Melatonin in Combination with Albendazole or Albendazole Sulfoxide Produces a Synergistic Cytotoxicity against Malignant Glioma Cells through Autophagy and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Drugs, and Antibodies
2.2. Glioma Cells and Cell Culture
2.3. Concentration-Effect and Combination Study
2.4. Determination of Cell Death Mechanisms
2.4.1. Apoptosis Detection with Annexin V and 7-AAD Double Stain
2.4.2. Evaluation of Autophagy
Detection of Acidic Vesicular Organelles
LC3 immunofluorescence Staining
2.5. Proliferation Assay
2.6. Statistical Analysis
3. Results
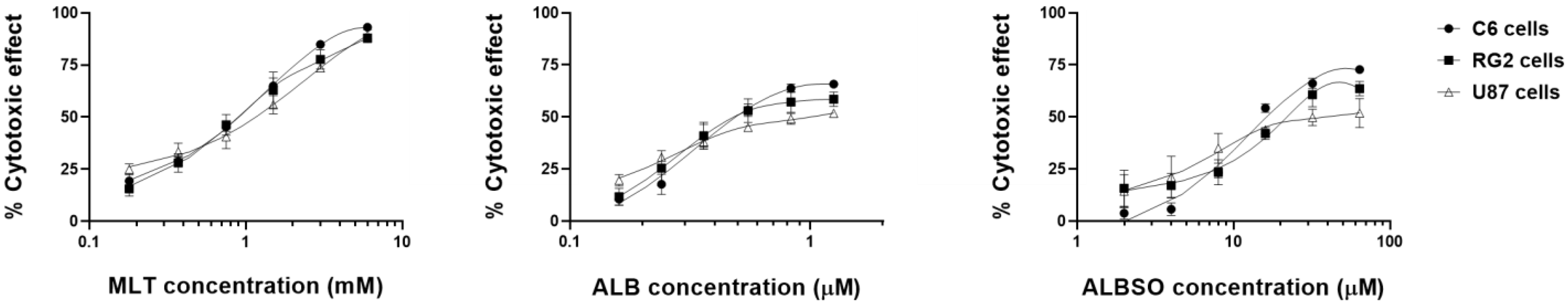
3.1. ALB, ALBSO, and MLT Induced a Cytotoxic Effect on C6, RG2, and U87 Cell Lines
3.2. The Combination of MLT with ALB or ALBSO Induced a Synergistic Cytotoxicity
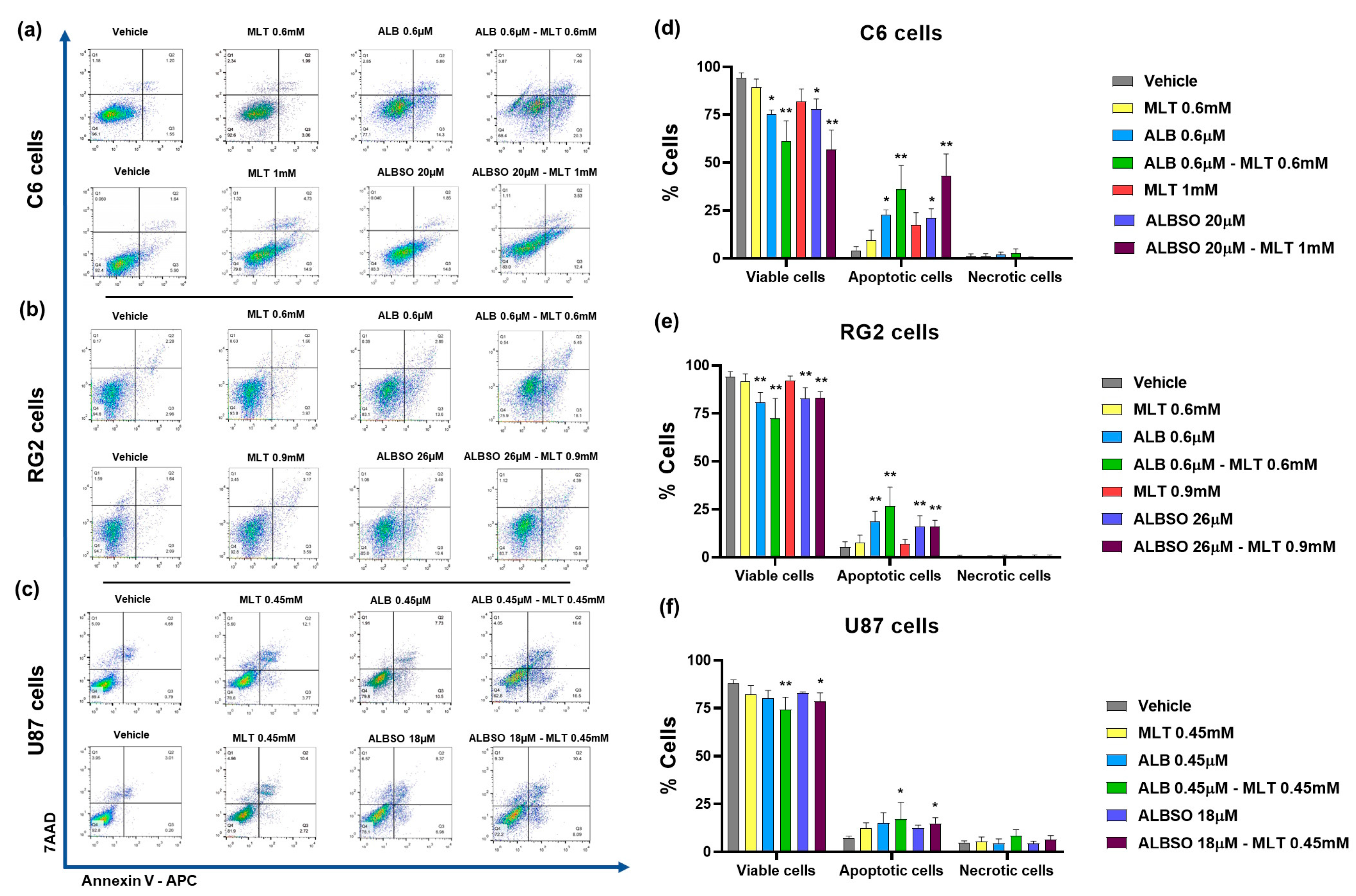
3.3. Effect of the Combinations of MLT with ALB or ALBSO on the Cell Death Mechanisms
3.3.1. The Combinations of MLT with ALB or ALBSO Induced Apoptosis
3.3.2. The Combinations of MLT with ALB or ALBSO Induced Autophagy
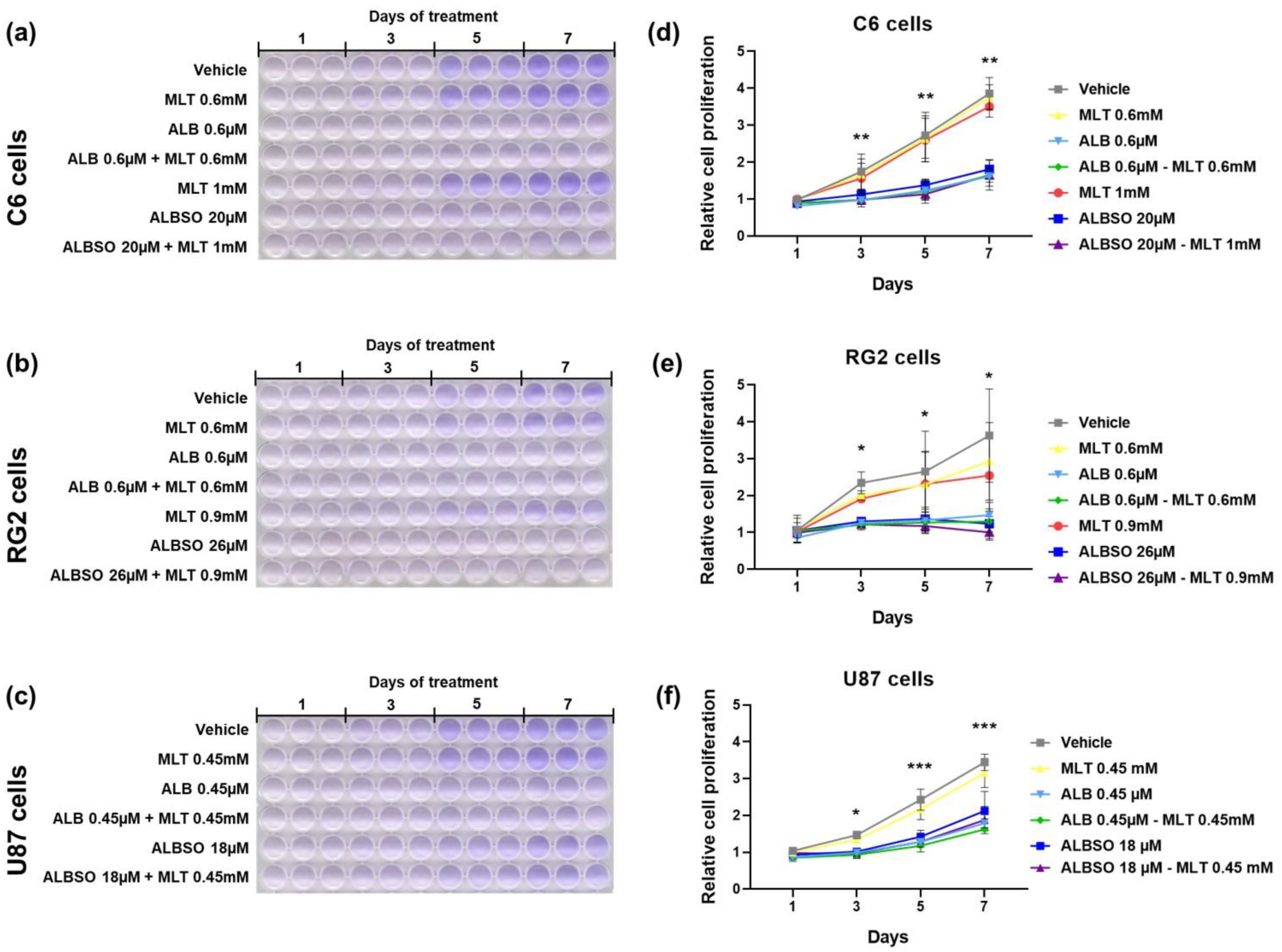
3.4. The Treatment with ALB and ALBSO Inhibited Proliferation, Independently of MLT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Wick, W.; Osswald, M.; Wick, A.; Winkler, F. Treatment of glioblastoma in adults. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418790452. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.V.R.; Batista, C.; Afonso, B.H.; Alexandre-Moreira, M.S.; Dubois, L.G.; Pontes, B.; Moura Neto, V.; Mendes, F.A. Obstacles to Glioblastoma Treatment Two Decades after Temozolomide. Cancers 2022, 14, 3203. [Google Scholar] [CrossRef]
- Bhatia, P.; Bernier, M.; Sanghvi, M.; Moaddel, R.; Schwarting, R.; Ramamoorthy, A.; Wainer, I.W. Breast cancer resistance protein (BCRP/ABCG2) localises to the nucleus in glioblastoma multiforme cells. Xenobiotica 2012, 42, 748–755. [Google Scholar] [CrossRef]
- Lin, F.; de Gooijer, M.C.; Roig, E.M.; Buil, L.C.; Christner, S.M.; Beumer, J.H.; Würdinger, T.; Beijnen, J.H.; van Tellingen, O. ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clin. Cancer Res. 2014, 20, 2703–2713. [Google Scholar] [CrossRef] [PubMed]
- Panic, G.; Duthaler, U.; Speich, B.; Keiser, J. Repurposing drugs for the treatment and control of helminth infections. Int. J. Parasitol. Drugs Drug. Resist. 2014, 4, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Son, D.S.; Lee, E.S.; Adunyah, S.E. The Antitumor Potentials of Benzimidazole Anthelmintics as Repurposing Drugs. Immune Netw. 2020, 20, e29. [Google Scholar] [CrossRef]
- Castro, L.S.; Kviecinski, M.R.; Ourique, F.; Parisotto, E.B.; Grinevicius, V.M.; Correia, J.F.; Wilhelm Filho, D.; Pedrosa, R.C. Albendazole as a promising molecule for tumor control. Redox Biol. 2016, 10, 90–99. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Akhter, J.; Wang, L.; Lu, Y.; Morris, D.L. Antitumor activity of albendazole against the human colorectal cancer cell line HT-29: In vitro and in a xenograft model of peritoneal carcinomatosis. Cancer Chemother. Pharm. 2005, 55, 425–432. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Liu, X.; Khandelwal, V.K.M.; Xiaolei, S.; Xiang, W.; Franklin, G. Solid Lipid Nanoparticles of Albendazole for Enhancing Cellular Uptake and Cytotoxicity against U-87 MG Glioma Cell Lines. Molecules 2017, 22, 2040. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Szwajcer, M.; Chin, M.; Liauw, W.; Seef, J.; Galettis, P.; Morris, D.L.; Links, M. Phase I clinical trial to determine maximum tolerated dose of oral albendazole in patients with advanced cancer. Cancer Chemother. Pharm. 2010, 65, 597–605. [Google Scholar] [CrossRef]
- Rawden, H.C.; Kokwaro, G.O.; Ward, S.A.; Edwards, G. Relative contribution of cytochromes P-450 and flavin-containing monoxygenases to the metabolism of albendazole by human liver microsomes. Br. J. Clin. Pharm. 2000, 49, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A.; Neul, D.; Clouser-Roche, A.; Dalvie, D.; Wester, M.R.; Jiang, Y.; Jones, J.P.; Freiwald, S.; Zientek, M.; Totah, R.A. Identification of novel substrates for human cytochrome P450 2J2. Drug. Metab. Dispos. 2010, 38, 347–356. [Google Scholar] [CrossRef]
- Jung, H.; Cárdenas, G.; Sciutto, E.; Fleury, A. Medical treatment for neurocysticercosis: Drugs, indications and perspectives. Curr. Top. Med. Chem. 2008, 8, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.; Jung, H. Pharmacokinetic optimisation of the treatment of neurocysticercosis. Clin. Pharm. 1998, 34, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Ortiz, R.; Méndez-Lucio, O.; Medina-Franco, J.L.; Castillo, R.; Yépez-Mulia, L.; Hernández-Luis, F.; Hernández-Campos, A. Towards the identification of the binding site of benzimidazoles to β-tubulin of Trichinella spiralis: Insights from computational and experimental data. J. Mol. Graph. Model. 2013, 41, 12–19. [Google Scholar] [CrossRef]
- Yang, M.H.; Ha, I.J.; Um, J.Y.; Ahn, K.S. Albendazole Exhibits Anti-Neoplastic Actions against Gastric Cancer Cells by Affecting STAT3 and STAT5 Activation by Pleiotropic Mechanism(s). Biomedicines 2021, 9, 362. [Google Scholar] [CrossRef]
- Kim, U.; Shin, C.; Kim, C.Y.; Ryu, B.; Kim, J.; Bang, J.; Park, J.H. Albendazole exerts antiproliferative effects on prostate cancer cells by inducing reactive oxygen species generation. Oncol. Lett. 2021, 21, 395. [Google Scholar] [CrossRef] [PubMed]
- Macchi, M.M.; Bruce, J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocr. 2004, 25, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Korkmaz, A. Clinical aspects of melatonin. Saudi Med. J. 2008, 29, 1537–1547. [Google Scholar]
- Hardeland, R. Melatonin metabolism in the central nervous system. Curr. Neuropharmacol. 2010, 8, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dickson, E.J.; Jung, S.R.; Koh, D.S.; Hille, B. High membrane permeability for melatonin. J. Gen. Physiol. 2016, 147, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharm. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Favero, G.; Rodella, L.F.; Rezzani, R. Melatonin’s Antineoplastic Potential Against Glioblastoma. Cells 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.J.; Xu, D.P.; Li, H.B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [PubMed]
- Martín, V.; Sanchez-Sanchez, A.M.; Herrera, F.; Gomez-Manzano, C.; Fueyo, J.; Alvarez-Vega, M.A.; Antolín, I.; Rodriguez, C. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br. J. Cancer 2013, 108, 2005–2012. [Google Scholar] [CrossRef]
- Hsiao, S.Y.; Tang, C.H.; Chen, P.C.; Lin, T.H.; Chao, C.C. Melatonin Inhibits EMT in Bladder Cancer by Targeting Autophagy. Molecules 2022, 27, 8649. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharm. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Zembruski, N.C.; Stache, V.; Haefeli, W.E.; Weiss, J. 7-Aminoactinomycin D for apoptosis staining in flow cytometry. Anal. Biochem. 2012, 429, 79–81. [Google Scholar] [CrossRef]
- Kanzawa, T.; Kondo, Y.; Ito, H.; Kondo, S.; Germano, I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003, 63, 2103–2108. [Google Scholar] [PubMed]
- Romano-Feinholz, S.; Salazar-Ramiro, A.; Munoz-Sandoval, E.; Magana-Maldonado, R.; Hernandez Pedro, N.; Rangel Lopez, E.; Gonzalez Aguilar, A.; Sanchez Garcia, A.; Sotelo, J.; Perez de la Cruz, V.; et al. Cytotoxicity induced by carbon nanotubes in experimental malignant glioma. Int. J. Nanomed. 2017, 12, 6005–6026. [Google Scholar] [CrossRef]
- Runwal, G.; Stamatakou, E.; Siddiqi, F.H.; Puri, C.; Zhu, Y.; Rubinsztein, D.C. LC3-positive structures are prominent in autophagy-deficient cells. Sci. Rep. 2019, 9, 10147. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, 087379. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Baek, S.H.; Ha, I.J.; Ahn, K.S. Regulation of apoptosis and autophagy by albendazole in human colon adenocarcinoma cells. Biochimie 2022, 198, 155–166. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, W.; Sun, T.; Wang, L.; Du, C.; Hu, Y.; Liu, W.; Feng, F.; Chen, Y.; Sun, H. Therapeutic strategies of glioblastoma (GBM): The current advances in the molecular targets and bioactive small molecule compounds. Acta Pharm. Sin. B 2022, 12, 1781–1804. [Google Scholar] [CrossRef]
- Mudduluru, G.; Walther, W.; Kobelt, D.; Dahlmann, M.; Treese, C.; Assaraf, Y.G.; Stein, U. Repositioning of drugs for intervention in tumor progression and metastasis: Old drugs for new targets. Drug. Resist. Updat. 2016, 26, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Ramesh, S. Molecular Targets for Combined Therapeutic Strategies to Limit Glioblastoma Cell Migration and Invasion. Front. Pharm. 2020, 11, 358. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zheng, Z.; Liu, Q.X.; Lu, X.; Zhou, D.; Zhang, J.; Zheng, H.; Dai, J.G. Repositioning of Antiparasitic Drugs for Tumor Treatment. Front. Oncol. 2021, 11, 670804. [Google Scholar] [CrossRef]
- González-Hernández, I.; Ruiz-Olmedo, M.I.; Cárdenas, G.; Jung-Cook, H. A simple LC-MS/MS method to determine plasma and cerebrospinal fluid levels of albendazole metabolites (albendazole sulfoxide and albendazole sulfone) in patients with neurocysticercosis. Biomed. Chromatogr. 2012, 26, 267–272. [Google Scholar] [CrossRef]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105–112. [Google Scholar] [PubMed]
- Martín, V.; Herrera, F.; Carrera-Gonzalez, P.; García-Santos, G.; Antolín, I.; Rodriguez-Blanco, J.; Rodriguez, C. Intracellular signaling pathways involved in the cell growth inhibition of glioma cells by melatonin. Cancer Res. 2006, 66, 1081–1088. [Google Scholar] [CrossRef]
- Ariey-Bonnet, J.; Carrasco, K.; Le Grand, M.; Hoffer, L.; Betzi, S.; Feracci, M.; Tsvetkov, P.; Devred, F.; Collette, Y.; Morelli, X.; et al. In silico molecular target prediction unveils mebendazole as a potent MAPK14 inhibitor. Mol. Oncol. 2020, 14, 3083–3099. [Google Scholar] [CrossRef]
- Gu, J.; Lu, Z.; Ji, C.; Chen, Y.; Liu, Y.; Lei, Z.; Wang, L.; Zhang, H.T.; Li, X. Melatonin inhibits proliferation and invasion via repression of miRNA-155 in glioma cells. Biomed. Pharm. 2017, 93, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhu, Y.; Gao, C.; Ling, C.; Qin, J.; Wang, Q.; Huang, Y.; Lu, W.; Wang, J. Menthol-modified BSA nanoparticles for glioma targeting therapy using an energy restriction strategy. NPG Asia Mater. 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Belaz, K.R.A.; Denadai, M.; Almeida, A.P.; Lima, R.T.; Vasconcelos, M.H.; Pinto, M.M.; Cass, Q.B.; Oliveira, R.V. Enantiomeric resolution of albendazole sulfoxide by semipreparative HPLC and in vitro study of growth inhibitory effects on human cancer cell lines. J. Pharm. Biomed. Anal. 2012, 66, 100–108. [Google Scholar] [CrossRef]
- Ehteda, A.; Galettis, P.; Pillai, K.; Morris, D.L. Combination of albendazole and 2-methoxyestradiol significantly improves the survival of HCT-116 tumor-bearing nude mice. BMC Cancer 2013, 13, 86. [Google Scholar] [CrossRef]
- Thomé, M.P.; Filippi-Chiela, E.C.; Villodre, E.S.; Migliavaca, C.B.; Onzi, G.R.; Felipe, K.B.; Lenz, G. Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J. Cell. Sci. 2016, 129, 4622–4632. [Google Scholar] [CrossRef]
- Jo, S.B.; Sung, S.J.; Choi, H.S.; Park, J.S.; Hong, Y.K.; Joe, Y.A. Modulation of Autophagy is a Potential Strategy for Enhancing the Anti-Tumor Effect of Mebendazole in Glioblastoma Cells. Biomol. Ther. 2022, 30, 616–624. [Google Scholar] [CrossRef]
- He, Q.; Yin, Y.; Pan, X.; Wu, Y.; Li, X. Albendazole-induced autophagy blockade contributes to elevated apoptosis in cholangiocarcinoma cells through AMPK/mTOR activation. Toxicol. Appl. Pharm. 2022, 454, 116214. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, W.; Xu, C.; Zhou, X.; Leng, X.; Li, Y. Melatonin suppresses serum starvation-induced autophagy of ovarian granulosa cells in premature ovarian insufficiency. BMC Womens Health 2022, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liang, F.; Zheng, J.; Zhou, K.; Chen, S.; Yu, J.; Zhang, J. Melatonin Regulates Apoptosis and Autophagy Via ROS-MST1 Pathway in Subarachnoid Hemorrhage. Front. Mol. Neurosci. 2018, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, V.F.; Pessoa, A.S.; Padovan, C.Z.; Abrantes, D.C.; Gomes, F.H.; Maticoli, M.A.; de Menezes, M.L. Oxidation of melatonin by AAPH-derived peroxyl radicals: Evidence of a pro-oxidant effect of melatonin. Biochim. Biophys. Acta 2009, 1790, 787–792. [Google Scholar] [CrossRef]
- Yao, K.C.; Komata, T.; Kondo, Y.; Kanzawa, T.; Kondo, S.; Germano, I.M. Molecular response of human glioblastoma multiforme cells to ionizing radiation: Cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J. Neurosurg. 2003, 98, 378–384. [Google Scholar] [CrossRef]
- Bata, N.; Cosford, N.D.P. Cell Survival and Cell Death at the Intersection of Autophagy and Apoptosis: Implications for Current and Future Cancer Therapeutics. ACS Pharm. Transl. Sci. 2021, 4, 1728–1746. [Google Scholar] [CrossRef]
- Fang, Z.; Jung, K.H.; Yan, H.H.; Kim, S.J.; Rumman, M.; Park, J.H.; Han, B.; Lee, J.E.; Kang, Y.W.; Lim, J.H.; et al. Melatonin Synergizes with Sorafenib to Suppress Pancreatic Cancer via Melatonin Receptor and PDGFR-β/STAT3 Pathway. Cell. Physiol. Biochem. 2018, 47, 1751–1768. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, W.; Xue, Z.; Liu, X.; Feng, Z.; Zhang, Y.; Li, W.; Zhang, Q.; Chen, A.; Huang, B.; et al. Thiabendazole Inhibits Glioblastoma Cell Proliferation and Invasion Targeting Mini-chromosome Maintenance Protein 2. J. Pharm. Exp. Ther. 2022, 380, 63–75. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, Q.; Hu, R.; Li, Y.; Peng, Y.; Liu, H.; Ye, M.; Zhang, B.; Zhang, P.; Liu-Smith, F.; et al. Novel therapeutic strategy for melanoma based on albendazole and the CDK4/6 inhibitor palbociclib. Sci. Rep. 2022, 12, 5706. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes. Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Cho, H.Y.; Jahanian, A.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H.; Chen, T.C. Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg. Focus. 2014, 37, E12. [Google Scholar] [CrossRef]
- Magaña-Maldonado, R.; Manoutcharian, K.; Hernández-Pedro, N.Y.; Rangel-López, E.; Pérez-De la Cruz, V.; Rodríguez-Balderas, C.; Sotelo, J.; Pineda, B. Concomitant treatment with pertussis toxin plus temozolomide increases the survival of rats bearing intracerebral RG2 glioma. J. Cancer Res. Clin. Oncol. 2014, 140, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kuang, X.; Zhang, G.; Liang, L.; Liu, D.; Hu, B.; Xie, Z.; Li, H.; Liu, H.; Ye, M.; et al. Albendazole induces immunotherapy response by facilitating ubiquitin-mediated PD-L1 degradation. J. Immunother. Cancer 2022, 10, e003819. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef] [PubMed]
- deSouza, R.M.; Shaweis, H.; Han, C.; Sivasubramaniam, V.; Brazil, L.; Beaney, R.; Sadler, G.; Al-Sarraj, S.; Hampton, T.; Logan, J.; et al. Has the survival of patients with glioblastoma changed over the years? Br. J. Cancer 2016, 114, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Chavarria, V.; Ortiz-Islas, E.; Salazar, A.; Pérez-de la Cruz, V.; Espinosa-Bonilla, A.; Figueroa, R.; Ortíz-Plata, A.; Sotelo, J.; Sánchez-García, F.J.; Pineda, B. Lactate-Loaded Nanoparticles Induce Glioma Cytotoxicity and Increase the Survival of Rats Bearing Malignant Glioma Brain Tumor. Pharmaceutics 2022, 14, 327. [Google Scholar] [CrossRef]
- Wu, S.; Calero-Pérez, P.; Arús, C.; Candiota, A.P. Anti-PD-1 Immunotherapy in Preclinical GL261 Glioblastoma: Influence of Therapeutic Parameters and Non-Invasive Response Biomarker Assessment with MRSI-Based Approaches. Int. J. Mol. Sci. 2020, 21, 8775. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Cerón, M.; Chavarria, V.; Ríos, C.; Pineda, B.; Palomares-Alonso, F.; Rojas-Tomé, I.S.; Jung-Cook, H. Melatonin in Combination with Albendazole or Albendazole Sulfoxide Produces a Synergistic Cytotoxicity against Malignant Glioma Cells through Autophagy and Apoptosis. Brain Sci. 2023, 13, 869. https://doi.org/10.3390/brainsci13060869
Hernández-Cerón M, Chavarria V, Ríos C, Pineda B, Palomares-Alonso F, Rojas-Tomé IS, Jung-Cook H. Melatonin in Combination with Albendazole or Albendazole Sulfoxide Produces a Synergistic Cytotoxicity against Malignant Glioma Cells through Autophagy and Apoptosis. Brain Sciences. 2023; 13(6):869. https://doi.org/10.3390/brainsci13060869
Chicago/Turabian StyleHernández-Cerón, Miguel, Víctor Chavarria, Camilo Ríos, Benjamin Pineda, Francisca Palomares-Alonso, Irma Susana Rojas-Tomé, and Helgi Jung-Cook. 2023. "Melatonin in Combination with Albendazole or Albendazole Sulfoxide Produces a Synergistic Cytotoxicity against Malignant Glioma Cells through Autophagy and Apoptosis" Brain Sciences 13, no. 6: 869. https://doi.org/10.3390/brainsci13060869
APA StyleHernández-Cerón, M., Chavarria, V., Ríos, C., Pineda, B., Palomares-Alonso, F., Rojas-Tomé, I. S., & Jung-Cook, H. (2023). Melatonin in Combination with Albendazole or Albendazole Sulfoxide Produces a Synergistic Cytotoxicity against Malignant Glioma Cells through Autophagy and Apoptosis. Brain Sciences, 13(6), 869. https://doi.org/10.3390/brainsci13060869