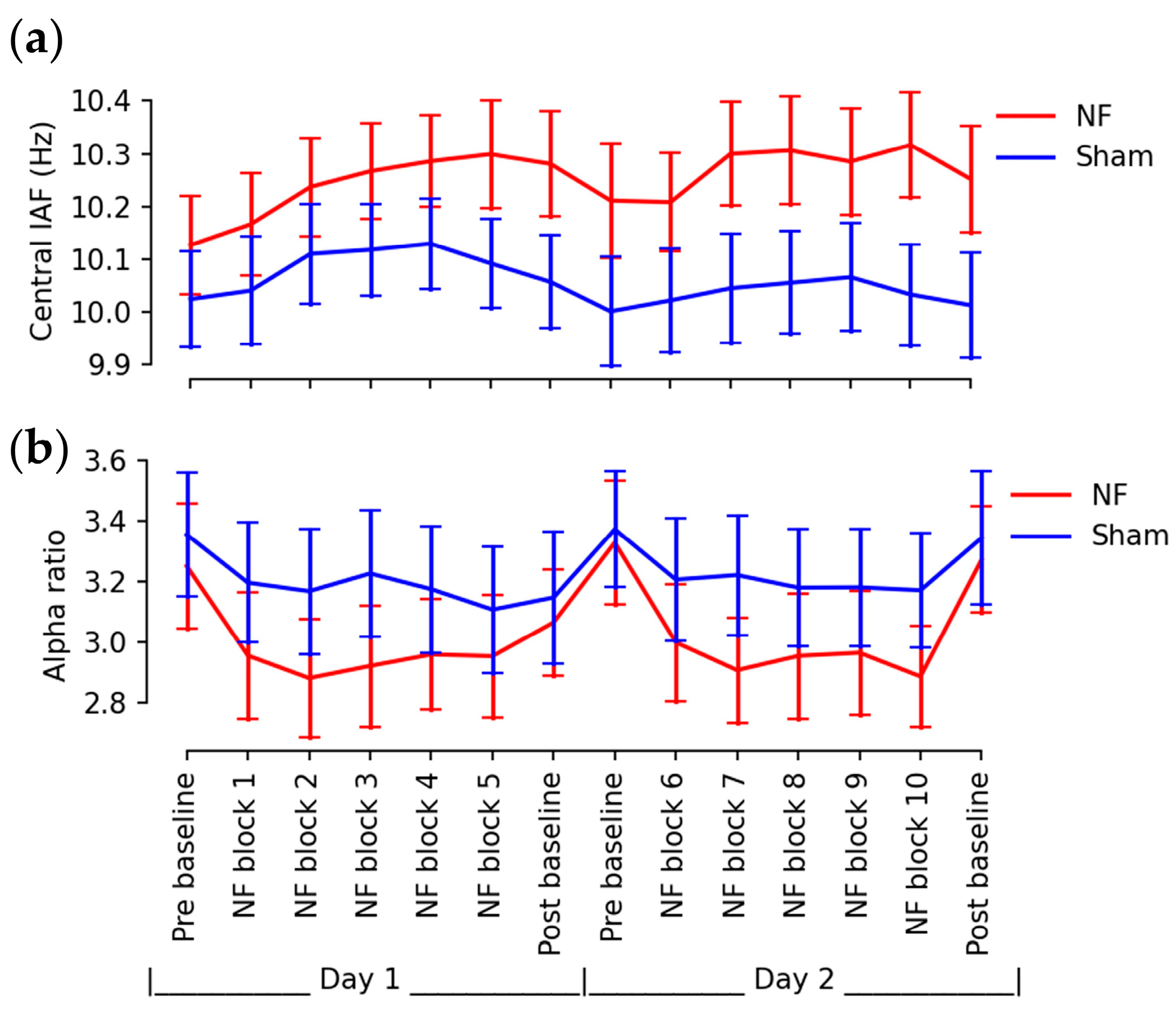
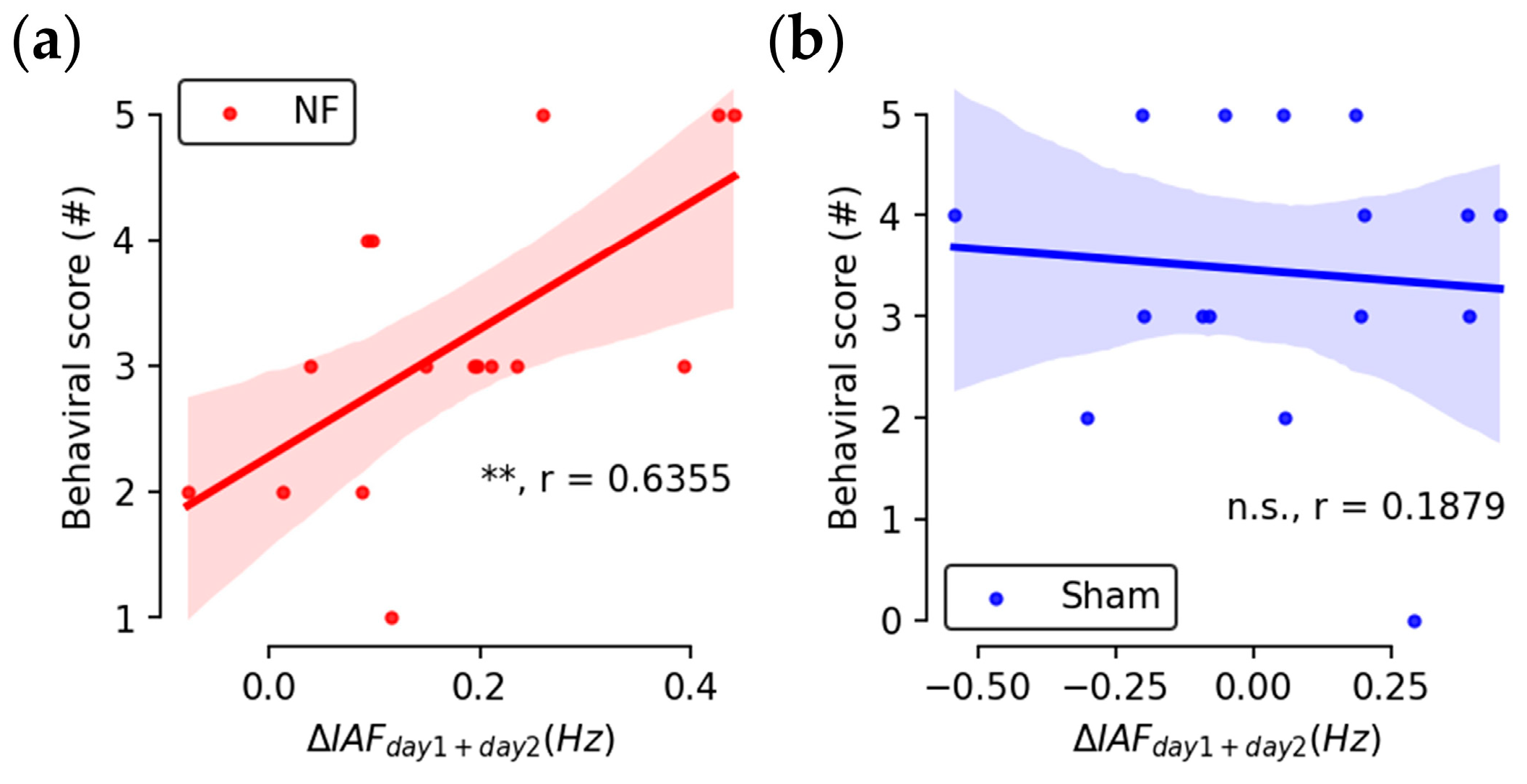
3.1. Up-Regulated IAFs
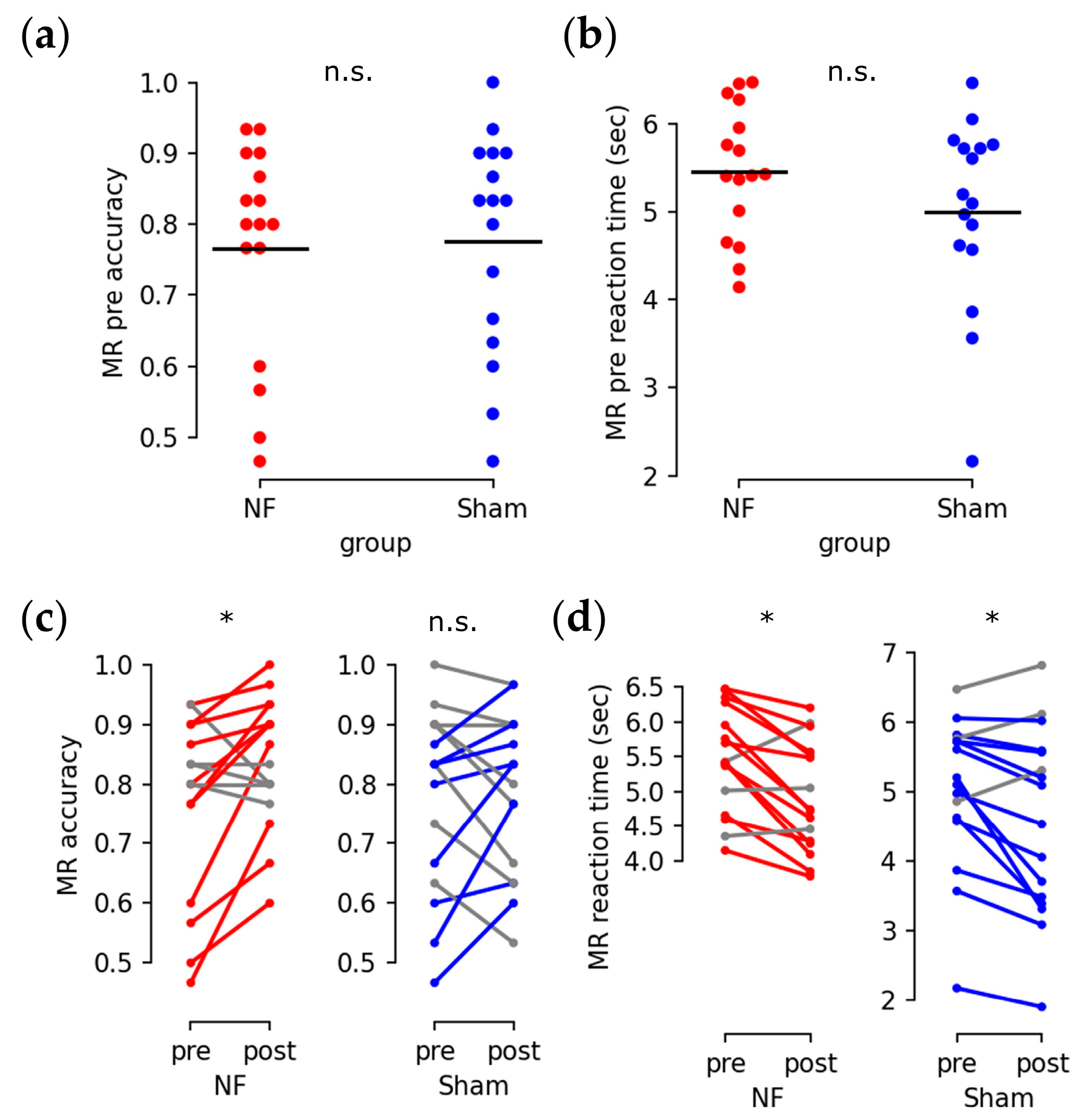
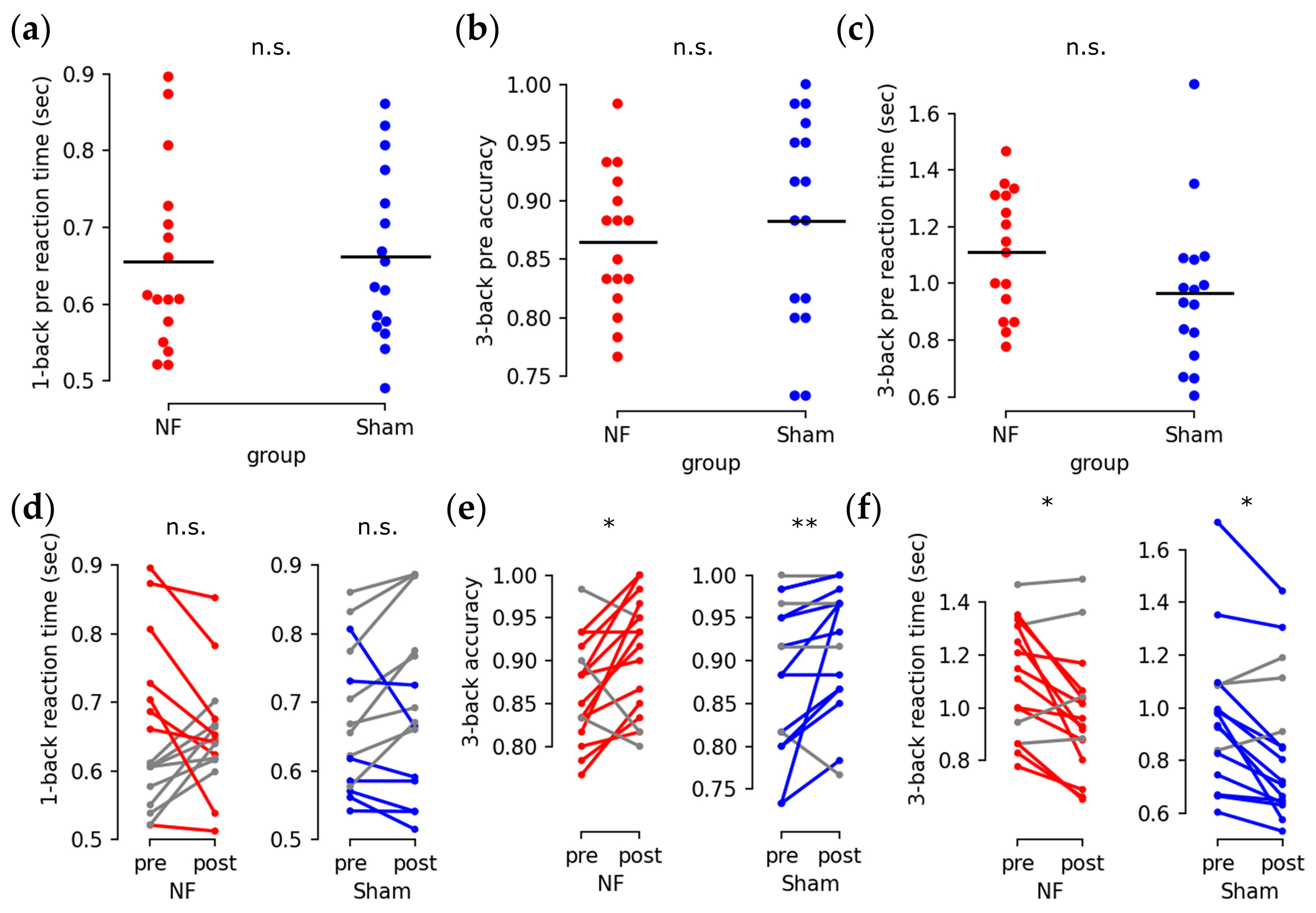
3.2. Enhanced Behavioral Performance
3.3. Fatigue and Adverse Side Effects
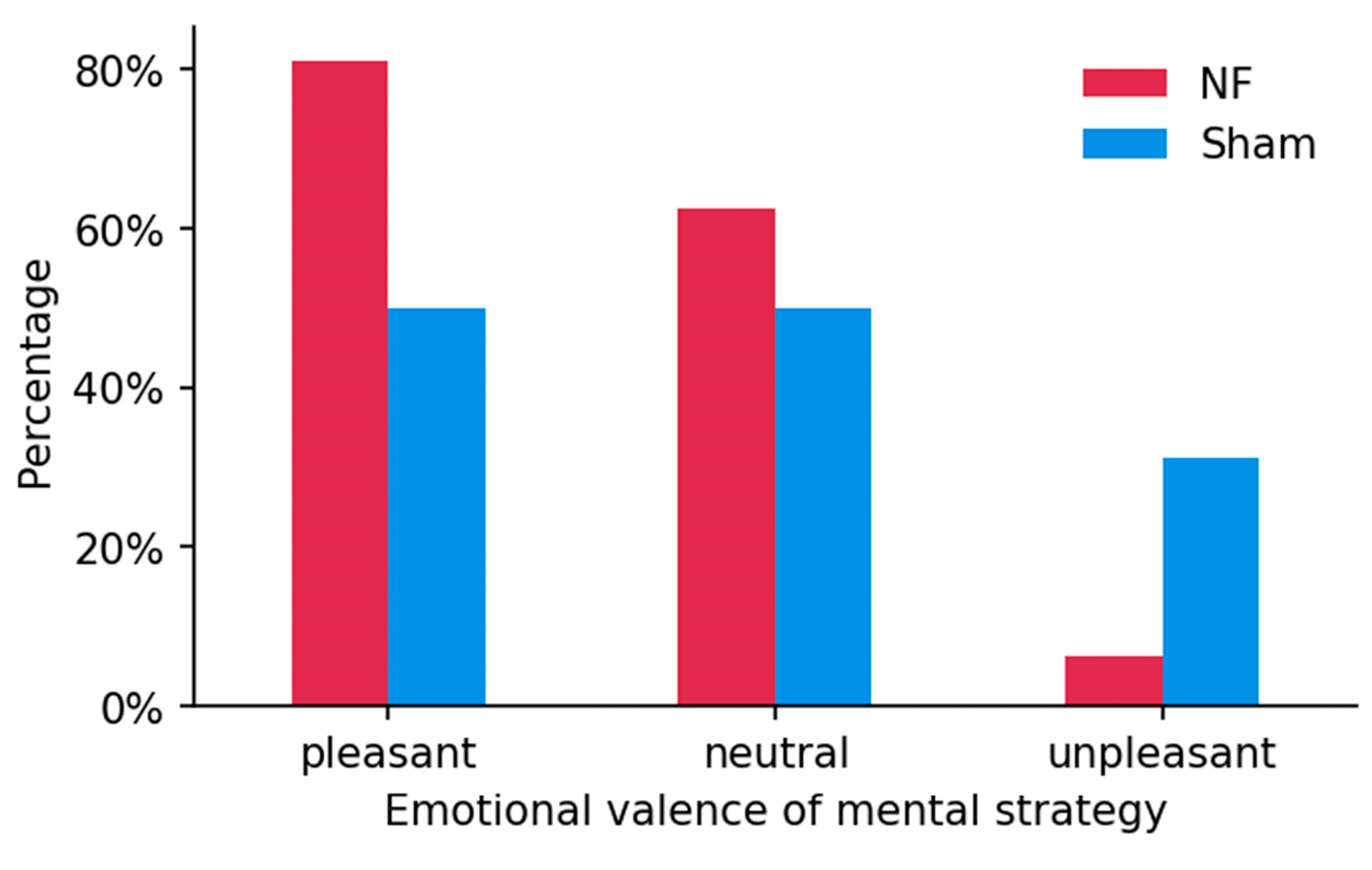
3.4. Mental Strategies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimesch, W. EEG Alpha and Theta Oscillations Reflect Cognitive and Memory Performance: A Review and Analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Draguhn, A. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. Alpha-Band Oscillations, Attention, and Controlled Access to Stored Information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- De Vries, I.E.J.; Slagter, H.A.; Olivers, C.N.L. Oscillatory Control over Representational States in Working Memory. Trends Cogn. Sci. 2020, 24, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Chikhi, S.; Matton, N.; Blanchet, S. EEG Power Spectral Measures of Cognitive Workload: A Meta-analysis. Psychophysiology 2022, 59, e14009. [Google Scholar] [CrossRef]
- Von Stein, A.; Chiang, C.; König, P. Top-down Processing Mediated by Interareal Synchronization. Proc. Natl. Acad. Sci. USA 2000, 97, 14748–14753. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG Alpha Oscillations: The Inhibition–Timing Hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Riddle, J.; Scimeca, J.M.; Cellier, D.; Dhanani, S.; D’Esposito, M. Causal Evidence for a Role of Theta and Alpha Oscillations in the Control of Working Memory. Curr. Biol. 2020, 30, 1748–1754.e4. [Google Scholar] [CrossRef]
- Posthuma, D.; Neale, M.C.; Boomsma, D.I.; de Geus, E.J.C. Are Smarter Brains Running Faster? Heritability of Alpha Peak Frequency, IQ, and Their Interrelation. Behav. Genet. 2001, 31, 567–579. [Google Scholar] [CrossRef]
- Smit, C.M.; Wright, M.J.; Hansell, N.K.; Geffen, G.M.; Martin, N.G. Genetic Variation of Individual Alpha Frequency (IAF) and Alpha Power in a Large Adolescent Twin Sample. Int. J. Psychophysiol. 2006, 61, 235–243. [Google Scholar] [CrossRef]
- Roubicek, J. The Electroencephalogram in the Middle-Aged and the Elderly. J. Am. Geriatr. Soc. 1977, 25, 145–152. [Google Scholar] [CrossRef]
- Richard Clark, C.; Veltmeyer, M.D.; Hamilton, R.J.; Simms, E.; Paul, R.; Hermens, D.; Gordon, E. Spontaneous Alpha Peak Frequency Predicts Working Memory Performance across the Age Span. Int. J. Psychophysiol. 2004, 53, 1–9. [Google Scholar] [CrossRef]
- Moretti, D. Individual Analysis of EEG Frequency and Band Power in Mild Alzheimer’s Disease. Clin. Neurophysiol. 2004, 115, 299–308. [Google Scholar] [CrossRef]
- Bonanni, L.; Thomas, A.; Tiraboschi, P.; Perfetti, B.; Varanese, S.; Onofrj, M. EEG Comparisons in Early Alzheimer’s Disease, Dementia with Lewy Bodies and Parkinson’s Disease with Dementia Patients with a 2-Year Follow-Up. Brain 2008, 131, 690–705. [Google Scholar] [CrossRef]
- Schumacher, J.; Thomas, A.J.; Peraza, L.R.; Firbank, M.; Cromarty, R.; Hamilton, C.A.; Donaghy, P.C.; O’Brien, J.T.; Taylor, J.-P. EEG Alpha Reactivity and Cholinergic System Integrity in Lewy Body Dementia and Alzheimer’s Disease. Alz Res. Ther. 2020, 12, 46. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Neural Oscillations and Brain Stimulation in Alzheimer’s Disease. Prog. Neurobiol. 2020, 194, 101878. [Google Scholar] [CrossRef]
- Babiloni, C.; Noce, G.; Ferri, R.; Lizio, R.; Lopez, S.; Lorenzo, I.; Tucci, F.; Soricelli, A.; Zurrón, M.; Díaz, F.; et al. Resting State Alpha Electroencephalographic Rhythms Are Affected by Sex in Cognitively Unimpaired Seniors and Patients with Alzheimer’s Disease and Amnesic Mild Cognitive Impairment: A Retrospective and Exploratory Study. Cereb. Cortex 2022, 32, 2197–2215. [Google Scholar] [CrossRef]
- Klimesch, W. EEG-Alpha Rhythms and Memory Processes. Int. J. Psychophysiol. 1997, 26, 319–340. [Google Scholar] [CrossRef]
- Wianda, E.; Ross, B. The Roles of Alpha Oscillation in Working Memory Retention. Brain Behav. 2019, 9, e01263. [Google Scholar] [CrossRef]
- Ghazi, T.R.; Blacker, K.J.; Hinault, T.T.; Courtney, S.M. Modulation of Peak Alpha Frequency Oscillations During Working Memory Is Greater in Females Than Males. Front. Hum. Neurosci. 2021, 15, 626406. [Google Scholar] [CrossRef] [PubMed]
- Jann, K.; Koenig, T.; Dierks, T.; Boesch, C.; Federspiel, A. Association of Individual Resting State EEG Alpha Frequency and Cerebral Blood Flow. NeuroImage 2010, 51, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, I.S.; Lynn, P.A.; Schermitzler, B.; Sponheim, S.R. Individual Alpha Peak Frequency Is Slower in Schizophrenia and Related to Deficits in Visual Perception and Cognition. Sci. Rep. 2021, 11, 17852. [Google Scholar] [CrossRef] [PubMed]
- Hülsdünker, T.; Mierau, A.; Strüder, H.K. Higher Balance Task Demands Are Associated with an Increase in Individual Alpha Peak Frequency. Front. Hum. Neurosci. 2016, 9, 695. [Google Scholar] [CrossRef]
- Grandy, T.H.; Werkle-Bergner, M.; Chicherio, C.; Lövdén, M.; Schmiedek, F.; Lindenberger, U. Individual Alpha Peak Frequency Is Related to Latent Factors of General Cognitive Abilities. NeuroImage 2013, 79, 10–18. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Sauseng, P.; Doppelmayr, M.; Schabus, M.; Klimesch, W. Increasing Individual Upper Alpha Power by Neurofeedback Improves Cognitive Performance in Human Subjects. Appl. Psychophysiol. Biofeedback 2005, 30, 1–10. [Google Scholar] [CrossRef]
- Angelakis, E.; Stathopoulou, S.; Frymiare, J.L.; Green, D.L.; Lubar, J.F.; Kounios, J. EEG Neurofeedback: A Brief Overview and an Example of Peak Alpha Frequency Training for Cognitive Enhancement in the Elderly. Clin. Neuropsychol. 2007, 21, 110–129. [Google Scholar] [CrossRef]
- Escolano, C.; Aguilar, M.; Minguez, J. EEG-Based Upper Alpha Neurofeedback Training Improves Working Memory Performance. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2327–2330. [Google Scholar]
- Zoefel, B.; Huster, R.J.; Herrmann, C.S. Neurofeedback Training of the Upper Alpha Frequency Band in EEG Improves Cognitive Performance. NeuroImage 2011, 54, 1427–1431. [Google Scholar] [CrossRef]
- Nan, W.; Rodrigues, J.P.; Ma, J.; Qu, X.; Wan, F.; Mak, P.-I.; Mak, P.U.; Vai, M.I.; Rosa, A. Individual Alpha Neurofeedback Training Effect on Short Term Memory. Int. J. Psychophysiol. 2012, 86, 83–87. [Google Scholar] [CrossRef]
- Escolano, C.; Navarro-Gil, M.; Garcia-Campayo, J.; Congedo, M.; Minguez, J. The Effects of Individual Upper Alpha Neurofeedback in ADHD: An Open-Label Pilot Study. Appl. Psychophysiol. Biofeedback 2014, 39, 193–202. [Google Scholar] [CrossRef]
- Guez, J.; Rogel, A.; Getter, N.; Keha, E.; Cohen, T.; Amor, T.; Gordon, S.; Meiran, N.; Todder, D. Influence of Electroencephalography Neurofeedback Training on Episodic Memory: A Randomized, Sham-Controlled, Double-Blind Study. Memory 2015, 23, 683–694. [Google Scholar] [CrossRef]
- Quaedflieg, C.W.E.M.; Smulders, F.T.Y.; Meyer, T.; Peeters, F.; Merckelbach, H.; Smeets, T. The Validity of Individual Frontal Alpha Asymmetry EEG Neurofeedback. Soc. Cogn. Affect. Neurosci. 2016, 11, 33–43. [Google Scholar] [CrossRef]
- Lavy, Y.; Dwolatzky, T.; Kaplan, Z.; Guez, J.; Todder, D. Neurofeedback Improves Memory and Peak Alpha Frequency in Individuals with Mild Cognitive Impairment. Appl. Psychophysiol. Biofeedback 2019, 44, 41–49. [Google Scholar] [CrossRef]
- Grosselin, F.; Breton, A.; Yahia-Cherif, L.; Wang, X.; Spinelli, G.; Hugueville, L.; Fossati, P.; Attal, Y.; Navarro-Sune, X.; Chavez, M.; et al. Alpha Activity Neuromodulation Induced by Individual Alpha-Based Neurofeedback Learning in Ecological Context: A Double-Blind Randomized Study. Sci. Rep. 2021, 11, 18489. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Gerloff, C. Enhancing Cognitive Performance with Repetitive Transcranial Magnetic Stimulation at Human Individual Alpha Frequency: RTMS and Individual Alpha Frequency. Eur. J. Neurosci. 2003, 17, 1129–1133. [Google Scholar] [CrossRef]
- Weisz, N.; Lüchinger, C.; Thut, G.; Müller, N. Effects of Individual Alpha RTMS Applied to the Auditory Cortex and Its Implications for the Treatment of Chronic Tinnitus: Effects of Individual Alpha RTMS. Hum. Brain Mapp. 2014, 35, 14–29. [Google Scholar] [CrossRef]
- Corlier, J.; Carpenter, L.L.; Wilson, A.C.; Tirrell, E.; Gobin, A.P.; Kavanaugh, B.; Leuchter, A.F. The Relationship between Individual Alpha Peak Frequency and Clinical Outcome with Repetitive Transcranial Magnetic Stimulation (RTMS) Treatment of Major Depressive Disorder (MDD). Brain Stimul. 2019, 12, 1572–1578. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Schneider, T.R.; Rach, S.; Trautmann-Lengsfeld, S.A.; Engel, A.K.; Herrmann, C.S. Entrainment of Brain Oscillations by Transcranial Alternating Current Stimulation. Curr. Biol. 2014, 24, 333–339. [Google Scholar] [CrossRef]
- Notbohm, A.; Herrmann, C.S. Flicker Regularity Is Crucial for Entrainment of Alpha Oscillations. Front. Hum. Neurosci. 2016, 10, 503. [Google Scholar] [CrossRef]
- Rogel, A.; Guez, J.; Getter, N.; Keha, E.; Cohen, T.; Amor, T.; Todder, D. Transient Adverse Side Effects During Neurofeedback Training: A Randomized, Sham-Controlled, Double Blind Study. Appl. Psychophysiol. Biofeedback 2015, 40, 209–218. [Google Scholar] [CrossRef]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a Fatigue Scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, A. Intelligenz-Struktur-Test: IST 70; Verlag für Psychologie Hogrefe: Göttingen, Germany, 1970. [Google Scholar]
- Gittler, G.; GmbH, S. Raumvorstellungsdiagnostikum: Adaptiver Dreidimensionaler Würfeltest; Beltz: Shibuya City, Tokyo, 1990. [Google Scholar]
- Hockey, A.; Geffen, G. The Concurrent Validity and Test?Retest Reliability of a Visuospatial Working Memory Task. Intelligence 2004, 32, 591–605. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Buschkuehl, M.; Perrig, W.J.; Meier, B. The Concurrent Validity of the N-Back Task as a Working Memory Measure. Memory 2010, 18, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H.; Braun, M. Automated Cortical Projection of EEG Sensors: Anatomical Correlation via the International 10–10 System. NeuroImage 2009, 46, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Vallat, R. Pingouin: Statistics in Python. JOSS 2018, 3, 1026. [Google Scholar] [CrossRef]
- Thibault, R.T.; Lifshitz, M.; Raz, A. Neurofeedback or Neuroplacebo? Brain 2017, 140, 862–864. [Google Scholar] [CrossRef]
- Ros, T.; Enriquez-Geppert, S.; Zotev, V.; Young, K.D.; Wood, G.; Whitfield-Gabrieli, S.; Wan, F.; Vuilleumier, P.; Vialatte, F.; Van De Ville, D.; et al. Consensus on the Reporting and Experimental Design of Clinical and Cognitive-Behavioural Neurofeedback Studies (CRED-Nf Checklist). Brain 2020, 143, 1674–1685. [Google Scholar] [CrossRef]
- Bazanova, O.M.; Aftanas, L.I. Individual EEG Alpha Activity Analysis for Enhancement Neurofeedback Efficiency: Two Case Studies. J. Neurother. 2010, 14, 244–253. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, X.; Sourina, O.; Bazanova, O. Individual Theta/Beta Based Algorithm for Neurofeedback Games to Improve Cognitive Abilities. In Transactions on Computational Science XXVI; Gavrilova, M.L., Tan, C.J.K., Iglesias, A., Shinya, M., Galvez, A., Sourin, A., Eds.; Lecture Notes in Computer Science; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2016; Volume 9550, pp. 57–73. ISBN 978-3-662-49246-8. [Google Scholar]
- Van Son, D.; Van Der Does, W.; Band, G.P.H.; Putman, P. EEG Theta/Beta Ratio Neurofeedback Training in Healthy Females. Appl. Psychophysiol. Biofeedback 2020, 45, 195–210. [Google Scholar] [CrossRef]
- Gharabaghi, A.; Kraus, D.; Leão, M.T.; Spüler, M.; Walter, A.; Bogdan, M.; Rosenstiel, W.; Naros, G.; Ziemann, U. Coupling Brain-Machine Interfaces with Cortical Stimulation for Brain-State Dependent Stimulation: Enhancing Motor Cortex Excitability for Neurorehabilitation. Front. Hum. Neurosci. 2014, 8, 122. [Google Scholar] [CrossRef]
- Fleury, M.; Lioi, G.; Barillot, C.; Lécuyer, A. A Survey on the Use of Haptic Feedback for Brain-Computer Interfaces and Neurofeedback. Front. Neurosci. 2020, 14, 528. [Google Scholar] [CrossRef]
- Shabani, F.; Nisar, S.; Philamore, H.; Matsuno, F. Haptic vs. Visual Neurofeedback for Brain Training: A Proof-of-Concept Study. IEEE Trans. Haptics 2021, 14, 297–302. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.-Z.; Nan, W.; Pun, S.H.; Vai, M.I.; Rosa, A.; Wan, F. Modulating Individual Alpha Frequency through Short-Term Neurofeedback for Cognitive Enhancement in Healthy Young Adults. Brain Sci. 2023, 13, 926. https://doi.org/10.3390/brainsci13060926
Li B-Z, Nan W, Pun SH, Vai MI, Rosa A, Wan F. Modulating Individual Alpha Frequency through Short-Term Neurofeedback for Cognitive Enhancement in Healthy Young Adults. Brain Sciences. 2023; 13(6):926. https://doi.org/10.3390/brainsci13060926
Chicago/Turabian StyleLi, Ben-Zheng, Wenya Nan, Sio Hang Pun, Mang I. Vai, Agostinho Rosa, and Feng Wan. 2023. "Modulating Individual Alpha Frequency through Short-Term Neurofeedback for Cognitive Enhancement in Healthy Young Adults" Brain Sciences 13, no. 6: 926. https://doi.org/10.3390/brainsci13060926
APA StyleLi, B.-Z., Nan, W., Pun, S. H., Vai, M. I., Rosa, A., & Wan, F. (2023). Modulating Individual Alpha Frequency through Short-Term Neurofeedback for Cognitive Enhancement in Healthy Young Adults. Brain Sciences, 13(6), 926. https://doi.org/10.3390/brainsci13060926






