Cerebrospinal Fluid Chloride Is Associated with Disease Activity of Relapsing–Remitting Multiple Sclerosis: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
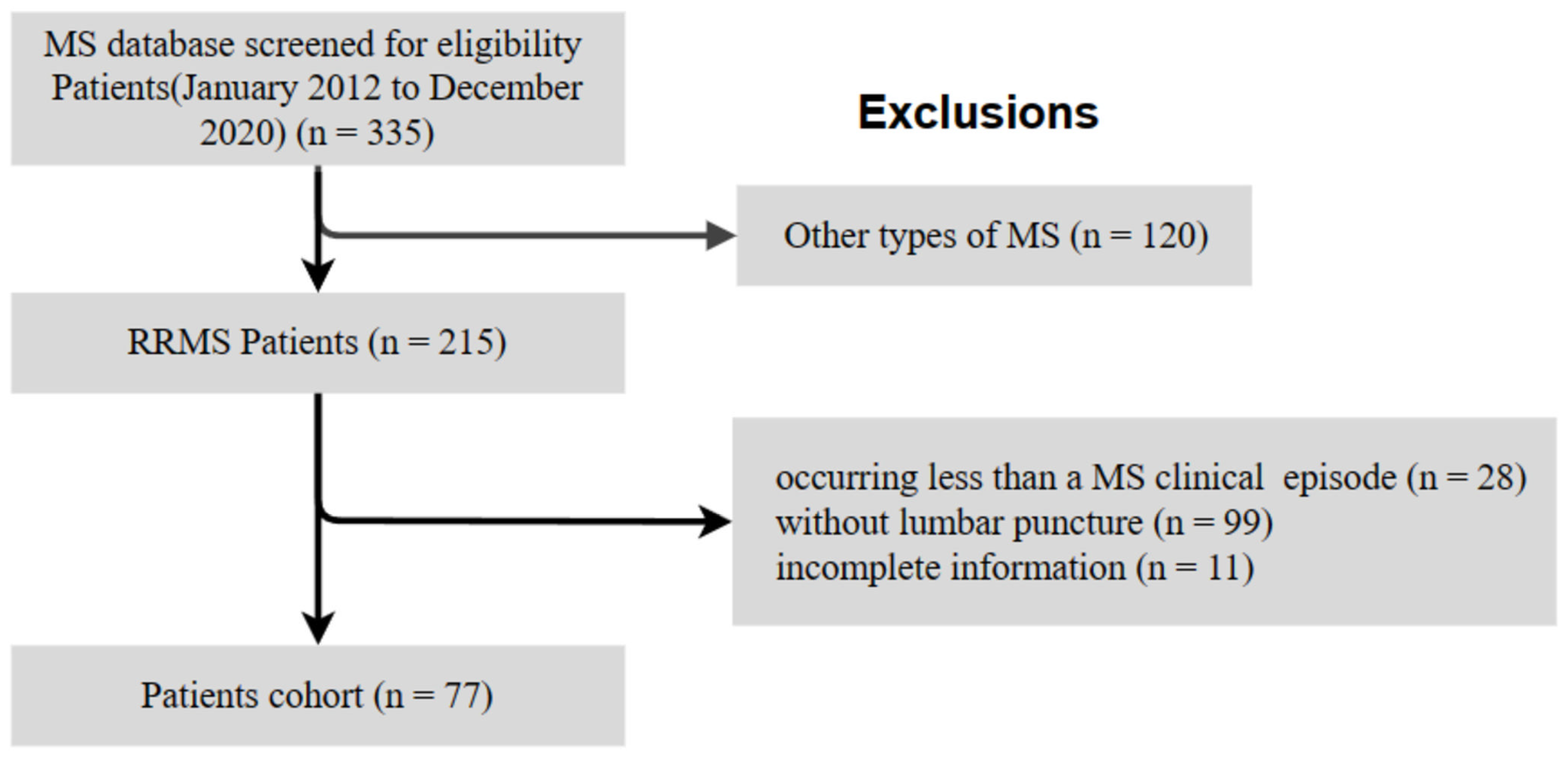
2.1. Study Design and Participants
2.2. Outcome Definition
2.3. Statistical Analysis
3. Results
3.1. Demographics and CSF Results at the Time of the First LP
3.2. CSF Findings and MS Relapse Risk
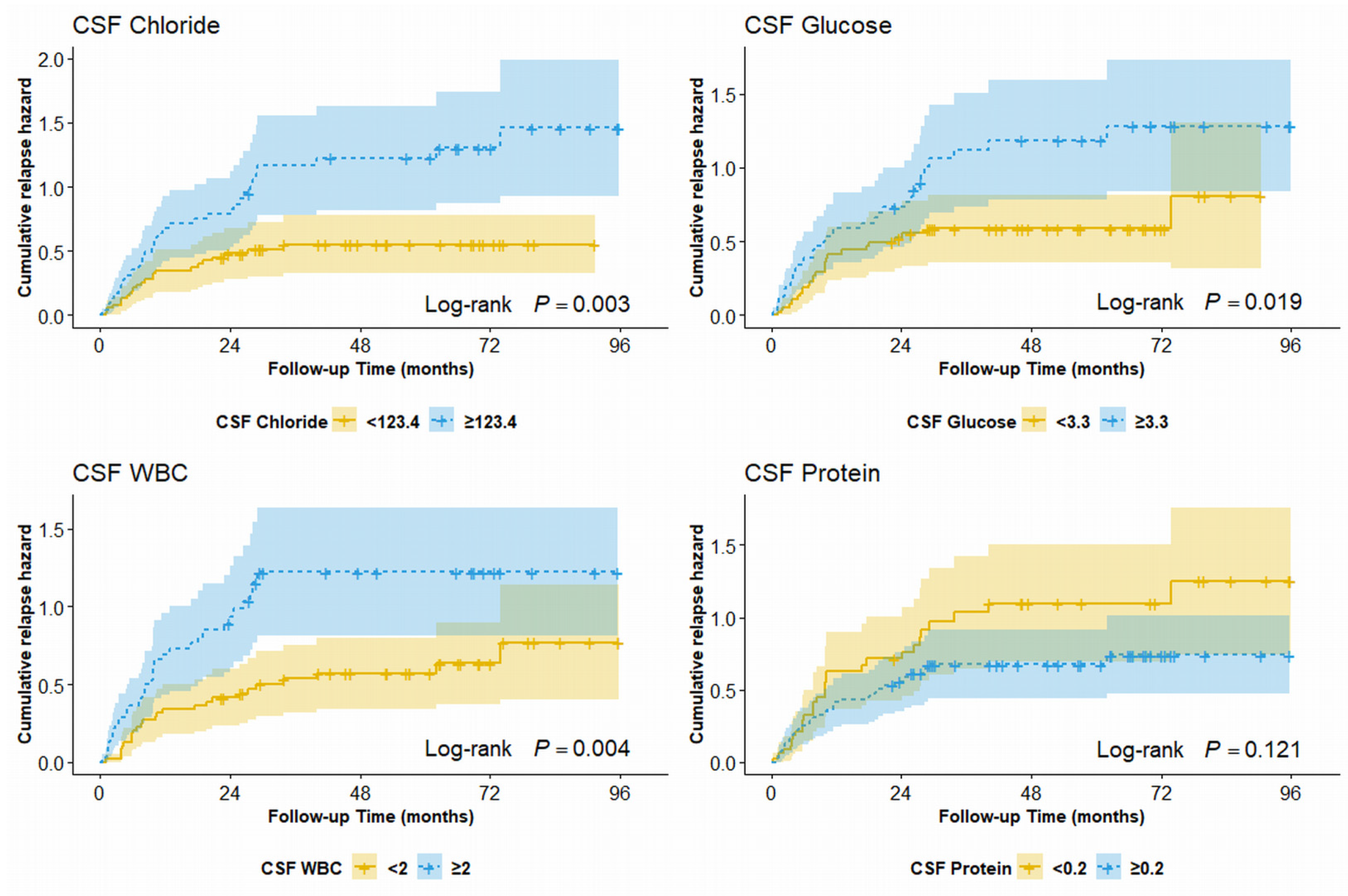
3.3. Cumulative Hazard Resulting in the First Relapse
3.4. CSF Findings and Disease Course
3.5. CSF Findings and Presence of CSF OCBs at Presentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS, Third Edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple Sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, J.; Zhou, H.; Deshpande, C. Annual Cost Burden by Level of Relapse Severity in Patients with Multiple Sclerosis. Adv. Ther. 2021, 38, 758–771. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, Regional, and National Burden of Multiple Sclerosis 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390. [Google Scholar] [CrossRef]
- Rotstein, D.; Montalban, X. Reaching an Evidence-Based Prognosis for Personalized Treatment of Multiple Sclerosis. Nat. Rev. Neurol. 2019, 15, 287–300. [Google Scholar] [CrossRef]
- Yamout, B.; Sahraian, M.; Bohlega, S.; Al-Jumah, M.; Goueider, R.; Dahdaleh, M.; Inshasi, J.; Hashem, S.; Alsharoqi, I.; Khoury, S.; et al. Consensus Recommendations for the Diagnosis and Treatment of Multiple Sclerosis: 2019 Revisions to the MENACTRIMS Guidelines. Mult. Scler. Relat. Disord. 2020, 37, 101459. [Google Scholar] [CrossRef]
- Shahan, B. Cerebrospinal Fluid Analysis. Am. Fam. Physician 2021, 103, 422–428. [Google Scholar]
- Tintoré, M.; Rovira, A.; Río, J.; Tur, C.; Pelayo, R.; Nos, C.; Téllez, N.; Perkal, H.; Comabella, M.; Sastre-Garriga, J.; et al. Do Oligoclonal Bands Add Information to MRI in First Attacks of Multiple Sclerosis? Neurology 2008, 70, 1079–1083. [Google Scholar] [CrossRef]
- Kermode, A.G.; Thompson, A.J.; Tofts, P.; MacManus, D.G.; Kendall, B.E.; Kingsley, D.P.; Moseley, I.F.; Rudge, P.; McDonald, W.I. Breakdown of the Blood-Brain Barrier Precedes Symptoms and Other MRI Signs of New Lesions in Multiple Sclerosis. Pathogenetic and Clinical Implications. Brain 1990, 113, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Lotan, I.; Benninger, F.; Mendel, R.; Hellmann, M.A.; Steiner, I. Does CSF Pleocytosis Have a Predictive Value for Disease Course in MS? Neurol. Neuroimmunol. Neuroinflamm 2019, 6, e584. [Google Scholar] [CrossRef] [PubMed]
- Albanese, M.; Zagaglia, S.; Landi, D.; Boffa, L.; Nicoletti, C.G.; Marciani, M.G.; Mandolesi, G.; Marfia, G.A.; Buttari, F.; Mori, F.; et al. Cerebrospinal Fluid Lactate Is Associated with Multiple Sclerosis Disease Progression. J. Neuroinflamm. 2016, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Diem, L.; Bürge, M.; Leichtle, A.; Hakim, A.; Chan, A.; Salmen, A.; Evangelopoulos, M.-E.; Hoepner, R. Pathological Cerebrospinal Fluid Protein Concentration and Albumin Quotient at Relapse Predicts Short-Term Disability Progression in Multiple Sclerosis: A Retrospective Single Center Observational Study. Ther. Adv. Neurol. Disord. 2020, 13, 175628642097590. [Google Scholar] [CrossRef]
- Sapko, K.; Jamroz-Wiśniewska, A.; Marciniec, M.; Kulczyński, M.; Szczepańska-Szerej, A.; Rejdak, K. Biomarkers in Multiple Sclerosis: A Review of Diagnostic and Prognostic Factors. Neurol. Neurochir. Pol. 2020, 54, 252–258. [Google Scholar] [CrossRef]
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [Google Scholar] [CrossRef]
- Schoen, E.J. Spinal Fluid Chloride: A Test 40 Years Past Its Time. JAMA 1984, 251, 37–38. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, J.; Xing, X.; Tian, C.; Huang, X.; Yu, S. Significance of Chloride Contents in Cerebrospinal Fluid and Plasma and Their Ratio in Early Diagnosis and Differential Diagnosis of Central Nervous System Infections. Med. J. Chin. People’s Lib. Army 2014, 39, 401–405. [Google Scholar]
- Deidda, G.; Bozarth, I.F.; Cancedda, L. Modulation of GABAergic Transmission in Development and Neurodevelopmental Disorders: Investigating Physiology and Pathology to Gain Therapeutic Perspectives. Front. Cell. Neurosci. 2014, 8, 119. [Google Scholar] [CrossRef]
- Glykys, J.; Dzhala, V.; Egawa, K.; Kahle, K.T.; Delpire, E.; Staley, K. Chloride Dysregulation, Seizures, and Cerebral Edema: A Relationship with Therapeutic Potential. Trends Neurosci. 2017, 40, 276–294. [Google Scholar] [CrossRef]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular PH Regulation by Acid-Base Transporters in Mammalian Neurons. Front. Physiol. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Doyon, N.; Vinay, L.; Prescott, S.A.; De Koninck, Y. Chloride Regulation: A Dynamic Equilibrium Crucial for Synaptic Inhibition. Neuron 2016, 89, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Galeffi, F.; Sah, R.; Pond, B.B.; George, A.; Schwartz-Bloom, R.D. Changes in Intracellular Chloride after Oxygen-Glucose Deprivation of the Adult Hippocampal Slice: Effect of Diazepam. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 4478–4488. [Google Scholar] [CrossRef] [PubMed]
- Pond, B.B.; Berglund, K.; Kuner, T.; Feng, G.; Augustine, G.J.; Schwartz-Bloom, R.D. The Chloride Transporter Na(+)-K(+)-Cl- Cotransporter Isoform-1 Contributes to Intracellular Chloride Increases after in Vitro Ischemia. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 1396–1406. [Google Scholar] [CrossRef]
- Papp, E.; Rivera, C.; Kaila, K.; Freund, T.F. Relationship between Neuronal Vulnerability and Potassium-Chloride Cotransporter 2 Immunoreactivity in Hippocampus Following Transient Forebrain Ischemia. Neuroscience 2008, 154, 677–689. [Google Scholar] [CrossRef]
- Depienne, C.; Bugiani, M.; Dupuits, C.; Galanaud, D.; Touitou, V.; Postma, N.; van Berkel, C.; Polder, E.; Tollard, E.; Darios, F.; et al. Brain White Matter Oedema Due to ClC-2 Chloride Channel Deficiency: An Observational Analytical Study. Lancet Neurol. 2013, 12, 659–668. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Roxburgh, R.H.S.R.; Seaman, S.R.; Masterman, T.; Hensiek, A.E.; Sawcer, S.J.; Vukusic, S.; Achiti, I.; Confavreux, C.; Coustans, M.; le Page, E.; et al. Multiple Sclerosis Severity Score: Using Disability and Disease Duration to Rate Disease Severity. Neurology 2005, 64, 1144–1151. [Google Scholar] [CrossRef]
- Alboukadel, K.; Marcin, K.; Przemyslaw, B. Survminer: Drawing Survival Curves Using “Ggplot2.” R Package Version 0.4.9. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 1 November 2020).
- Andersen, P.K.; Gill, R.D. Cox’s Regression Model for Counting Processes: A Large Sample Study. Ann. Stat. 1982, 10, 1100–1120. [Google Scholar] [CrossRef]
- Amorim, L.D.A.F.; Cai, J. Modelling Recurrent Events: A Tutorial for Analysis in Epidemiology. Int. J. Epidemiol. 2015, 44, 324–333. [Google Scholar] [CrossRef]
- Therneau, T.M. A Package for Survival Analysis in R. R Package Version 3.3-1. 2022. Available online: https://CRAN.R-project.org/package=survival (accessed on 1 November 2020).
- Kalincik, T.; Vivek, V.; Jokubaitis, V.; Lechner-Scott, J.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; Grand’Maison, F.; Hupperts, R.; Oreja-Guevara, C.; et al. Sex as a Determinant of Relapse Incidence and Progressive Course of Multiple Sclerosis. Brain 2013, 136, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.; Williams, O.; Willis, M.; Hrastelj, J.; Rimmer, A.; Joseph, F.; Tomassini, V.; Wardle, M.; Pickersgill, T.; Robertson, N.; et al. Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients with Multiple Sclerosis. JAMA Neurol. 2019, 76, 536. [Google Scholar] [CrossRef] [PubMed]
- Zeydan, B.; Kantarci, O.H. Impact of Age on Multiple Sclerosis Disease Activity and Progression. Curr. Neurol. Neurosci. Rep. 2020, 20, 24. [Google Scholar] [CrossRef]
- Skaper, S.D. Ion Channels on Microglia: Therapeutic Targets for Neuroprotection. CNS Neurol. Disord. Drug. Targets 2011, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, F.; Baumann, C.; Epstein, J.; Kerschen, P.; Garot, T.; Mathey, G.; Debouverie, M.; LORSEP Group. Older Age at Multiple Sclerosis Onset Is an Independent Factor of Poor Prognosis: A Population-Based Cohort Study. Neuroepidemiology 2017, 48, 179–187. [Google Scholar] [CrossRef]
- Pawelec, G. Hallmarks of Human “Immunosenescence”: Adaptation or Dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef]
- Pardo, G.; Jones, D.E. The Sequence of Disease-Modifying Therapies in Relapsing Multiple Sclerosis: Safety and Immunologic Considerations. J. Neurol. 2017, 264, 2351–2374. [Google Scholar] [CrossRef]
- Mathur, D.; López-Rodas, G.; Casanova, B.; Marti, M.B. Perturbed Glucose Metabolism: Insights into Multiple Sclerosis Pathogenesis. Front. Neurol. 2014, 5, 250. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, Q.; Wen, H.; Zhang, Y. Disease Modifying Therapies in Relapsing-Remitting Multiple Sclerosis: A Systematic Review and Network Meta-Analysis. Autoimmun. Rev. 2021, 20, 102826. [Google Scholar] [CrossRef]
- Deisenhammer, F. The Cerebrospinal Fluid in Multiple Sclerosis. Front. Immunol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Konen, F.F.; Schwenkenbecher, P.; Jendretzky, K.F.; Gingele, S.; Witte, T.; Sühs, K.-W.; Grothe, M.; Hannich, M.J.; Süße, M.; Skripuletz, T. Kappa Free Light Chains in Cerebrospinal Fluid in Inflammatory and Non-Inflammatory Neurological Diseases. Brain Sci. 2022, 12, 475. [Google Scholar] [CrossRef] [PubMed]

| Variable | RRMS Patients (n = 77) |
|---|---|
| Age at disease onset (years) | 27.0 [22.0, 34.0] |
| Sex (%) | |
| Male | 27.0 (35.1) |
| Female | 50.0 (64.9) |
| Baseline EDSS score | 2.5 [1.5, 3.5] |
| Maintenance therapies a (%) | |
| Disease-modifying therapies | 28.0 (36.4) |
| No disease-modifying therapy | 49.0 (63.6) |
| Annualized relapse rate | 0.5 [0.2, 0.7] |
| Follow-up time (months) | 55.9 [41.2, 71.0] |
| Time between first attack and LP (months) | 22.3 [3.4, 57.2] |
| Time between first attack and therapy | 37.0 [7.0, 382.0] |
| Oligoclonal band (%) | |
| Oligoclonal band + | 38.0 (49.4) |
| Oligoclonal band- | 39.0 (50.6) |
| CSF data | |
| CSF chloride (mmol/L) | 123.2 [121.5, 124.5] |
| CSF glucose (mmol/L) | 3.3 [3.1, 3.8] |
| Number of CSF WBCs (cells/µL) | 2.0 [0.0, 6.0] |
| CSF protein (mmol/L) | 0.2 [0.2, 0.3] |
| Serum data | |
| Glucose (mmol/L) | 4.7 [4.3, 5.7] |
| Chloride (mmol/L) | 104.5 [102.4, 105.5] |
| WBC count, ×103 (μL) | 7.1 [5.8, 9.4] |
| Protein (g/L) | 64.4 [61.4, 67.5] |
| Ratio | |
| CSF/serum chloride | 0.8 [0.8, 0.9] |
| CSF/serum glucose | 1.5 [1.3, 1.7] |
| CSF/serum protein | 287.4 [215.0, 379.0] |
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Age at disease onset | 0.98 (0.95–0.99) | 0.041 |
| Sex | ||
| Male | Reference | - |
| Female | 1.11 (0.62–1.99) | 0.719 |
| Maintenance therapies | ||
| Disease-modifying therapy | Reference | - |
| No disease-modifying therapy | 2.35 (1.44–3.83) | 0.001 |
| Variable | Univariate | Multivariate a | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| CSF chloride | 1.16 (1.06–1.27) | 0.001 | 1.14 (1.06–1.22) | 0.001 |
| CSF glucose | 1.45 (1.15–1.82) | 0.002 | 1.46 (1.23–1.74) | <0.001 |
| CSF protein | 0.29 (0.02–5.43) | 0.407 | 0.97 (0.05–17.16) | 0.983 |
| CSF WBC | 1.04 (0.99–1.10) | 0.108 | 1.06 (1.02–1.11) | 0.004 |
| Serum chloride | 1.02 (0.89–1.16) | 0.784 | 1.01 (0.91–1.11) | 0.895 |
| Serum glucose | 1.05 (0.88–1.25) | 0.601 | 1.02 (0.89–1.18) | 0.780 |
| Serum protein | 1.05 (1.01–1.09) | 0.021 | 1.05 (1.01–1.08) | 0.006 |
| Serum WBC | 0.99 (0.90–1.08) | 0.776 | 0.98 (0.92–1.05) | 0.647 |
| CSF/serum chloride | 0.01 (0.01–271.02) | 0.249 | 0.01 (0.01–9.71) | 0.128 |
| CSF/serum glucose | 0.70 (0.34–1.44) | 0.330 | 0.60 (0.34–1.07) | 0.081 |
| CSF/serum protein | 1.00 (1.00–1.00) | 0.007 | 1.00 (1.00–1.00) | 0.047 |
| Variable | First Lumbar Puncture | First Relapse | ||
|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | |
| CSF chloride | 0.12 | 0.281 | 0.02 | 0.872 |
| CSF glucose | 0.13 | 0.243 | 0.17 | 0.137 |
| CSF WBC | 0.26 | 0.021 | 0.16 | 0.153 |
| CSF protein | 0.07 | 0.542 | 0.02 | 0.857 |
| Serum chloride | −0.10 | 0.364 | 0.02 | 0.872 |
| Serum glucose | 0.07 | 0.537 | 0.17 | 0.137 |
| Serum protein | 0.02 | 0.885 | 0.16 | 0.153 |
| Serum WBC | 0.03 | 0.782 | 0.02 | 0.857 |
| CSF/serum chloride | −0.07 | 0.521 | −0.22 | 0.052 |
| CSF/serum glucose | −0.06 | 0.631 | −0.03 | 0.787 |
| CSF/serum protein | 0.12 | 0.281 | 0.02 | 0.872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Lu, Y.; Fu, Y.; Liu, Z.; Kermode, A.G.; Qiu, W.; Ling, L.; Liu, C. Cerebrospinal Fluid Chloride Is Associated with Disease Activity of Relapsing–Remitting Multiple Sclerosis: A Retrospective Cohort Study. Brain Sci. 2023, 13, 924. https://doi.org/10.3390/brainsci13060924
Fang X, Lu Y, Fu Y, Liu Z, Kermode AG, Qiu W, Ling L, Liu C. Cerebrospinal Fluid Chloride Is Associated with Disease Activity of Relapsing–Remitting Multiple Sclerosis: A Retrospective Cohort Study. Brain Sciences. 2023; 13(6):924. https://doi.org/10.3390/brainsci13060924
Chicago/Turabian StyleFang, Xingwei, Yaxin Lu, Yongmei Fu, Zifeng Liu, Allan G. Kermode, Wei Qiu, Li Ling, and Chunxin Liu. 2023. "Cerebrospinal Fluid Chloride Is Associated with Disease Activity of Relapsing–Remitting Multiple Sclerosis: A Retrospective Cohort Study" Brain Sciences 13, no. 6: 924. https://doi.org/10.3390/brainsci13060924
APA StyleFang, X., Lu, Y., Fu, Y., Liu, Z., Kermode, A. G., Qiu, W., Ling, L., & Liu, C. (2023). Cerebrospinal Fluid Chloride Is Associated with Disease Activity of Relapsing–Remitting Multiple Sclerosis: A Retrospective Cohort Study. Brain Sciences, 13(6), 924. https://doi.org/10.3390/brainsci13060924







