Efficacy of Functional Remediation on Cognitive and Psychosocial Functioning in Patients with Bipolar Disorder: Study Protocol for a Randomized Controlled Study
Abstract
1. Background
Trial Aims and Objectives
2. Methods
2.1. Trial Governance
2.2. Trial Design and Setting
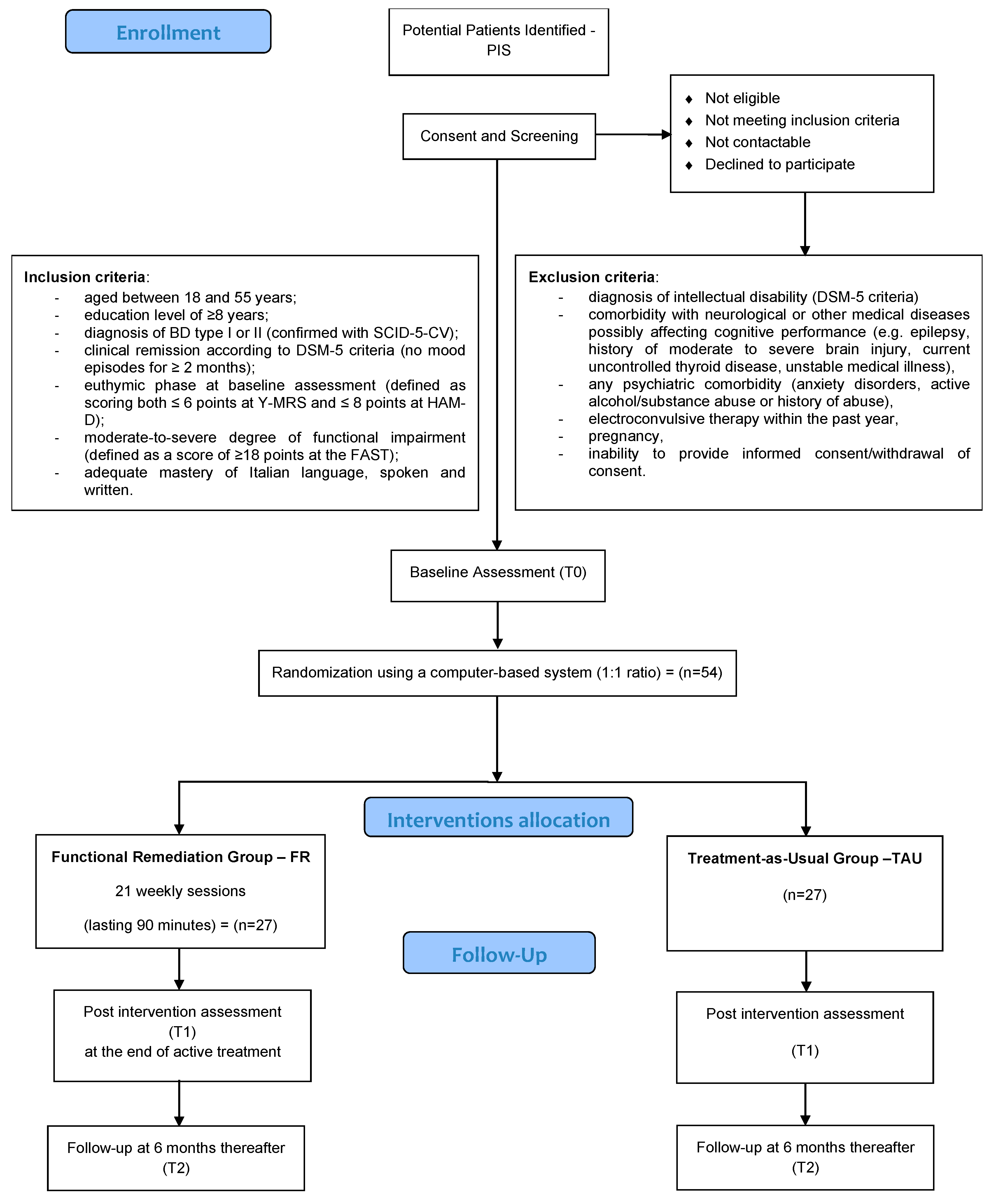
2.3. Participants
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Procedures
2.4.1. Screening and Randomization Phase
2.4.2. Allocation and Blinding
2.4.3. Intervention Phase
Functional Remediation
2.5. Treatment as Usual
2.6. Assessment Phase
2.7. Outcome Measures
3. Measurement
Statistical Analyses
Power—Sample Size Calculation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Carvalho, A.F.; Firth, J.; Vieta, E.; Ropper, A.H. Bipolar Disorder. N. Engl. J. Med. 2020, 383, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Vieta, E.; Berk, M.; Schulze, T.G.; Carvalho, A.F.; Suppes, T.; Calabrese, J.R.; Gao, K.; Miskowiak, K.W.; Grande, I. Bipolar disorders. Nat. Rev. Dis. Prim. 2018, 4, 18008. [Google Scholar] [CrossRef] [PubMed]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Disability 2011. Available online: http://www.who.int/about/ (accessed on 20 February 2023).
- Merikangas, K.R.; Jin, R.; He, J.-P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch. Gen. Psychiatry 2011, 68, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Bourne, C.; Aydemir, O.; Balanzá-Martínez, V.; Bora, E.; Brissos, S.; Cavanagh, J.T.O.; Clark, L.; Cubukcuoglu, Z.; Dias, V.V.; Dittmann, S.; et al. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: An individual patient data meta-analysis. Acta Psychiatr. Scand. 2013, 128, 149–162. [Google Scholar] [CrossRef]
- Torres, I.J.; Boudreau, V.G.; Yatham, L.N. Neuropsychological functioning in euthymic bipolar disorder: A meta-analysis. Acta Psychiatr. Scand. 2007, 116, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Burdick, K.E.; Ketter, T.A.; Goldberg, J.F.; Calabrese, J.R. Assessing cognitive function in bipolar disorder: Challenges and recommendations for clinical trial design. J. Clin. Psychiatry 2015, 76, e342–e350. [Google Scholar] [CrossRef]
- Rosa, A.R.; Bonnín, C.M.; Vázquez, G.H.; Reinares, M.; Solé, B.; Tabarés-Seisdedos, R.; Balanzá-Martínez, V.; González-Pinto, A.; Sánchez-Moreno, J.; Vieta, E. Functional impairment in bipolar II disorder: Is it as disabling as bipolar I? J. Affect. Disord. 2010, 127, 71–76. [Google Scholar] [CrossRef]
- Martínez-Arán, A.; Vieta, E.; Colom, F.; Torrent, C.; Sánchez-Moreno, J.; Reinares, M.; Benabarre, A.; Goikolea, J.M.; Brugué, E.; Daban, C.; et al. Cognitive impairment in euthymic bipolar patients: Implications for clinical and functional outcome. Bipolar Disord. 2004, 6, 224–232. [Google Scholar] [CrossRef]
- Demmo, C.; Lagerberg, T.V.; Kvitland, L.R.; Aminoff, S.R.; Hellvin, T.; Simonsen, C.; Haatveit, B.; Andreassen, O.A.; Melle, I.; Ueland, T. Neurocognitive functioning, clinical course and functional outcome in first-treatment bipolar I disorder patients with and without clinical relapse: A 1-year follow-up study. Bipolar Disord. 2018, 20, 228–237. [Google Scholar] [CrossRef]
- Bonnin, C.M.; Torrent, C.; Arango, C.; Amann, B.L.; Solé, B.; González-Pinto, A.; Crespo, J.M.; Tabarés-Seisdedos, R.; Reinares, M.; Ayuso-Mateos, J.L.; et al. Functional remediation in bipolar disorder: 1-year follow-up of neurocognitive and functional outcome. Br. J. Psychiatry 2016, 208, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bonnín, C.D.M.; Reinares, M.; Martínez-Arán, A.; Jiménez, E.; Sánchez-Moreno, J.; Solé, B.; Montejo, L.; Vieta, E. Improving functioning, quality of life, and well-being in patients with bipolar disorder. Int. J. Neuropsychopharmacol. 2019, 22, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, J.; Bonnin, C.M.; González-Pinto, A.; Amann, B.L.; Solé, B.; Balanzá-Martinez, V.; Arango, C.; Jiménez, E.; Tabarés-Seisdedos, R.; Garcia-Portilla, M.P.; et al. Factors associated with poor functional outcome in bipolar disorder: Sociodemographic, clinical, and neurocognitive variables. Acta Psychiatr. Scand. 2018, 138, 145–154. [Google Scholar] [CrossRef]
- Vieta, E.; Torrent, C. Functional Remediation: The Pathway from Remission to Recovery in Bipolar Disorder. World Psychiatry 2016, 15, 288–289. [Google Scholar] [CrossRef]
- Bora, E.; Hıdıroğlu, C.; Özerdem, A.; Kaçar, F.; Sarısoy, G.; Arslan, F.C.; Aydemir, Ö.; Tas, Z.C.; Vahip, S.; Atalay, A.; et al. Executive dysfunction and cognitive subgroups in a large sample of euthymic patients with bipolar disorder. Eur. Neuropsychopharmacol. 2016, 26, 1338–1347. [Google Scholar] [CrossRef]
- Jensen, J.H.; Knorr, U.; Vinberg, M.; Kessing, L.V.; Miskowiak, K.W. Discrete neurocognitive subgroups in fully or partially remitted bipolar disorder: Associations with functional abilities. J. Affect. Disord. 2016, 205, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.; Rabelo-Da-Ponte, F.D.; Bücker, J.; Czepielewski, L.; Hasse-Sousa, M.; Telesca, R.; Solé, B.; Reinares, M.; Vieta, E.; Rosa, A.R. Identifying cognitive subgroups in bipolar disorder: A cluster analysis. J. Affect. Disord. 2019, 246, 252–261. [Google Scholar] [CrossRef]
- Cotrena, C.; Branco, L.D.; Ponsoni, A.; Shansis, F.M.; Fonseca, R.P. Neuropsychological Clustering in Bipolar and Major Depressive Disorder. J. Int. Neuropsychol. Soc. 2017, 23, 584–593. [Google Scholar] [CrossRef]
- Lee, J.; Rizzo, S.; Altshuler, L.; Glahn, D.C.; Miklowitz, D.J.; Sugar, C.A.; Wynn, J.; Green, M.F. Deconstructing Bipolar Disorder and Schizophrenia: A cross-diagnostic cluster analysis of cognitive phenotypes. J. Affect. Disord. 2017, 209, 71–79. [Google Scholar] [CrossRef]
- Roux, P.; Raust, A.; Cannavo, A.S.; Aubin, V.; Aouizerate, B.; Azorin, J.-M.; Bellivier, F.; Belzeaux, R.; Bougerol, T.; Cussac, I.; et al. Cognitive profiles in euthymic patients with bipolar disorders: Results from the FACE-BD cohort. Bipolar Disord. 2017, 19, 146–153. [Google Scholar] [CrossRef]
- Russo, M.; Van Rheenen, T.E.; Shanahan, M.; Mahon, K.; Perez-Rodriguez, M.M.; Cuesta-Diaz, A.; Larsen, E.; Malhotra, A.K.; Burdick, K.E. Neurocognitive subtypes in patients with bipolar disorder and their unaffected siblings. Psychol. Med. 2017, 47, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.J.; Strejilevich, S.A.; Fassi, G.; Marengo, E.; Igoa, A. Theory of mind and facial emotion recognition in euthymic bipolar I and bipolar II disorders. Psychiatry Res. 2011, 189, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef] [PubMed]
- Murru, A.; Pacchiarotti, I.; Amann, B.; Nivoli, A.M.A.; Vieta, E.; Colom, F. Treatment adherence in bipolar i and schizoaffective disorder, bipolar type. J. Affect. Disord. 2013, 151, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Reinares, M.; Sánchez-Moreno, J.; Fountoulakis, K.N. Psychosocial interventions in bipolar disorder: What, for whom, and when. J. Affect. Disord. 2014, 156, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.; Barlati, S.; Ceraso, A.; Nibbio, G.; Ariu, C.; Deste, G.; Wykes, T. Effectiveness, Core Elements, and Moderators of Response of Cognitive Remediation for Schizophrenia: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. JAMA Psychiatry 2021, 78, 848–858. [Google Scholar] [CrossRef]
- Lejeune, J.A.; Northrop, A.; Kurtz, M.M. A Meta-Analysis of Cognitive Remediation for Schizophrenia: Efficacy and the Role of Participant and Treatment Factors. Schizophr. Bull. 2021, 47, 997–1006. [Google Scholar] [CrossRef]
- Yeo, H.; Yoon, S.; Lee, J.; Kurtz, M.M.; Choi, K. A Meta-Analysis of the Effects of Social-Cognitive Training in Schizophrenia: The Role of Treatment Characteristics and Study Quality. Br. J. Clin. Psychol. 2022, 61, 37–57. [Google Scholar] [CrossRef]
- Vita, A.; Gaebel, W.; Mucci, A.; Sachs, G.; Barlati, S.; Giordano, G.M.; Nibbio, G.; Nordentoft, M.; Wykes, T.; Galderisi, S. EPA Guidance on Treatment of Cognitive Impairment in Schizophrenia. Eur. Psychiatry 2022, 65, e57. [Google Scholar] [CrossRef]
- Van Rheenen, T.E.; Lewandowski, K.E.; Tan, E.J.; Ospina, L.H.; Ongur, D.; Neill, E.; Gurvich, C.; Pantelis, C.; Malhotra, A.K.; Rossell, S.L.; et al. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol. Med. 2017, 47, 1848–1864. [Google Scholar] [CrossRef]
- Bora, E.; Yucel, M.; Pantelis, C. Cognitive impairment in schizophrenia and affective psychoses: Implications for dsm-v criteria and beyond. Schizophr. Bulletin. 2010, 36, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Murray, R.M.; MacCabe, J.H. Do premorbid and post-onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta-Analysis. Psychol. Med. 2015, 45, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Durá, I.; Balanzá-Martínez, V.; Ruiz-Ruiz, J.C.; Martínez-Arán, A.; Girón, M.; Solé, B.; Sánchez-Moreno, J.; Gómez-Beneyto, M.; Vieta, E.; Tabarés-Seisdedos, R. Neurocognitive Training in Patients with Bipolar Disorders: Current Status and Perspectives. Psychother. Psychosom. 2012, 81, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.M.; Peckham, A.; Porter, R.; Hammar, A. Cognitive enhancement therapy for mood disorders: A new paradigm? Aust. New Zealand J. Psychiatry 2019, 53, 1148–1150. [Google Scholar] [CrossRef]
- Burdick, K.E.; Lewandowski, K.E.; van Rheenen, T.E. Entering the debate: Cognitive enhancement therapy for mood disorders: A new paradigm? Bipolar Disord. 2020, 22, 305–306. [Google Scholar] [CrossRef]
- Tsapekos, D.; Seccomandi, B.; Mantingh, T.; Cella, M.; Wykes, T.; Young, A. Cognitive enhancement interventions for people with bipolar disorder: A systematic review of methodological quality, treatment approaches, and outcomes. Bipolar Disord. 2020, 22, 216–230. [Google Scholar] [CrossRef]
- Miskowiak, K.; Burdick, K.; Martinez-Aran, A.; Bonnin, C.; Bowie, C.; Carvalho, A.; Gallagher, P.; Lafer, B.; López-Jaramillo, C.; Sumiyoshi, T.; et al. Methodological recommendations for cognition trials in bipolar disorder by the International Society for Bipolar Disorders Targeting Cognition Task Force. Bipolar Disord. 2017, 19, 614–626. [Google Scholar] [CrossRef]
- Torrent, C.; Bonnin, C.D.M.; Martínez-Arán, A.; Valle, J.; Amann, B.L.; González-Pinto, A.; Crespo, J.M.; Ibáñez, Á.; Garcia-Portilla, M.P.; Tabarés-Seisdedos, R.; et al. Efficacy of Functional Remediation in Bipolar Disorder: A Multicenter Randomized Controlled Study. Am. J. Psychiatry 2013, 170, 852–859. [Google Scholar] [CrossRef]
- Bernabei, L.; Bersani, F.S.; Pompili, E.; Chiaie, R.D.; Valente, D.; Corrado, A.; Vergnani, L.; Ferracuti, S.; Biondi, M.; De’Fornari, M.A.C. Cognitive remediation for the treatment of neuropsychological disturbances in subjects with euthymic bipolar disorder: Findings from a controlled study. J. Affect. Disord. 2020, 273, 576–585. [Google Scholar] [CrossRef]
- Strawbridge, R.; Tsapekos, D.; Hodsoll, J.; Mantingh, T.; Yalin, N.; McCrone, P.; Boadu, J.; Macritchie, K.; Cella, M.; Reeder, C.; et al. Cognitive remediation therapy for patients with bipolar disorder: A randomised proof-of-concept trial. Bipolar Disord. 2021, 23, 196–208. [Google Scholar] [CrossRef]
- Gomes, B.C.; Rocca, C.C.; Belizario, G.O.; Fernandes, F.d.B.F.; Valois, I.; Olmo, G.C.; Fachin, R.V.P.; Farhat, L.C.; Lafer, B. Cognitive behavioral rehabilitation for bipolar disorder patients: A randomized controlled trial. Bipolar Disord. 2019, 21, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Veeh, J.; Kopf, J.; Kittel-Schneider, S.; Deckert, J.; Reif, A. Cognitive remediation for bipolar patients with objective cognitive impairment: A naturalistic study. Int. J. Bipolar Disord. 2017, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Zyto, S.; Jabben, N.; Schulte, P.F.; Regeer, B.J.; Kupka, R.W. A pilot study of a combined group and individual functional remediation program for patients with bipolar i disorder. J. Affect. Disord. 2016, 194, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.E.; Sperry, S.H.; Cohen, B.M.; Norris, L.A.; Fitzmaurice, G.M.; Ongur, D.; Keshavan, M.S. Treatment to enhance cognition in bipolar disorder (TREC-BD): Efficacy of a randomized controlled trial of cognitive remediation versus active control. J. Clin. Psychiatry 2017, 78, 3199. [Google Scholar] [CrossRef]
- Deckersbach, T.; Nierenberg, A.A.; Kessler, R.; Lund, H.G.; Ametrano, R.M.; Sachs, G.; Rauch, S.L.; Dougherty, D. Cognitive rehabilitation for bipolar disorder: An open trial for employed patients with residual depressive symptoms. CNS Neurosci. Ther. 2010, 16, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arán, A.; Torrent, C.; Solé, B.; Mar Bonnín, C.; Rosa, A.R.; Sánchez-Moreno, J.; Vieta, E. Functional Remediation for Bipolar Disorder. Clin. Pract. Epidemiol. Ment. Health 2011, 7, 112–116. [Google Scholar] [CrossRef]
- Solé, B.; Bonnin, C.M.; Mayoral, M.; Amann, B.L.; Torres, I.; González-Pinto, A.; Jimenez, E.; Crespo, J.M.; Colom, F.; Tabarés-Seisdedos, R.; et al. Functional remediation for patients with bipolar II disorder: Improvement of functioning and subsyndromal symptoms. Eur. Neuropsychopharmacol. 2015, 25, 257–264. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA-J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- National Collaborating Centre for Mental Health. Bipolar Disorder the Nice Guideline on the Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care Updated Edition. Available online: www.nice.org.uk (accessed on 20 February 2023).
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Available online: www.annals.org (accessed on 20 February 2023).
- First, M.B.; Williams, J.B. SCID-5-CV Starter Kit. In Intervista Clinica Strutturata per i Disturbi del DSM-5—Versione per il Clinico; Fos-Sati, A., Borroni, S., Eds.; Raffaello Cortina: Milano, Italy, 2017. [Google Scholar]
- American Psychiatric Association (APA). DSM-5. Manuale Diagnostico e Statistico dei Disturbi Mentali, tr. it; Raffaello Cortina: Milano, Italy, 2014. [Google Scholar]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Brit. J. Psychiat. 2022. Available online: https://www.cambridge.org/core (accessed on 20 February 2023). [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiat. 1960, 23, 56. [Google Scholar] [CrossRef]
- Barbato, A.; Bossini, L.; Calugi, S.; D’Avanzo, B.; Fagiolini, A.; Koukouna, D.; Parabiaghi, A.; Rapisarda, F.; Rucci, P.; Vallarino, M. Validation of the Italian version of the Functioning Assessment Short Test (FAST) for bipolar disorder. Epidemiol. Psychiatr. Sci. 2013, 22, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Vieta, E.; Torrent, C.; Martínez-Arán, A. Functional Remediation for Bipolar Disorder; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Bang, H.; Flaherty, S.P.; Kolahi, J.; Park, J. Blinding assessment in clinical trials: A review of statistical methods and a proposal of blinding assessment protocol. Clin. Res. Regul. Aff. 2010, 27, 42–51. [Google Scholar] [CrossRef]
- Solé, B.; Bonnín, C.M.; Radua, J.; Montejo, L.; Hogg, B.; Jimenez, E.; Reinares, M.; Valls, E.; Varo, C.; Pacchiarotti, I.; et al. Long-term outcome predictors after functional remediation in patients with bipolar disorder. Psychol. Med. 2022, 52, 314–322. [Google Scholar] [CrossRef]
- Tsapekos, D.; Strawbridge, R.; Cella, M.; Young, A.H.; Wykes, T. Does cognitive improvement translate into functional changes? Exploring the transfer mechanisms of cognitive remediation therapy for euthymic people with bipolar disorder. Psychol. Med. 2021, 53, 936–944. [Google Scholar] [CrossRef]
- Rossetti, M.G.; Bonivento, C.; Garzitto, M.; Caletti, E.; Perlini, C.; Piccin, S.; Lazzaretti, M.; Marinelli, V.; Sala, M.; Abbiati, V.; et al. The brief assessment of cognition in affective disorders: Normative data for the Italian population. J. Affect. Disord. 2019, 252, 245–252. [Google Scholar] [CrossRef]
- Keefe, R.S.; Goldberg, T.E.; Harvey, P.D.; Gold, J.M.; Poe, M.P.; Coughenour, L. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004, 68, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Keefe, R.S.; Fox, K.H.; Davis, V.G.; Kennel, C.; Walker, T.M.; Burdick, K.E.; Harvey, P.D. The Brief Assessment of Cognition in Affective Disorders (BAC-A):Performance of patients with bipolar depression and healthy controls. J. Affect. Disord. 2014, 166, 86–92. [Google Scholar] [CrossRef]
- Leucht, S.; Samara, M.; Heres, S.; Davis, J.M. Dose Equivalents for Antipsychotic Drugs: The DDD Method. Schizophr. Bull. 2016, 42, S90–S94. [Google Scholar] [CrossRef]
- Erdfelder, E.; Buchner, A. GPOWER: A general power analysis program. Behav. Res. Methods Instrum. Comput. 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Varo, C.; Jimenez, E.; Solé, B.; Bonnín, C.; Torrent, C.; Valls, E.; Morilla, I.; Lahera, G.; Martínez-Arán, A.; Vieta, E.; et al. Social cognition in bipolar disorder: Focus on emotional intelligence. J. Affect. Disord. 2017, 217, 210–217. [Google Scholar] [CrossRef]
- Vita, A.; Barlati, S.; Ceraso, A.; Deste, G.; Nibbio, G.; Wykes, T. Acceptability of Cognitive Remediation for Schizophrenia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychol. Med. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nibbio, G.; Barlati, S.; Cacciani, P.; Corsini, P.; Mosca, A.; Ceraso, A.; Deste, G.; Vita, A. Evidence-Based Integrated Intervention in Patients with Schizophrenia: A Pilot Study of Feasibility and Effectiveness in a Real-World Rehabilitation Setting. Int. J. Environ. Res. Public Health 2020, 17, 3352. [Google Scholar] [CrossRef] [PubMed]
- Montemagni, C.; Del Favero, E.; Riccardi, C.; Canta, L.; Toye, M.; Zanalda, E.; Rocca, P. Effects of Cognitive Remediation on Cognition, Metacognition, and Social Cognition in Patients with Schizophrenia. Front. Psychiatry 2021, 12, 649737. [Google Scholar] [CrossRef]
- Magyari, M.; Sorensen, P.S. Comorbidity in Multiple Sclerosis. Front. Neurol. 2020, 11, 851. [Google Scholar] [CrossRef]
- Kosmidis, M.H.; Bozikas, V.P.; Giannouli, V.; Karavatos, A.; Fokas, K. Familial Comorbidity of Bipolar Disorder and Multiple Sclerosis: Genetic Susceptibility, Coexistence or Causal Relationship? Behav. Neurol. 2012, 25, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Cohen, J.; Stuve, O.; Trojano, M.; Sørensen, P.S.; Reingold, S.; Cutter, G.; Reider, N. A Systematic Review of the Incidence and Prevalence of Comorbidity in Multiple Sclerosis: Overview. Mult. Scler. 2015, 21, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Salagre, E.; Dodd, S.; Aedo, A.; Rosa, A.; Amoretti, S.; Pinzón-Espinosa, J.; Reinares, M.; Berk, M.; Kapczinski, F.P.; Vieta, E.; et al. Toward Precision Psychiatry in Bipolar Disorder: Staging 2.0. Front. Psychiatry 2018, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Verdolini, N.; Vieta, E. Resilience, prevention and positive psychiatry. Acta Psychiatr. Scand. 2021, 143, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, R.; Miskowiak, K.W.; Hasler, G. Evaluating endophenotypes for bipolar disorder. Int. J. Bipolar Disord. 2021, 9, 17. [Google Scholar] [CrossRef]
- Ching, C.R.K.; Hibar, D.P.; Gurholt, T.P.; Nunes, A.; Thomopoulos, S.I.; Abé, C.; Agartz, I.; Brouwer, R.M.; Cannon, D.M.; Zwarte, S.M.C.; et al. What we learn about bipolar disorder from large-scale neuroimaging: Findings and future directions from the ENIGMA Bipolar Disorder Working Group. Hum. Brain Mapp. 2020, 43, 56–82. [Google Scholar] [CrossRef]
- Toma, S.; MacIntosh, B.J.; Swardfager, W.; Goldstein, B.I. Cerebral blood flow in bipolar disorder: A systematic review. J. Affect. Disord. 2018, 241, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Hibar, D.P.; for the ENIGMA Bipolar Disorder Working Group; Westlye, L.T.; Doan, N.T.; Jahanshad, N.; Cheung, J.W.; Ching, C.R.K.; Versace, A.; Bilderbeck, A.C.; Uhlmann, A.; et al. Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol. Psychiatry 2018, 23, 932–942. [Google Scholar] [CrossRef]
- Duan, S.; Zghoul, T.; Wang, Y.; Chen, R. Longitudinal cognitive performance in patients with Bipolar Disorder. Bipolar Disord. 2020, 22, 303–304. [Google Scholar] [CrossRef]
- Szmulewicz, A.; Valerio, M.P.; Martino, D.J. Longitudinal analysis of cognitive performances in recent-onset and late-life Bipolar Disorder: A systematic review and meta-analysis. Bipolar Disord. 2020, 22, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Montejo, L.; Torrent, C.; Jiménez, E.; Martínez-Arán, A.; Blumberg, H.P.; Burdick, K.E.; Chen, P.; Dols, A.; Eyler, L.T.; Forester, B.P.; et al. Cognition in older adults with bipolar disorder: An ISBD task force systematic review and meta-analysis based on a comprehensive neuropsychological assessment. Bipolar Disord. 2022, 24, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Kucyi, A.; Alsuwaidan, M.T.; Liauw, S.S.; McIntyre, R.S. Aerobic Physical Exercise as a Possible Treatment for Neurocognitive Dysfunction in Bipolar Disorder. Postgrad. Med. 2010, 122, 107–116. [Google Scholar] [CrossRef]
- Melo, M.C.A.; Daher, E.D.F.; Albuquerque, S.G.C.; de Bruin, V.M.S. Exercise in Bipolar Patients: A Systematic Review. J. Affect. Disord. 2016, 198, 32–38. [Google Scholar] [CrossRef]
- Souza de Sa Filho, A.; Marcos de Souza Moura, A.; Khede Lamego, M.; Barbosa Ferreira Rocha, N.; Paes, F.; Cristina Oliveira, A.; Lattari, E.; Rimes, R.; Manochio, J.; Budde, H.; et al. Potential Therapeutic Effects of Physical Exercise for Bipolar Disorder. CNS Neurol. Disord.-Drug Targets 2015, 14, 1255–1259. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Liu, H.-C.; Cheng, Y.-C.; Li, H.; Huang, C.-C.; Ding, Y.-W.; Huang, M.-C.; Chiu, C.-C.; Tu, Y.-K.; Kuo, P.-H. Effect of Pharmacological and Neurostimulation Interventions for Cognitive Domains in Patients with Bipolar Disorder: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Clin. Epidemiol. 2021, 13, 1039–1049. [Google Scholar] [CrossRef]
- Dai, Y.; Ding, H.; Lu, X.; Wu, X.; Xu, C.; Jiang, T.; Ming, L.; Xia, Z.; Song, C.; Shen, H.; et al. CCRT and Aerobic Exercise: A Randomised Controlled Study of Processing Speed, Cognitive Flexibility, and Serum BDNF Expression in Schizophrenia. Schizophrenia 2022, 8, 84. [Google Scholar] [CrossRef]
- Deste, G.; Corbo, D.; Nibbio, G.; Italia, M.; Dell’Ovo, D.; Calzavara-Pinton, I.; Lisoni, J.; Barlati, S.; Gasparotti, R.; Vita, A. Impact of Physical Exercise Alone or in Combination with Cognitive Remediation on Cognitive Functions in People with Schizophrenia: A Qualitative Critical Review. Brain Sci. 2023, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Nuechterlein, K.H.; McEwen, S.C.; Ventura, J.; Subotnik, K.L.; Turner, L.R.; Boucher, M.; Casaus, L.R.; Distler, M.G.; Hayata, J.N. Aerobic Exercise Enhances Cognitive Training Effects in First-Episode Schizophrenia: Randomized Clinical Trial Demonstrates Cognitive and Functional Gains. Psychol. Med. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accardo, V.; Barlati, S.; Ceraso, A.; Nibbio, G.; Vieta, E.; Vita, A. Efficacy of Functional Remediation on Cognitive and Psychosocial Functioning in Patients with Bipolar Disorder: Study Protocol for a Randomized Controlled Study. Brain Sci. 2023, 13, 708. https://doi.org/10.3390/brainsci13050708
Accardo V, Barlati S, Ceraso A, Nibbio G, Vieta E, Vita A. Efficacy of Functional Remediation on Cognitive and Psychosocial Functioning in Patients with Bipolar Disorder: Study Protocol for a Randomized Controlled Study. Brain Sciences. 2023; 13(5):708. https://doi.org/10.3390/brainsci13050708
Chicago/Turabian StyleAccardo, Vivian, Stefano Barlati, Anna Ceraso, Gabriele Nibbio, Eduard Vieta, and Antonio Vita. 2023. "Efficacy of Functional Remediation on Cognitive and Psychosocial Functioning in Patients with Bipolar Disorder: Study Protocol for a Randomized Controlled Study" Brain Sciences 13, no. 5: 708. https://doi.org/10.3390/brainsci13050708
APA StyleAccardo, V., Barlati, S., Ceraso, A., Nibbio, G., Vieta, E., & Vita, A. (2023). Efficacy of Functional Remediation on Cognitive and Psychosocial Functioning in Patients with Bipolar Disorder: Study Protocol for a Randomized Controlled Study. Brain Sciences, 13(5), 708. https://doi.org/10.3390/brainsci13050708







