Neural Responses to a Working Memory Task in Acute Depressed and Remitted Phases in Bipolar Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Behavioral Results
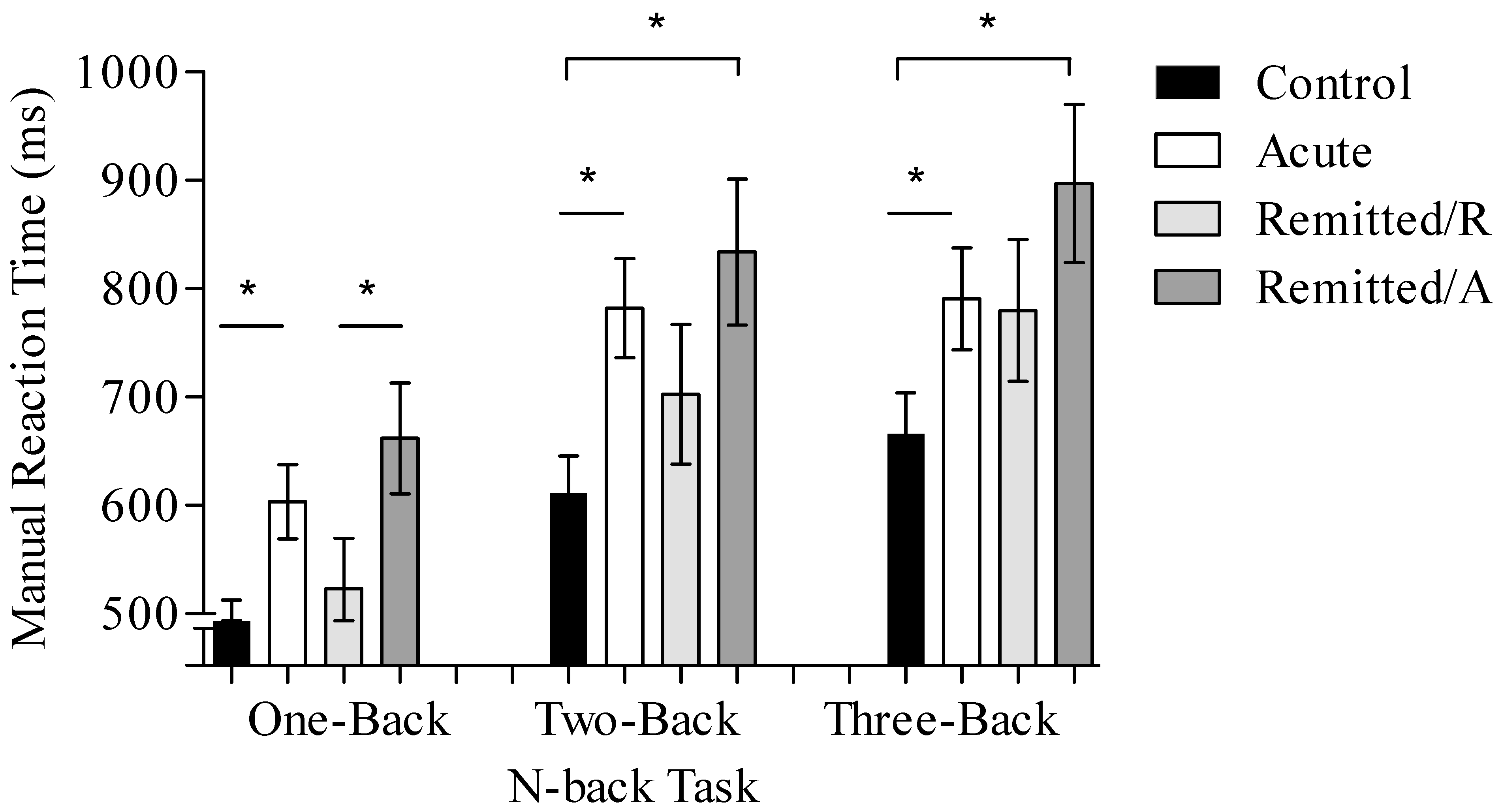
3.1.1. Reaction Time
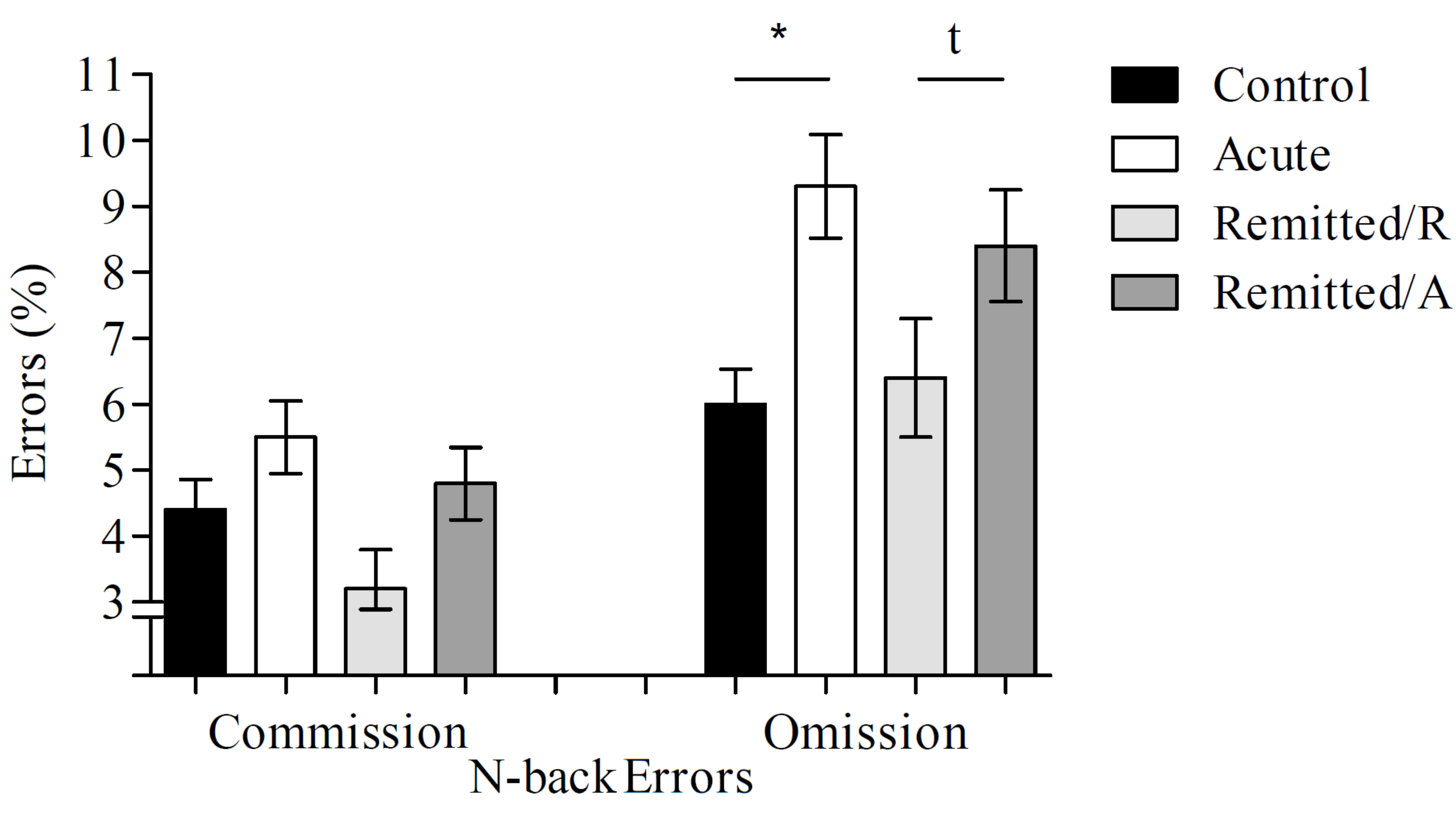
3.1.2. Commission Errors
3.1.3. Omission Errors
3.2. Imaging Results
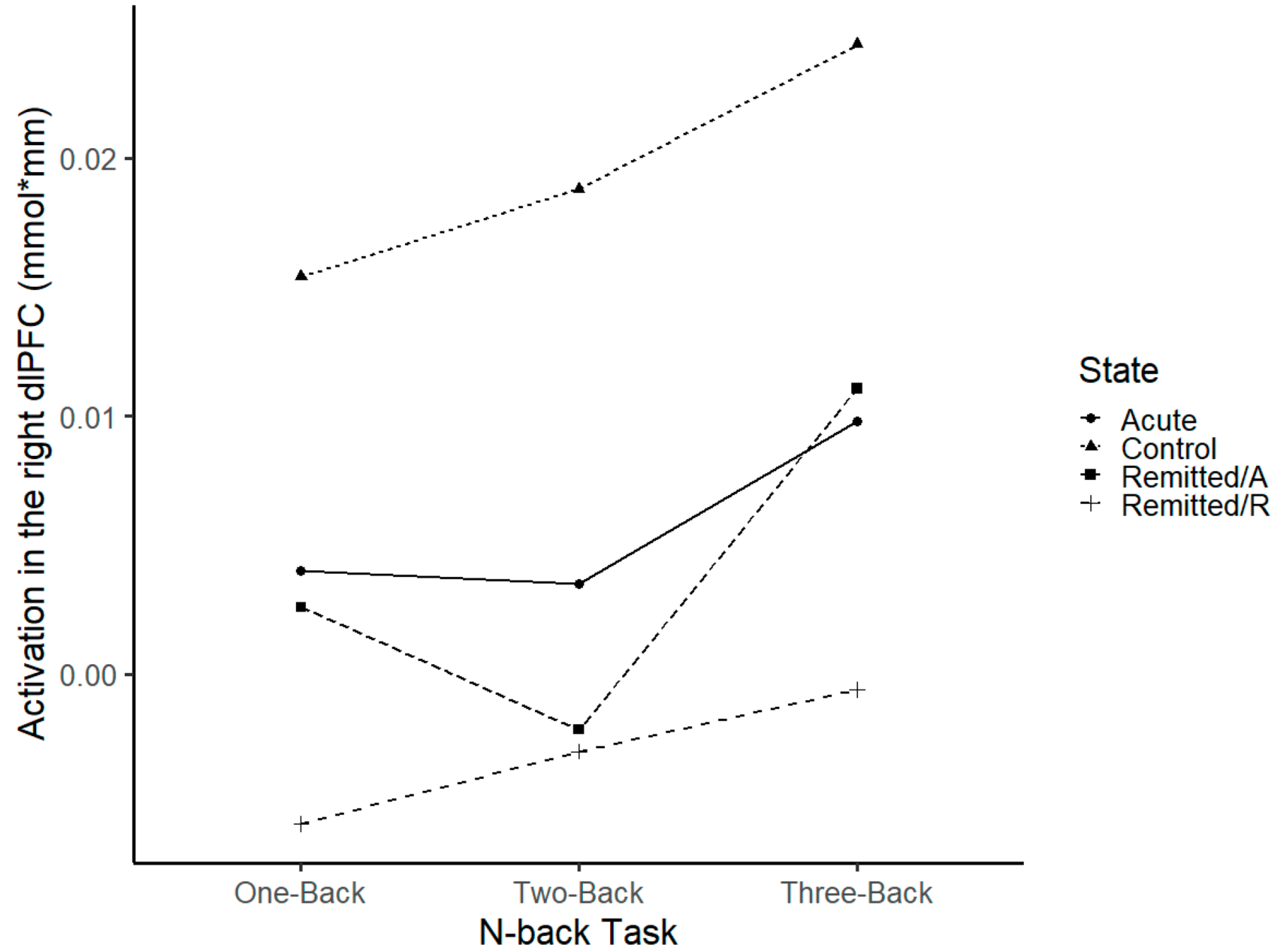
3.2.1. dlPFC
3.2.2. vlPFC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sole, B.; Bonnin, C.M.; Torrent, C.; Martinez-Aran, A.; Popovic, D.; Tabarés-Seisdedos, R.; Vieta, E. Neurocognitive Impairment across the Bipolar Spectrum. CNS Neurosci. Ther. 2012, 18, 194–200. [Google Scholar] [CrossRef]
- Dickinson, T.; Becerra, R.; Coombes, J. Executive Functioning Deficits among Adults with Bipolar Disorder (Types I and II): A Systematic Review and Meta-Analysis. J. Affect. Disord. 2017, 218, 407–427. [Google Scholar] [CrossRef]
- Lima, I.M.M.; Peckham, A.D.; Johnson, S.L. Cognitive Deficits in Bipolar Disorders: Implications for Emotion. Clin. Psychol. Rev. 2018, 59, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Yep, R.; Soncin, S.; Brien, D.C.; Coe, B.C.; Marin, A.; Munoz, D.P. Using an Emotional Saccade Task to Characterize Executive Functioning and Emotion Processing in Attention-Deficit Hyperactivity Disorder and Bipolar Disorder. Brain Cogn. 2018, 124, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.M.; Gerraty, R.T. A Meta-Analytic Investigation of Neurocognitive Deficits in Bipolar Illness: Profile and Effects of Clinical State. Neuropsychology 2009, 23, 551–562. [Google Scholar] [CrossRef]
- Mann-Wrobel, M.C.M.C.; Carreno, J.T.J.T.; Dickinson, D. Meta-Analysis of Neuropsychological Functioning in Euthymic Bipolar Disorder: An Update and Investigation of Moderator Variables. Bipolar Disord. 2011, 13, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Volkert, J.; Schiele, M.A.; Kazmaier, J.; Glaser, F.; Zierhut, K.C.; Kopf, J.; Kittel-Schneider, S.; Reif, A. Cognitive Deficits in Bipolar Disorder: From Acute Episode to Remission. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 225–237. [Google Scholar] [CrossRef]
- Cullen, B.; Ward, J.; Graham, N.A.; Deary, I.J.; Pell, J.P.; Smith, D.J.; Evans, J.J. Prevalence and Correlates of Cognitive Impairment in Euthymic Adults with Bipolar Disorder: A Systematic Review. J. Affect. Disord. 2016, 205, 165–181. [Google Scholar] [CrossRef]
- Cremaschi, L.; Penzo, B.; Palazzo, M.; Dobrea, C.; Cristoffanini, M.; Dell’Osso, B.; Altamura, A.C. Assessing Working Memory via N-Back Task in Euthymic Bipolar i Disorder Patients: A Review of Functional Magnetic Resonance Imaging Studies. Neuropsychobiology 2013, 68, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Plichta, M.M.; Schwarz, A.J.; Grimm, O.; Morgen, K.; Mier, D.; Haddad, L.; Gerdes, A.B.M.; Sauer, C.; Tost, H.; Esslinger, C.; et al. Test-Retest Reliability of Evoked BOLD Signals from a Cognitive-Emotive FMRI Test Battery. NeuroImage 2012, 60, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- Kopf, J.; Schecklmann, M.; Hahn, T.; Dresler, T.; Dieler, A.C.; Herrmann, M.J.; Fallgatter, A.J.; Reif, A. NOS1 Ex1f-VNTR Polymorphism Influences Prefrontal Brain Oxygenation during a Working Memory Task. NeuroImage 2011, 57, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Kopf, J.; Dresler, T.; Reicherts, P.; Herrmann, M.J.; Reif, A. The Effect of Emotional Content on Brain Activation and the Late Positive Potential in a Word N-Back Task. PLoS ONE 2013, 8, e75598. [Google Scholar] [CrossRef] [PubMed]
- Schecklmann, M.; Ehlis, A.C.; Plichta, M.M.; Dresler, T.; Heine, M.; Boreatti-Hümmer, A.; Romanos, M.; Jacob, C.; Pauli, P.; Fallgatter, A.J. Working Memory and Response Inhibition as One Integral Phenotype of Adult ADHD? A Behavioral and Imaging Correlational Investigation. J. Atten. Disord. 2013, 17, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, R.D.; Sagar, R.; Mehta, M. Neuropsychological Performance in Euthymic Indian Patients with Bipolar Disorder Type I: Correlation between Quality of Life and Global Functioning. Psychiatry Clin. Neurosci. 2012, 66, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lana, S.; Goikolea, J.M.; Bonnin, C.M.; Sarró, S.; Segura, B.; Amann, B.L.; Monté, G.C.; Moro, N.; Fernandez-Corcuera, P.; Maristany, T.; et al. Structural and Functional Brain Correlates of Cognitive Impairment in Euthymic Patients with Bipolar Disorder. PLoS ONE 2016, 11, e0158867. [Google Scholar] [CrossRef]
- Zhu, Y.; Quan, W.; Wang, H.; Ma, Y.; Yan, J.; Zhang, H.; Dong, W.; Yu, X. Prefrontal Activation during a Working Memory Task Differs between Patients with Unipolar and Bipolar Depression: A Preliminary Exploratory Study. J. Affect. Disord. 2018, 225, 64–70. [Google Scholar] [CrossRef]
- Fernández-Corcuera, P.; Salvador, R.; Monté, G.C.; Salvador Sarró, S.; Goikolea, J.M.; Amann, B.; Moro, N.; Sans-Sansa, B.; Ortiz-Gil, J.; Vieta, E.; et al. Bipolar Depressed Patients Show Both Failure to Activate and Failure to De-Activate during Performance of a Working Memory Task. J. Affect. Disord. 2013, 148, 170–178. [Google Scholar] [CrossRef]
- Pomarol-Clotet, E.; Alonso-Lana, S.; Moro, N.; Sarró, S.; Bonnin, M.C.; Goikolea, J.M.; Fernández-Corcuera, P.; Amann, B.L.; Romaguera, A.; Vieta, E.; et al. Brain Functional Changes across the Different Phases of Bipolar Disorder. Br. J. Psychiatry 2015, 206, 136–144. [Google Scholar] [CrossRef]
- McKenna, B.S.; Sutherland, A.N.; Legenkaya, A.P.; Eyler, L.T. Abnormalities of Brain Response during Encoding into Verbal Working Memory among Euthymic Patients with Bipolar Disorder. Bipolar Disord. 2015, 17, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Jogia, J.; Dima, D.; Kumari, V.; Frangou, S. Frontopolar Cortical Inefficiency May Underpin Reward and Working Memory Dysfunction in Bipolar Disorder. World J. Biol. Psychiatry 2012, 13, 605–615. [Google Scholar] [CrossRef]
- Frangou, S.; Kington, J.; Raymont, V.; Shergill, S.S. Examining Ventral and Dorsal Prefrontal Function in Bipolar Disorder: A Functional Magnetic Resonance Imaging Study. Eur. Psychiatry 2008, 23, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Lagopoulos, J.; Ivanovski, B.; Malhi, G.S. An Event-Related Functional MRI Study of Working Memory in Euthymic Bipolar: Discovery Service for Universitaetsbibliothek Johann Christian Senckenberg. J. Psychiatry Neurosci. 2007, 32, 174–184. [Google Scholar] [PubMed]
- Schecklmann, M.; Dresler, T.; Beck, S.; Jay, J.T.; Febres, R.; Haeusler, J.; Jarczok, T.A.; Reif, A.; Plichta, M.M.; Ehlis, A.C.; et al. Reduced Prefrontal Oxygenation during Object and Spatial Visual Working Memory in Unpolar and Bipolar Depression. Psychiatry Res. Neuroimaging 2011, 194, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.M.C.M.; Holland, S.K.S.K.; Schmithorst, V.; Tuchfarber, M.J.M.J.; Strakowski, S.M.S.M. Changes in Neuronal Activation in Patients with Bipolar Disorder during Performance of a Working Memory Task. Bipolar Disord. 2004, 6, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Monks, P.J.; Thompson, J.M.; Bullmore, E.T.; Suckling, J.; Brammer, M.J.; Williams, S.C.R.; Simmons, A.; Giles, N.; Lloyd, A.J.; Harrison, C.L.; et al. A Functional MRI Study of Working Memory Task in Euthymic Bipolar Disorder: Evidence for Task-Specific Dysfunction. Bipolar Disord. 2004, 6, 550–564. [Google Scholar] [CrossRef]
- Townsend, J.; Bookheimer, S.Y.; Foland-Ross, L.C.; Sugar, C.A.; Altshuler, L.L. FMRI Abnormalities in Dorsolateral Prefrontal Cortex during a Working Memory Task in Manic, Euthymic and Depressed Bipolar Subjects. Psychiatry Res. Neuroimaging 2010, 182, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Dell’osso, B.; Cinnante, C.; Di Giorgio, A.; Cremaschi, L.; Palazzo, M.C.; Cristoffanini, M.; Fazio, L.; Dobrea, C.; Avignone, S.; Triulzi, F.; et al. Altered Prefrontal Cortex Activity during Working Memory Task in Bipolar Disorder: A Functional Magnetic Resonance Imaging Study in Euthymic Bipolar I and II Patients. J. Affect. Disord. 2015, 184, 116–122. [Google Scholar] [CrossRef]
- Hamilton, L.S.; Altshuler, L.L.; Townsend, J.; Bookheimer, S.Y.; Phillips, O.R.; Fischer, J.; Woods, R.P.; Mazziotta, J.C.; Toga, A.W.; Nuechterlein, K.H.; et al. Alterations in Functional Activation in Euthymic Bipolar Disorder and Schizophrenia During a Working Memory Task Liberty. Hum. Brain Mapp. 2009, 30, 3958–3969. [Google Scholar] [CrossRef]
- Kopf, J.; Glöckner, S.; Schecklmann, M.; Dresler, T.; Plichta, M.M.; Veeh, J.; Kittel-Schneider, S.; Reif, A. Neural Correlates of Response Inhibition in Patients with Bipolar Disorder during Acute versus Remitted Phase. World J. Biol. Psychiatry 2018, 20, 637–646. [Google Scholar] [CrossRef]
- McGuffin, P.; Farmer, A.; Harvey, I. A Polydiagnostic Application of Operational Criteria in Studies of Psychotic Illness: Development and Reliability of the Opcrit System. Arch. Gen. Psychiatry 1991, 48, 764–770. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Asberg, M. A New Depression Scale Designed to Be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Lehrl, S.; Triebig, G.; Fischer, B. Multiple Choice Vocabulary Test MWT as a Valid and Short Test to Estimate Premorbid Intelligence. Acta Neurol. Scand. 1995, 91, 335–345. [Google Scholar] [CrossRef]
- Lecrubier, Y.; Sheehan, D.; Hergueta, T.; Weiller, E. The Mini International Neuropsychiatric Interview. Eur. Psychiatry 2002, 13, 198s. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-Back Working Memory Paradigm: A Meta-Analysis of Normative Functional Neuroimaging Studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y. Hemodynamic Signals in FNIRS, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 225, ISBN 9780444637048. [Google Scholar]
- Obrig, H.; Villringer, A. Beyond the Visible–Imaging the Human Brain with Light. J. Cereb. Blood Flow Metab. 2003, 23, 1–18. [Google Scholar] [CrossRef]
- Jasper, H.H. The Ten-Twenty Electrode System of the International Federation. Electroenceph. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Logothetis, N.K.; Wandell, B.A. Interpreting the BOLD Signal. Annu. Rev. Physiol. 2004, 66, 735–769. [Google Scholar] [CrossRef]
- Cui, X.; Bray, S.; Reiss, A.L. Functional Near Infrared Spectroscopy (NIRS) Signal Improvement Based on Negative Correlation between Oxygenated and Deoxygenated Hemoglobin Dynamics. Neuroimage 2010, 49, 3039–3046. [Google Scholar] [CrossRef]
- Tsuzuki, D.; Jurcak, V.; Singh, A.K.; Okamoto, M.; Watanabe, E.; Dan, I. Virtual Spatial Registration of Stand-Alone FNIRS Data to MNI Space. NeuroImage 2007, 34, 1506–1518. [Google Scholar] [CrossRef]
- Strakowski, S.M.; DelBello, M.P.; Adler, C.M. The Functional Neuroanatomy of Bipolar Disorder: A Review of Neuroimaging Findings. Mol. Psychiatry 2005, 10, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Strakowski, S.M.; Adler, C.M.; Almeida, J.; Altshuler, L.L.; Blumberg, H.P.; Chang, K.D.; Delbello, M.P.; Frangou, S.; McIntosh, A.; Phillips, M.L.; et al. The Functional Neuroanatomy of Bipolar Disorder: A Consensus Model. Bipolar Disord. 2012, 14, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Hafeman, D.M.; Chang, K.D.; Garrett, A.S.; Sanders, E.M.; Phillips, M.L. Effects of Medication on Neuroimaging Findings in Bipolar Disorder: An Updated Review. Bipolar Disord. 2012, 14, 375–410. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, C.; Bhardwaj, A.; Sharma, M.; Kumar, S. Cognitive Deficits in Euthymic Patients with Bipolar Disorder: State or Trait Marker? J. Nerv. Ment. Dis. 2019, 207, 100–105. [Google Scholar] [CrossRef]
| Healthy Controls (n = 30) | Acute BD (n = 32) | Remitted/A BD (n = 15) | Remitted/R BD (n = 15) | |
|---|---|---|---|---|
| Sex ♂/♀ (n) | 10/20 | 19/17 | 10/5 | 10/5 |
| Age (years) | 42.3 ± 10.7 | 42 ± 11.2 | 36 ± 11 | 36 ± 11 |
| MADRS (score) | - | 14.6 ± 10.3 | 18.2 ± 7.9 | 3.5 ± 1.5 |
| YMRS (score) | - | 3.4 ± 3.7 | 4 ± 3.5 | 1.1 ± 1.3 |
| Working Memory | ||||
| One-Back (RT in ms) | 485.81 ± 78.6 | 603.5 ± 146.3 | 661.89 ± 172.6 | 523.08 ± 103.2 |
| Two-Back (RT in ms) | 611.17 ± 119.9 | 782 ± 218.9 | 833.89 ± 264.5 | 702.48 ± 176.8 |
| Three-Back (RT in ms) | 665.91 ± 142 | 790.63 ± 237.5 | 896.88 ± 203.7 | 779.76 ± 200.1 |
| Commission errors (%) | 4.4 ± 3.5 | 5.5 ± 4.4 | 4.8 ± 4.1 | 3.2 ± 1.9 |
| Omission errors (%) | 6 ± 3.1 | 9.3 ± 4.7 | 8.4 ± 4.6 | 6.4 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopf, J.; Glöckner, S.; Althen, H.; Cevada, T.; Schecklmann, M.; Dresler, T.; Kittel-Schneider, S.; Reif, A. Neural Responses to a Working Memory Task in Acute Depressed and Remitted Phases in Bipolar Patients. Brain Sci. 2023, 13, 744. https://doi.org/10.3390/brainsci13050744
Kopf J, Glöckner S, Althen H, Cevada T, Schecklmann M, Dresler T, Kittel-Schneider S, Reif A. Neural Responses to a Working Memory Task in Acute Depressed and Remitted Phases in Bipolar Patients. Brain Sciences. 2023; 13(5):744. https://doi.org/10.3390/brainsci13050744
Chicago/Turabian StyleKopf, Juliane, Stefan Glöckner, Heike Althen, Thais Cevada, Martin Schecklmann, Thomas Dresler, Sarah Kittel-Schneider, and Andreas Reif. 2023. "Neural Responses to a Working Memory Task in Acute Depressed and Remitted Phases in Bipolar Patients" Brain Sciences 13, no. 5: 744. https://doi.org/10.3390/brainsci13050744
APA StyleKopf, J., Glöckner, S., Althen, H., Cevada, T., Schecklmann, M., Dresler, T., Kittel-Schneider, S., & Reif, A. (2023). Neural Responses to a Working Memory Task in Acute Depressed and Remitted Phases in Bipolar Patients. Brain Sciences, 13(5), 744. https://doi.org/10.3390/brainsci13050744







