Abstract
Background: Blood-brain barrier dysfunction in active multiple sclerosis (MS) lesions leads to pathological changes in the cerebrospinal fluid (CSF). This study aimed to investigate the possible association between routine CSF findings, especially CSF chloride, at the time of the first lumbar puncture and the relapse risk and disability progression of relapsing–remitting MS (RRMS). Methods: This retrospective study included 77 patients with RRMS at the MS Center of our institution from January 2012 to December 2020. The Anderson and Gill (AG) model and Spearman correlation analysis were used to explore predictors of relapse and disability during follow-up. Results: In the multivariate AG model, patients with elevated CSF chloride level (hazard ratio [HR], 1.1; 95% confidence interval [CI]: 1.06–1.22; p = 0.001) had a high risk of MS relapse. Using median values of CSF chloride (123.2 mmol/L) as a cut-off, patients with CSF chloride level ≥ 123.2 mmol/L had a 120% increased relapse risk compared with those with CSF chloride level < 123.2 mmol/L (HR = 2.20; 95% CI: 1.19–4.05; p = 0.012). Conclusions: Elevated CSF chloride levels might be a biologically unfavorable predictive factor for disease relapse in RRMS.
1. Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating, and neurodegenerative disease of the central nervous system (CNS). It is the primary cause of non-traumatic neurological disability among young adults, with a higher prevalence observed in women. The prevalence of MS varies globally, with the highest prevalence reported in northern regions and lower prevalence observed in equatorial countries. Recent data suggest that the prevalence of MS has been on the rise over the last few decades [1].
MS has three distinct clinical courses: relapsing–remitting MS (RRMS), secondary progressive MS (SPMS), and primary progressive MS (PPMS). In addition, clinically isolated syndrome (CIS) often progresses to MS, especially when symptoms are accompanied by CNS lesions. RRMS is the most common phenotype [2] and is characterized by unpredictable clinical relapses and remissions and gradual disability progression [3]. Early RRMS is usually marked by acute episodes of neurological deficits known as relapses, which rely on the location of the CNS region affected by the acute inflammatory demyelinating lesions and the extent of the inflammatory process.
Relapses are associated with a considerable increase in economic costs as well as a deterioration in health-related quality of life and functional abilities, which are some of the biggest concerns associated with the disease [4]. RRMS causes the accumulation of irreversible disability over time, further increasing the burden on patients and society [5].
Currently, RRMS remains incurable due to the unclear pathogenesis. However, with the development of increasingly effective therapies for RRMS, disease-modifying therapies could modify the course of disease through suppression or modulation of immune function [6,7]. They exert anti-inflammatory activity primarily in the relapsing phase of MS, reduce the rate of relapses, reduce accumulation of MRI lesions, and stabilize, delay, and in some cases modestly improve, disability. Nonetheless, the clinical course of RRMS varies greatly across patients and is unpredictable at the initial disease stages, making the treatment challenging for clinicians. Therefore, exploring effective biomarkers to predict disease activity over time is a prerequisite to creating a therapeutic algorithm for patients presenting with a highly active course in the early stages of the disease.
The diagnosis of MS typically involves a comprehensive evaluation of the patient’s medical history and a neurological examination, which aims to determine if there is dissemination in time and space of certain clinical symptoms while excluding other conditions with similar symptoms. Supportive diagnostic evidence may be obtained through paraclinical tests including Magnetic Resonance Imaging (MRI), evoked potential studies, and analysis of cerebrospinal fluid (CSF). These tests can provide supportive diagnostic information that helps clinicians confirm or rule out a diagnosis of MS [8]. Recently, the McDonald diagnostic criteria have been updated to allow the diagnosis of clinically definite MS (CDMS) after a single typical attack in patients who have MRI evidence of dissemination in space and the presence of oligoclonal bands (OCBs) in the CSF [3]. The new diagnostic guidelines underscore the significance of OCBs and highlight the growing importance of examining CSF.
Cerebrospinal fluid (CSF) analysis is a diagnostic or evaluative tool for many conditions affecting the CNS [9]; it has been proven that the presence of OCBs in patients with clinically isolated syndrome doubles the risk of a second clinical attack [10]. Theoretically, blood-brain barrier dysfunction in active MS lesions leads to pathological changes in the CSF, which could help predict disease relapse and disability progression [11]. Attempts have been made to correlate CSF parameters and clinical aspects of the disease in various studies, such as that of Lotan et al. [12], who reported that CSF pleocytosis at presentation is associated with a higher annual relapse rate and a more rapid progression of neurologic disability. Albanese et al. [13] found that CSF lactate is associated with MS disease progression. Diem et al. [14] found that increased CSF protein concentration have a negative impact on EDSS after relapse. CSF immunoglobulin free light chains, CSF neurofilament levels, and other CSF biomarkers have also been proposed as prognostic biomarkers [15,16].
CSF chloride is a measure of the concentration of chloride ions present in the cerebrospinal fluid that surrounds the brain and spinal cord. It is an important electrolyte in the CSF and plays a role in maintaining the proper balance of fluids and electrolytes in the central nervous system. Abnormal levels of CSF chloride may indicate underlying neurological conditions [17,18] such as meningitis, encephalitis, or hydrocephalus. However, very few studies have assessed the association between the levels of CSF chloride and MS disease activity.
Chloride ions are the primary permeant anions in mammals, and homeostasis of their flux across cell membranes is important for a range of neurophysiological processes [19,20,21]. The concentration of chloride ions in neurons is very low; thus, small changes in the concentration greatly impact the transmembrane gradient [22]. Several studies have highlighted that abnormal chloride homeostasis is associated with neuronal trauma or brain disorders such as hypoxic–ischemic encephalopathy, brain edema, and post-traumatic seizures [23,24,25]. The role of chloride ions in the pathogenesis of MS has not been fully elucidated; however, emerging human and animal models directly link intra-myelinic edema and ion homeostasis [26].
Therefore, this study aimed to investigate the possible association between routine CSF findings (at the time of the first lumbar puncture [LP] during the initial presentation), especially CSF chloride, and the relapse risk and disability progression of MS.
2. Materials and Methods
2.1. Study Design and Participants
This retrospective study, which followed the STROBE guidelines [27], was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (grant number: [2020]02-423), and all participants provided written informed consent.
We retrospectively reviewed the clinical data of 335 patients with MS who attended the Neurology Department of the Third Affiliated Hospital of Sun Yat-sen University from January 2012 to December 2020. We included patients of all ages who fulfilled all the following criteria: diagnosed with RRMS according to the 2017 modified McDonald criteria [3]; without diagnosis of other systemic diseases; with at least one MS clinical episode; and had undergone LP during the initial diagnostic process. Patients with a different type of MS, those without LP, and those with incomplete information were excluded. As of the end of the study, no patients had died or moved. Figure 1 shows a flowchart of the inclusion and exclusion of data.
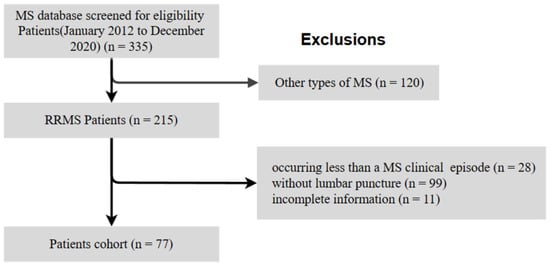
Figure 1.
Flowchart of the study cohort. MS, multiple sclerosis; RRMS, relapsing–remitting multiple sclerosis.
We collected the demographic and clinical data of eligible patients, including age at disease onset, sex, disease course phenotype, maintenance therapy, subsequent relapse (start and stop date, outcome), expanded disability status scale (EDSS) score, and laboratory examination data. Baseline characteristics were collected when patients were admitted for treatment due to a first attack or relapse during the study period and all relapses were recorded and archived by patient medical records during the follow-up period. For an individual subject, regular follow-up is set at 3 months, 6 months, and 1 year after the first day of MS diagnosis. If symptoms are stable, maintain annual follow-up. At least one senior neurologist calculated the EDSS score throughout the disease duration period.
CSF was evaluated for the number of white blood cells, protein (g/L), chlorine and glucose levels (mmol/L), and quotient of CSF and serum glucose, chlorine, and protein levels. For analysis, maintenance therapies were classified into two categories: disease-modifying therapies (DMTs) such as interferon beta, fingolimod, mitoxantrone, rituximab, and dimethyl fumarate, and non-DMTs, such as azathioprine, mycophenolate mofetil, methotrexate, tacrolimus, cyclophosphamide, corticosteroids, and intravenous immunoglobulin administration. The duration of MS was calculated from the time of the patient-reported first clinical manifestation of the disease.
2.2. Outcome Definition
Attack/relapse was defined as a monophasic clinical episode with patient-reported symptoms, increase in EDSS, and objective findings reflecting a focal or multifocal inflammatory demyelinating event in the central nervous system, developing acutely or sub-acutely, with a duration of at least 24 h, with or without recovery, and in the absence of fever or infection [3]. ‘First attack’ was defined as the inaugural attack that marked the disease onset and ‘recurrent relapses’ as all attacks following the first attack.
Disease progression was evaluated by calculating multiple sclerosis severity score (MSSS), which is an algorithm that relates EDSS scores to the distribution of disability in patients with comparable disease duration [28].
2.3. Statistical Analysis
Baseline characteristics, including patient demographics, baseline EDSS score, annualized relapse rate, laboratory values at the time of the first LP, time between the first attack and LP, and maintenance therapies, are presented as counts and percentages for discrete variables and median (interquartile range, IQR) for continuous data. The correlation between the MSSS and CSF findings for the entire study population was calculated using the Spearman correlation coefficient. Survival curves were generated by estimating the cumulative hazard to reach the first relapse for RRMS patients stratified by the median of CSF findings using the Kaplan–Meier method expressing 95% confidence intervals (CIs) with the Survminer package [29].
To assess the risk of relapse over the entire disease course in patients, the Anderson and Gill model was used using the survival package [30,31,32]. Univariate and multivariate analyzes were performed, and multivariate models were adjusted for known MS prognostic markers, including demographic markers (sex and age at disease onset), and maintenance therapies [33,34,35].
All statistical analyzes were performed using R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/ (accessed on 1 November 2020)), and p-values < 0.05 were considered significant.
3. Results
3.1. Demographics and CSF Results at the Time of the First LP
The cohort included 77 patients (50 women and 27 men; sex ratio, 1.85:1) who underwent LP at the first clinical episode, with a mean age of 28.8 ± 12.6 years. The mean follow-up period was 57.2 ± 19.7 months (range: 22.1–97.7 months).
At the time of the first LP in our cohort, the CSF chloride levels were in the range of 117.4–130.0 mmol/L. The range of the CSF leukocyte count was 0–28.0 cells/µL and correlated with CSF chloride level (rs = 0.23; p = 0.040). No significant difference was noted in CSF findings between male and female patients. The demographics and CSF results at the time of the LP are summarized in Table 1.

Table 1.
Demographic and clinical features of relapsing–remitting multiple sclerosis patients.
3.2. CSF Findings and MS Relapse Risk
In addition to the index events, 112 subsequent demyelinating relapses were registered, with a median number of relapses of 2 (IQR: 1, 3). Overall, 45 patients (58.4%) had at least one relapse, and Figure 2 shows the number of relapses along the disease course.

Figure 2.
Relapse distribution along the whole disease cohort.
From univariate analysis, the risk of relapse decreased with the age of onset. Patients not treated with DMT had a greater risk of relapse over the entire disease course than those treated with DMT (Table 2). In addition, elevated CSF chloride level, CSF glucose level, serum protein level, and protein quotient were associated with a higher risk of relapse (Table 3).

Table 2.
Univariate analysis for adjusted variables.

Table 3.
Univariate and multivariate Cox regression analyzes for recurrent events.
In the multivariate analysis, the model was adjusted by including age at onset, sex, and maintenance treatment. Elevated CSF chloride level, CSF glucose level, CSF white blood cell (WBC) count, serum protein level, and protein quotient level were associated with a high risk of relapse. However, the remaining serum and quotient indicators had no significant effect on the risk of relapse (Table 3).
3.3. Cumulative Hazard Resulting in the First Relapse
To distinguish the potential relapse risk of patients with MS, we used the median values of CSF findings as cut-off values. Patients with CSF chloride level ≥ 123.2 mmol/L had a 120% increased risk of relapse compared with those with CSF chloride level < 123.2 mmol/L (HR = 2.20; 95% CI: 1.19–4.05; p = 0.012). Patients with CSF WBC count ≥ 2 cells/µL had an 84% increased risk of relapse compared with those with CSF WBC count < 2 cells/µL (HR = 1.84; 95% CI: 1.02–3.32; p = 0.043). Patients with CSF glucose level ≥ 3.3 mmol/L had a 141% increased risk of relapse compared with those with CSF glucose level < 3.3 mmol/L (HR = 2.41; 95% CI: 1.30–4.49; p = 0.006). The cumulative hazard of the relapse events is shown in Figure 3.
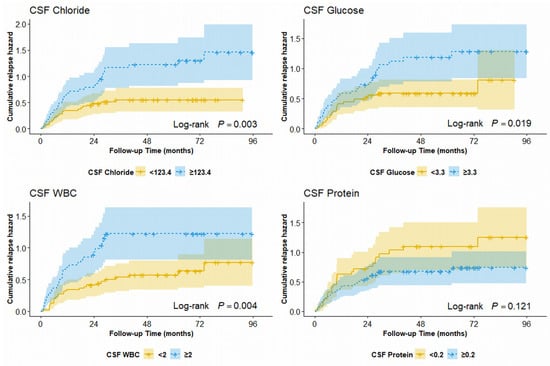
Figure 3.
Cumulative hazard of first relapse for RRMS patients stratified by median of CSF findings. RRMS, relapsing–remitting multiple sclerosis; CSF, cerebrospinal fluid.
3.4. CSF Findings and Disease Course
Next, we calculated the MSSS for each patient at the time of sampling and the first relapse, and correlated the data with the initial CSF findings. Significant correlation was only determined between CSF WBC and the MSSS in the patients with MS (Table 4). When stratifying by maintenance therapies, we also found a positive correlation between CSF WBC and MSSS in the not-DMT group (Tables S1 and S2).

Table 4.
Correlation between CSF findings and the Multiple Sclerosis Severity Score at the time of the first lumbar puncture and first relapse.
3.5. CSF Findings and Presence of CSF OCBs at Presentation
The relationship between CSF findings and the presence of CSF OCBs at presentation was also examined. CSF OCBs were present in 49.4% of the 77 patients included in the study. There was no significant difference between the CSF findings of patients with positive OCBs and those without positive OCBs (Table S3). Elevated CSF chloride level, CSF WBC count, and CSF glucose level were associated with a high risk of relapse in the multivariate stratifying patients by the presence or absence of OCB (Table S4).
4. Discussion
In this monocentric and retrospective study from 2012 to 2020, we analyzed the data of patients with RRMS who underwent their first LP and confirmed that an increased CSF chloride concentration has a negative impact on the risk of relapse. This finding may have therapeutic implications and indicates the need for a more aggressive therapeutic approach.
A previous study substantiated the concept that chloride channels are essential for ion and water homeostasis in the brain. Astrocytes are pivotal to maintaining ion and water homeostasis in the brain and play a significant role in potential action generation and impulse conduction [36]. Loss of function of the chloride channel is linked to brain white matter edema, which radiological correlates and functional models have clearly demonstrated [26].
Interestingly, using the Anderson and Gill model to assess the risk of recurrent events, we found that patients with elevated CSF chloride levels were at a high risk of relapse. Furthermore, we observed that the relapse risk of patients with CSF chloride level ≥ 123.2 mmol/L was more than double that of patients with CSF chloride level < 123.2 mmol/L for the first relapse, when using median CSF chloride concentrations as the cut-off point. This finding provides useful information for individual patients tailor the specific agent to address a more active course. The ability of elevated CSF chlorides at the time of the first LP to predict disease relapse could be correlated with the breakage of ions and water homeostasis in the brain, which might impact action potential generation and impulse conduction. Elevated CSF chlorides might also represent an innate response to infection, probably viral, triggering an initial relapse; whether they represent a marker or a factor of pathogenic significance is still unknown.
Two other covariates, age at onset and maintenance treatment approach, were associated with the risk of relapse, which was in accordance with previous studies: increasing age was associated with fewer relapses [35,37]. The decline in clinical and subclinical disease activity with aging is a clinical phenomenon strongly associated with the progressive loss of the innate and adaptive immune system’s efficiency with aging, which may be triggered by an adaptive or a dysregulated response [38]. Currently, several DMTs are available for the treatment of RRMS and are considered to reduce the immune-mediated inflammatory process of the central nervous system, which translates into demonstrable improvements in clinical and radiological outcomes [39]. The present study found that DMT is superior to no-DMT for relapse control, which indicates that a suitable therapeutic regime is more favorable for relapse control.
In addition, we found that an increased leukocyte count in CSF and increased protein quotient were also related to a higher risk of relapse, which is consistent with the recent reports [12,14]. We also observed a positive correlation between leukocyte count in CSF and MSSS, especially in patients who did not receive DMT. These results suggest that an increased leukocyte count in CSF may reflect a more active disease in patients, highlighting the importance of early DMT. Perturbed glucose metabolism is implicated in neurodegenerative disorders like Alzheimer’s, Parkinson’s, and Huntington’s. However, little is known about its role in MS pathology. We found that an increase in glucose level in the CSF was also related to a higher risk of relapse, which may be related to mitochondrial aberrations and impaired glucose metabolism in MS [40].
Remarkable advancements have been made in the treatment of MS, thanks to an improved understanding of its pathogenesis and course. Immunosuppressive therapies have proven effective for controlling relapses and delaying disability progression in patients with RRMS [41]. In the past, high-dose IFN-b-1a and glatiramer acetate were typically preferred as first-line options; however, recent studies recommend using highly effective disease-modifying therapies as the initial approach for most patients with active RRMS [6]. This approach supersedes the traditional “treat to target” method which involves using a moderately effective treatment initially and then advancing to a more potent agent when breakthrough disease occurs. Observational studies suggest that early use of high-efficacy therapy can lead to better long-term outcomes. However, due to significant disease heterogeneity, clinical and biological markers are necessary to develop personalized treatment plans based on each patient’s specific risk of relapse and disability progression.
As diagnostic criteria for MS continue to improve, CSF testing has become increasingly important [3]. In addition to assisting in the differential diagnosis of neurological disorders, CSF tests are significant sources of supportive diagnostic evidence. OCBs in the CSF are present in up to 90% of MS patients [42], while various studies have identified other CSF biomarkers associated with MS relapse and disability progression [12,14,43]. This study found that CSF chloride, glucose, and white blood cell count at the first onset were associated with a higher risk of relapse, which may predict a more active disease course and eventually indicate the need for a more active therapeutic approach.
This study has some limitations. The primary limitations of this study are its retrospective nature and the associated possibility of recall bias. Additionally, we could not further confirm the correlation with the clinical results due to the lack of magnetic resonance imaging and paraclinical data. Thirdly, the sample size of our study was relatively small, and we only included Asian populations. Lastly, due to the limited sample size, further categorization of DMT into first-line and second-line drugs was not possible. Further prospective studies with larger sample sizes are required to validate our findings.
5. Conclusions
Patients with RRMS with elevated CSF chloride level at the time of the first LP (especially those with CSF chloride level ≥123.2 mmol/L) were likely to show a high risk of relapse, indicating the need for a more active immunosuppressive treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci13060924/s1, Table S1: Correlation between CSF findings and the Multiple Sclerosis Severity Score at the time of the first lumbar puncture stratifying patients by maintenance therapies; Table S2: Correlation between CSF findings and the Multiple Sclerosis Severity Score at the first relapse stratifying patients by maintenance therapies; Table S3: CSF findings baseline feature in patients with the presence or absence of OCB; Table S4: Univariate and multivariate Cox regression analyses for recurrent events stratifying patients by the presence or absence of OCB
Author Contributions
Conceptualization, C.L. and L.L.; methodology, X.F., Y.L. and Y.F.; software, X.F., Y.L. and Y.F.; validation, All Authors; formal analysis, X.F., Y.L. and Y.F.; investigation, X.F., Y.L., A.G.K. and Y.F.; resources, C.L., Z.L. and W.Q.; data curation, C.L., Z.L. and W.Q.; writing—original draft preparation, X.F., Y.L. and Y.F.; writing—review and editing, A.G.K., C.L. and L.L.; visualization, X.F., Y.L. and Y.F.; supervision, A.G.K., C.L. and L.L.; project administration, C.L. and L.L.; funding acquisition, C.L. and W.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Medical Science and Technology Foundation of Guangdong under Grant number A2022136 and Guangzhou Science and Technology Plan Project under Grant number 202102010263.
Institutional Review Board Statement
The study was approved by the Third Affiliated Hospital of the Sun Yat-sen University Research Ethics Committee, and written informed consents were obtained from all participants (grant number: [2020]02-423).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS, Third Edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple Sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, J.; Zhou, H.; Deshpande, C. Annual Cost Burden by Level of Relapse Severity in Patients with Multiple Sclerosis. Adv. Ther. 2021, 38, 758–771. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, Regional, and National Burden of Multiple Sclerosis 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390. [Google Scholar] [CrossRef]
- Rotstein, D.; Montalban, X. Reaching an Evidence-Based Prognosis for Personalized Treatment of Multiple Sclerosis. Nat. Rev. Neurol. 2019, 15, 287–300. [Google Scholar] [CrossRef]
- Yamout, B.; Sahraian, M.; Bohlega, S.; Al-Jumah, M.; Goueider, R.; Dahdaleh, M.; Inshasi, J.; Hashem, S.; Alsharoqi, I.; Khoury, S.; et al. Consensus Recommendations for the Diagnosis and Treatment of Multiple Sclerosis: 2019 Revisions to the MENACTRIMS Guidelines. Mult. Scler. Relat. Disord. 2020, 37, 101459. [Google Scholar] [CrossRef]
- Shahan, B. Cerebrospinal Fluid Analysis. Am. Fam. Physician 2021, 103, 422–428. [Google Scholar]
- Tintoré, M.; Rovira, A.; Río, J.; Tur, C.; Pelayo, R.; Nos, C.; Téllez, N.; Perkal, H.; Comabella, M.; Sastre-Garriga, J.; et al. Do Oligoclonal Bands Add Information to MRI in First Attacks of Multiple Sclerosis? Neurology 2008, 70, 1079–1083. [Google Scholar] [CrossRef]
- Kermode, A.G.; Thompson, A.J.; Tofts, P.; MacManus, D.G.; Kendall, B.E.; Kingsley, D.P.; Moseley, I.F.; Rudge, P.; McDonald, W.I. Breakdown of the Blood-Brain Barrier Precedes Symptoms and Other MRI Signs of New Lesions in Multiple Sclerosis. Pathogenetic and Clinical Implications. Brain 1990, 113, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Lotan, I.; Benninger, F.; Mendel, R.; Hellmann, M.A.; Steiner, I. Does CSF Pleocytosis Have a Predictive Value for Disease Course in MS? Neurol. Neuroimmunol. Neuroinflamm 2019, 6, e584. [Google Scholar] [CrossRef] [PubMed]
- Albanese, M.; Zagaglia, S.; Landi, D.; Boffa, L.; Nicoletti, C.G.; Marciani, M.G.; Mandolesi, G.; Marfia, G.A.; Buttari, F.; Mori, F.; et al. Cerebrospinal Fluid Lactate Is Associated with Multiple Sclerosis Disease Progression. J. Neuroinflamm. 2016, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Diem, L.; Bürge, M.; Leichtle, A.; Hakim, A.; Chan, A.; Salmen, A.; Evangelopoulos, M.-E.; Hoepner, R. Pathological Cerebrospinal Fluid Protein Concentration and Albumin Quotient at Relapse Predicts Short-Term Disability Progression in Multiple Sclerosis: A Retrospective Single Center Observational Study. Ther. Adv. Neurol. Disord. 2020, 13, 175628642097590. [Google Scholar] [CrossRef]
- Sapko, K.; Jamroz-Wiśniewska, A.; Marciniec, M.; Kulczyński, M.; Szczepańska-Szerej, A.; Rejdak, K. Biomarkers in Multiple Sclerosis: A Review of Diagnostic and Prognostic Factors. Neurol. Neurochir. Pol. 2020, 54, 252–258. [Google Scholar] [CrossRef]
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [Google Scholar] [CrossRef]
- Schoen, E.J. Spinal Fluid Chloride: A Test 40 Years Past Its Time. JAMA 1984, 251, 37–38. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, J.; Xing, X.; Tian, C.; Huang, X.; Yu, S. Significance of Chloride Contents in Cerebrospinal Fluid and Plasma and Their Ratio in Early Diagnosis and Differential Diagnosis of Central Nervous System Infections. Med. J. Chin. People’s Lib. Army 2014, 39, 401–405. [Google Scholar]
- Deidda, G.; Bozarth, I.F.; Cancedda, L. Modulation of GABAergic Transmission in Development and Neurodevelopmental Disorders: Investigating Physiology and Pathology to Gain Therapeutic Perspectives. Front. Cell. Neurosci. 2014, 8, 119. [Google Scholar] [CrossRef]
- Glykys, J.; Dzhala, V.; Egawa, K.; Kahle, K.T.; Delpire, E.; Staley, K. Chloride Dysregulation, Seizures, and Cerebral Edema: A Relationship with Therapeutic Potential. Trends Neurosci. 2017, 40, 276–294. [Google Scholar] [CrossRef]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular PH Regulation by Acid-Base Transporters in Mammalian Neurons. Front. Physiol. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Doyon, N.; Vinay, L.; Prescott, S.A.; De Koninck, Y. Chloride Regulation: A Dynamic Equilibrium Crucial for Synaptic Inhibition. Neuron 2016, 89, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Galeffi, F.; Sah, R.; Pond, B.B.; George, A.; Schwartz-Bloom, R.D. Changes in Intracellular Chloride after Oxygen-Glucose Deprivation of the Adult Hippocampal Slice: Effect of Diazepam. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 4478–4488. [Google Scholar] [CrossRef] [PubMed]
- Pond, B.B.; Berglund, K.; Kuner, T.; Feng, G.; Augustine, G.J.; Schwartz-Bloom, R.D. The Chloride Transporter Na(+)-K(+)-Cl- Cotransporter Isoform-1 Contributes to Intracellular Chloride Increases after in Vitro Ischemia. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 1396–1406. [Google Scholar] [CrossRef]
- Papp, E.; Rivera, C.; Kaila, K.; Freund, T.F. Relationship between Neuronal Vulnerability and Potassium-Chloride Cotransporter 2 Immunoreactivity in Hippocampus Following Transient Forebrain Ischemia. Neuroscience 2008, 154, 677–689. [Google Scholar] [CrossRef]
- Depienne, C.; Bugiani, M.; Dupuits, C.; Galanaud, D.; Touitou, V.; Postma, N.; van Berkel, C.; Polder, E.; Tollard, E.; Darios, F.; et al. Brain White Matter Oedema Due to ClC-2 Chloride Channel Deficiency: An Observational Analytical Study. Lancet Neurol. 2013, 12, 659–668. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Roxburgh, R.H.S.R.; Seaman, S.R.; Masterman, T.; Hensiek, A.E.; Sawcer, S.J.; Vukusic, S.; Achiti, I.; Confavreux, C.; Coustans, M.; le Page, E.; et al. Multiple Sclerosis Severity Score: Using Disability and Disease Duration to Rate Disease Severity. Neurology 2005, 64, 1144–1151. [Google Scholar] [CrossRef]
- Alboukadel, K.; Marcin, K.; Przemyslaw, B. Survminer: Drawing Survival Curves Using “Ggplot2.” R Package Version 0.4.9. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 1 November 2020).
- Andersen, P.K.; Gill, R.D. Cox’s Regression Model for Counting Processes: A Large Sample Study. Ann. Stat. 1982, 10, 1100–1120. [Google Scholar] [CrossRef]
- Amorim, L.D.A.F.; Cai, J. Modelling Recurrent Events: A Tutorial for Analysis in Epidemiology. Int. J. Epidemiol. 2015, 44, 324–333. [Google Scholar] [CrossRef]
- Therneau, T.M. A Package for Survival Analysis in R. R Package Version 3.3-1. 2022. Available online: https://CRAN.R-project.org/package=survival (accessed on 1 November 2020).
- Kalincik, T.; Vivek, V.; Jokubaitis, V.; Lechner-Scott, J.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; Grand’Maison, F.; Hupperts, R.; Oreja-Guevara, C.; et al. Sex as a Determinant of Relapse Incidence and Progressive Course of Multiple Sclerosis. Brain 2013, 136, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.; Williams, O.; Willis, M.; Hrastelj, J.; Rimmer, A.; Joseph, F.; Tomassini, V.; Wardle, M.; Pickersgill, T.; Robertson, N.; et al. Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients with Multiple Sclerosis. JAMA Neurol. 2019, 76, 536. [Google Scholar] [CrossRef] [PubMed]
- Zeydan, B.; Kantarci, O.H. Impact of Age on Multiple Sclerosis Disease Activity and Progression. Curr. Neurol. Neurosci. Rep. 2020, 20, 24. [Google Scholar] [CrossRef]
- Skaper, S.D. Ion Channels on Microglia: Therapeutic Targets for Neuroprotection. CNS Neurol. Disord. Drug. Targets 2011, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, F.; Baumann, C.; Epstein, J.; Kerschen, P.; Garot, T.; Mathey, G.; Debouverie, M.; LORSEP Group. Older Age at Multiple Sclerosis Onset Is an Independent Factor of Poor Prognosis: A Population-Based Cohort Study. Neuroepidemiology 2017, 48, 179–187. [Google Scholar] [CrossRef]
- Pawelec, G. Hallmarks of Human “Immunosenescence”: Adaptation or Dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef]
- Pardo, G.; Jones, D.E. The Sequence of Disease-Modifying Therapies in Relapsing Multiple Sclerosis: Safety and Immunologic Considerations. J. Neurol. 2017, 264, 2351–2374. [Google Scholar] [CrossRef]
- Mathur, D.; López-Rodas, G.; Casanova, B.; Marti, M.B. Perturbed Glucose Metabolism: Insights into Multiple Sclerosis Pathogenesis. Front. Neurol. 2014, 5, 250. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, Q.; Wen, H.; Zhang, Y. Disease Modifying Therapies in Relapsing-Remitting Multiple Sclerosis: A Systematic Review and Network Meta-Analysis. Autoimmun. Rev. 2021, 20, 102826. [Google Scholar] [CrossRef]
- Deisenhammer, F. The Cerebrospinal Fluid in Multiple Sclerosis. Front. Immunol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Konen, F.F.; Schwenkenbecher, P.; Jendretzky, K.F.; Gingele, S.; Witte, T.; Sühs, K.-W.; Grothe, M.; Hannich, M.J.; Süße, M.; Skripuletz, T. Kappa Free Light Chains in Cerebrospinal Fluid in Inflammatory and Non-Inflammatory Neurological Diseases. Brain Sci. 2022, 12, 475. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).