Effect of Obesity and Osteocalcin on Brain Glucose Metabolism in Healthy Participants
Abstract
1. Introduction
2. Subjects and Methods
2.1. Participants
2.2. Imaging Protocol
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
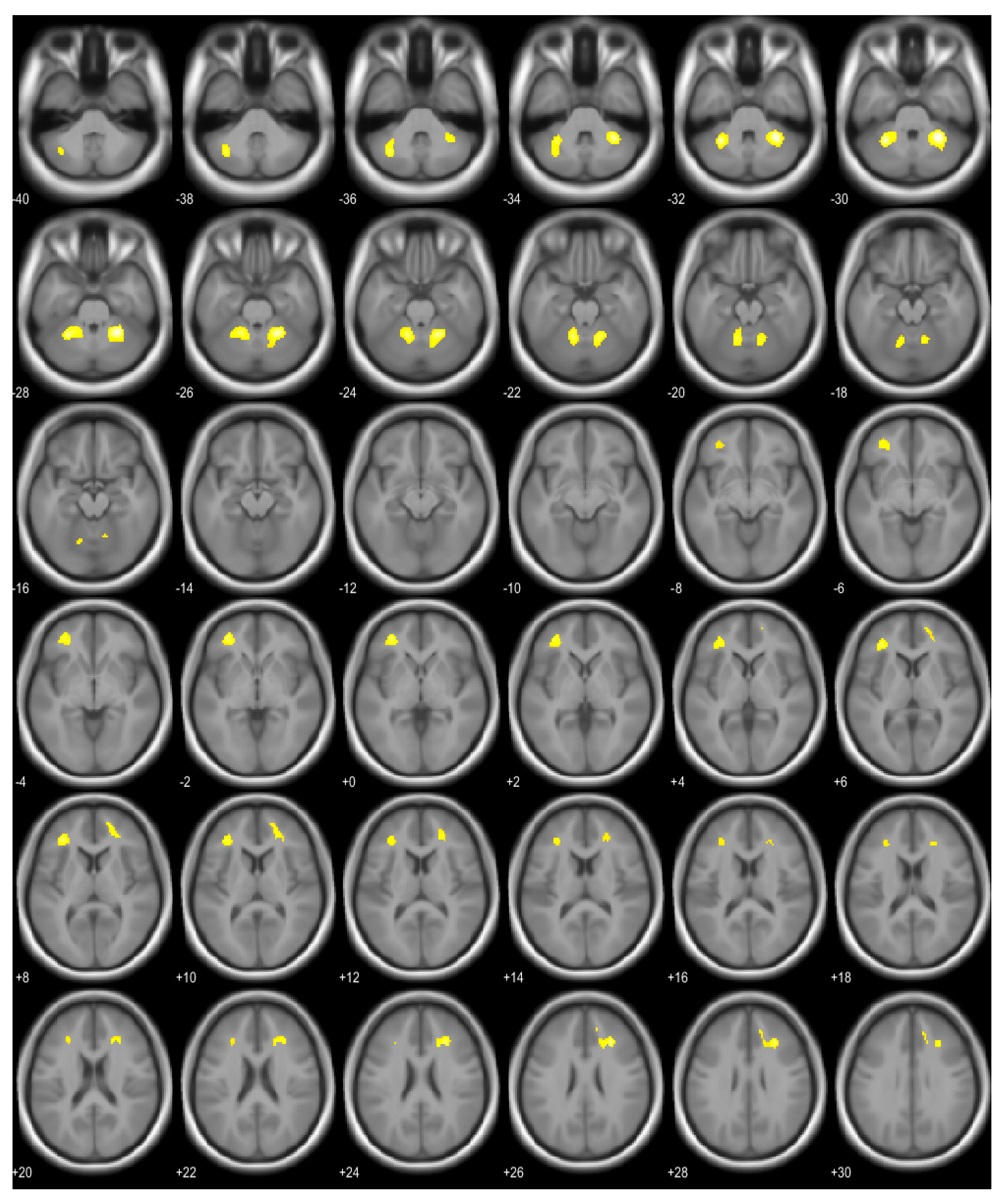
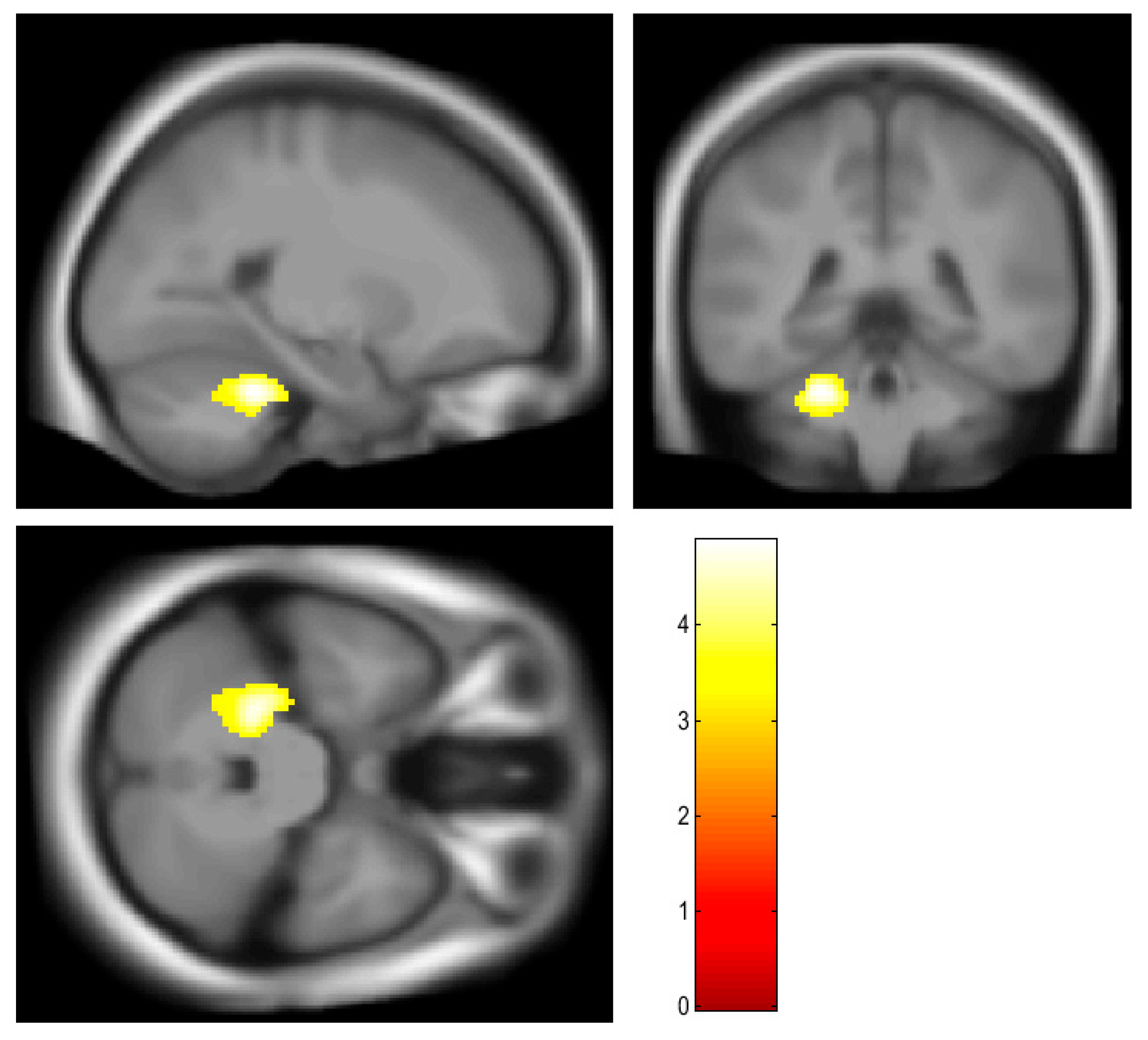
3.2. Brain Region Showing Correlation with BMI and Osteocalcin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Sui, S.X.; Pasco, J.A. Obesity and Brain Function: The Brain-Body Crosstalk. Medicina 2020, 56, 499. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Hay, P.; Campbell, L.; Trollor, J.N. A review of the association between obesity and cognitive function across the lifespan: Implications for novel approaches to prevention and treatment. Obes. Rev. 2011, 12, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.C.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Almuhaideb, A.; Papathanasiou, N.; Bomanji, J. 18F-FDG PET/CT imaging in oncology. Ann. Saudi Med. 2011, 31, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Guedj, E. The renaissance of functional (18)F-FDG PET brain activation imaging. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2338–2341. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.K.; Bohnen, N.I.; Wong, K.K.; Minoshima, S.; Frey, K.A. Brain PET in suspected dementia: Patterns of altered FDG metabolism. Radiographics 2014, 34, 684–701. [Google Scholar] [CrossRef]
- Pegueroles, J.; Pane, A.; Vilaplana, E.; Montal, V.; Bejanin, A.; Videla, L.; Carmona-Iragui, M.; Barroeta, I.; Ibarzabal, A.; Casajoana, A.; et al. Obesity impacts brain metabolism and structure independently of amyloid and tau pathology in healthy elderly. Alzheimers Dement. 2020, 12, e12052. [Google Scholar] [CrossRef]
- Tuulari, J.J.; Karlsson, H.K.; Hirvonen, J.; Hannukainen, J.C.; Bucci, M.; Helmio, M.; Ovaska, J.; Soinio, M.; Salminen, P.; Savisto, N.; et al. Weight loss after bariatric surgery reverses insulin-induced increases in brain glucose metabolism of the morbidly obese. Diabetes 2013, 62, 2747–2751. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Goldstein, R.Z.; Alia-Klein, N.; Logan, J.; Wong, C.; Thanos, P.K.; Ma, Y.; et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity 2009, 17, 60–65. [Google Scholar] [CrossRef]
- Rebelos, E.; Rinne, J.O.; Nuutila, P.; Ekblad, L.L. Brain Glucose Metabolism in Health, Obesity, and Cognitive Decline-Does Insulin Have Anything to Do with It? A Narrative Review. J. Clin. Med. 2021, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.C.; van der Eerden, B.C.J. Osteocalcin-A Versatile Bone-Derived Hormone. Front. Endocrinol. 2018, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Presotto, L.; Iaccarino, L.; Sala, A.; Vanoli, E.G.; Muscio, C.; Nigri, A.; Bruzzone, M.G.; Tagliavini, F.; Gianolli, L.; Perani, D.; et al. Low-dose CT for the spatial normalization of PET images: A validation procedure for amyloid-PET semi-quantification. Neuroimage Clin. 2018, 20, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi, V.S.; Prabhu, K.; Ramesh, M.; Venkatesan, V. The association of serum osteocalcin with the bone mineral density in post menopausal women. J. Clin. Diagn. Res. 2013, 7, 814–816. [Google Scholar] [CrossRef]
- Carlen, M. What constitutes the prefrontal cortex? Science 2017, 358, 478–482. [Google Scholar] [CrossRef]
- Lowe, C.J.; Reichelt, A.C.; Hall, P.A. The Prefrontal Cortex and Obesity: A Health Neuroscience Perspective. Trends Cogn. Sci. 2019, 23, 349–361. [Google Scholar] [CrossRef]
- Fernandez-Andujar, M.; Morales-Garcia, E.; Garcia-Casares, N. Obesity and Gray Matter Volume Assessed by Neuroimaging: A Systematic Review. Brain Sci. 2021, 11, 999. [Google Scholar] [CrossRef]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef]
- Lundgaard, I.; Li, B.; Xie, L.; Kang, H.; Sanggaard, S.; Haswell, J.D.; Sun, W.; Goldman, S.; Blekot, S.; Nielsen, M.; et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun. 2015, 6, 6807. [Google Scholar] [CrossRef]
- Bandala, C.; Cardenas-Rodriguez, N.; Reyes-Long, S.; Cortes-Altamirano, J.L.; Garciadiego-Cazares, D.; Lara-Padilla, E.; Ibanez-Cervantes, G.; Mancilla-Ramirez, J.; Gomez-Manzo, S.; Alfaro-Rodriguez, A. Trends in Gliosis in Obesity, and the Role of Antioxidants as a Therapeutic Alternative. Antioxidants 2022, 11, 1972. [Google Scholar] [CrossRef]
- Kullmann, S.; Abbas, Z.; Machann, J.; Shah, N.J.; Scheffler, K.; Birkenfeld, A.L.; Haring, H.U.; Fritsche, A.; Heni, M.; Preissl, H. Investigating obesity-associated brain inflammation using quantitative water content mapping. J. Neuroendocrinol. 2020, 32, e12907. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, E.R.; Parent, M.J.; Souza, D.G.; Leuzy, A.; Lecrux, C.; Kim, H.I.; Gauthier, S.; Pellerin, L.; Hamel, E.; Rosa-Neto, P. [(18)F]FDG PET signal is driven by astroglial glutamate transport. Nat. Neurosci. 2017, 20, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Salvado, G.; Mila-Aloma, M.; Shekari, M.; Ashton, N.J.; Operto, G.; Falcon, C.; Cacciaglia, R.; Minguillon, C.; Fauria, K.; Ninerola-Baizan, A.; et al. Reactive astrogliosis is associated with higher cerebral glucose consumption in the early Alzheimer’s continuum. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4567–4579. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Griffin, C.; Abrishami, S.; Eter, L.; Lanzetta, N.; Hak, L.; Clemente, J.; Agarwal, D.; Lerner, A.; Westerhoff, M.; et al. Sex hormones regulate metainflammation in diet-induced obesity in mice. J. Biol. Chem. 2021, 297, 101229. [Google Scholar] [CrossRef]
- Miller, C.N.; Brown, L.M.; Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Estrogens, inflammation and obesity: An overview. Front. Biol. 2012, 7, 40–47. [Google Scholar] [CrossRef]
- Brown, L.M.; Gent, L.; Davis, K.; Clegg, D.J. Metabolic impact of sex hormones on obesity. Brain Res. 2010, 1350, 77–85. [Google Scholar] [CrossRef]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-Garcia, J.M.; da Guarda, S.N.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Marien, P.; et al. Consensus paper: Roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef]
- Klein, A.P.; Ulmer, J.L.; Quinet, S.A.; Mathews, V.; Mark, L.P. Nonmotor Functions of the Cerebellum: An Introduction. AJNR Am. J. Neuroradiol. 2016, 37, 1005–1009. [Google Scholar] [CrossRef]
- Manolagas, S.C. Osteocalcin promotes bone mineralization but is not a hormone. PLoS Genet. 2020, 16, e1008714. [Google Scholar] [CrossRef]
- Shan, C.; Ghosh, A.; Guo, X.Z.; Wang, S.M.; Hou, Y.F.; Li, S.T.; Liu, J.M. Roles for osteocalcin in brain signalling: Implications in cognition- and motor-related disorders. Mol. Brain 2019, 12, 23. [Google Scholar] [CrossRef]
- Kanazawa, I.; Tanaka, S.; Sugimoto, T. The Association Between Osteocalcin and Chronic Inflammation in Patients with Type 2 Diabetes Mellitus. Calcif. Tissue Int. 2018, 103, 599–605. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Number of subjects | 179 |
| Age | 46.20 ± 6.22 |
| Height (cm) | 171.36 ± 4.99 |
| Weight (kg) | 71.93 ± 8.62 |
| BMI | 24.49 ± 2.64 |
| Fasting glucose level (mg/dL) | 92.97 ± 13.02 |
| HbA1C | 5.60 ± 0.54 |
| BMD | |
| Lumbar spine T-score | −0.04 ± 1.14 |
| Femur neck T-score | −0.06 ± 0.97 |
| Femur total T-score | 0.45 ± 0.90 |
| Osteocalcin (ng/mL) | 16.36 ± 5.08 |
| Brain Area | Voxel | T | p (FWE Corrected) | Coordinates (x, y, z) |
|---|---|---|---|---|
| Right cerebellum, anterior lobe | 521 | 6.55 | <0.001 | 28, −48, −30 |
| Left cerebellum, anterior and posterior lobe | 522 | 6.19 | <0.001 | −30, −50, −30 |
| Right middle frontal gyrus, right cingulate gyrus, right anterior cingulate | 297 | 5.78 | <0.001 | 28, 30, 26 |
| Left middle frontal gyrus, sub-gyral area of left frontal lobe | 368 | 5.66 | <0.001 | −34, 38, 8 |
| Brain Area | Voxel | T | p (FWE Corrected for Cluster Level) | Coordinates (x, y, z) |
|---|---|---|---|---|
| Left cerebellum, anterior lobe | 458 | 4.87 | 0.009 | −24, −40, −30 |
| Brain Area | Coefficient | S.E | t | p |
|---|---|---|---|---|
| Left cerebellum, 3 | −0.005101 | 0.004714 | −2.086 | 0.0384 |
| Left cerebellum, 4_5 | −0.002666 | 0.001218 | −2.189 | 0.0299 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Nam, H.-Y. Effect of Obesity and Osteocalcin on Brain Glucose Metabolism in Healthy Participants. Brain Sci. 2023, 13, 889. https://doi.org/10.3390/brainsci13060889
Shin S, Nam H-Y. Effect of Obesity and Osteocalcin on Brain Glucose Metabolism in Healthy Participants. Brain Sciences. 2023; 13(6):889. https://doi.org/10.3390/brainsci13060889
Chicago/Turabian StyleShin, Seunghyeon, and Hyun-Yeol Nam. 2023. "Effect of Obesity and Osteocalcin on Brain Glucose Metabolism in Healthy Participants" Brain Sciences 13, no. 6: 889. https://doi.org/10.3390/brainsci13060889
APA StyleShin, S., & Nam, H.-Y. (2023). Effect of Obesity and Osteocalcin on Brain Glucose Metabolism in Healthy Participants. Brain Sciences, 13(6), 889. https://doi.org/10.3390/brainsci13060889






