Gait Recovery in Spinal Cord Injury: A Systematic Review with Metanalysis Involving New Rehabilitative Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Data Extraction and Criteria Appraisal
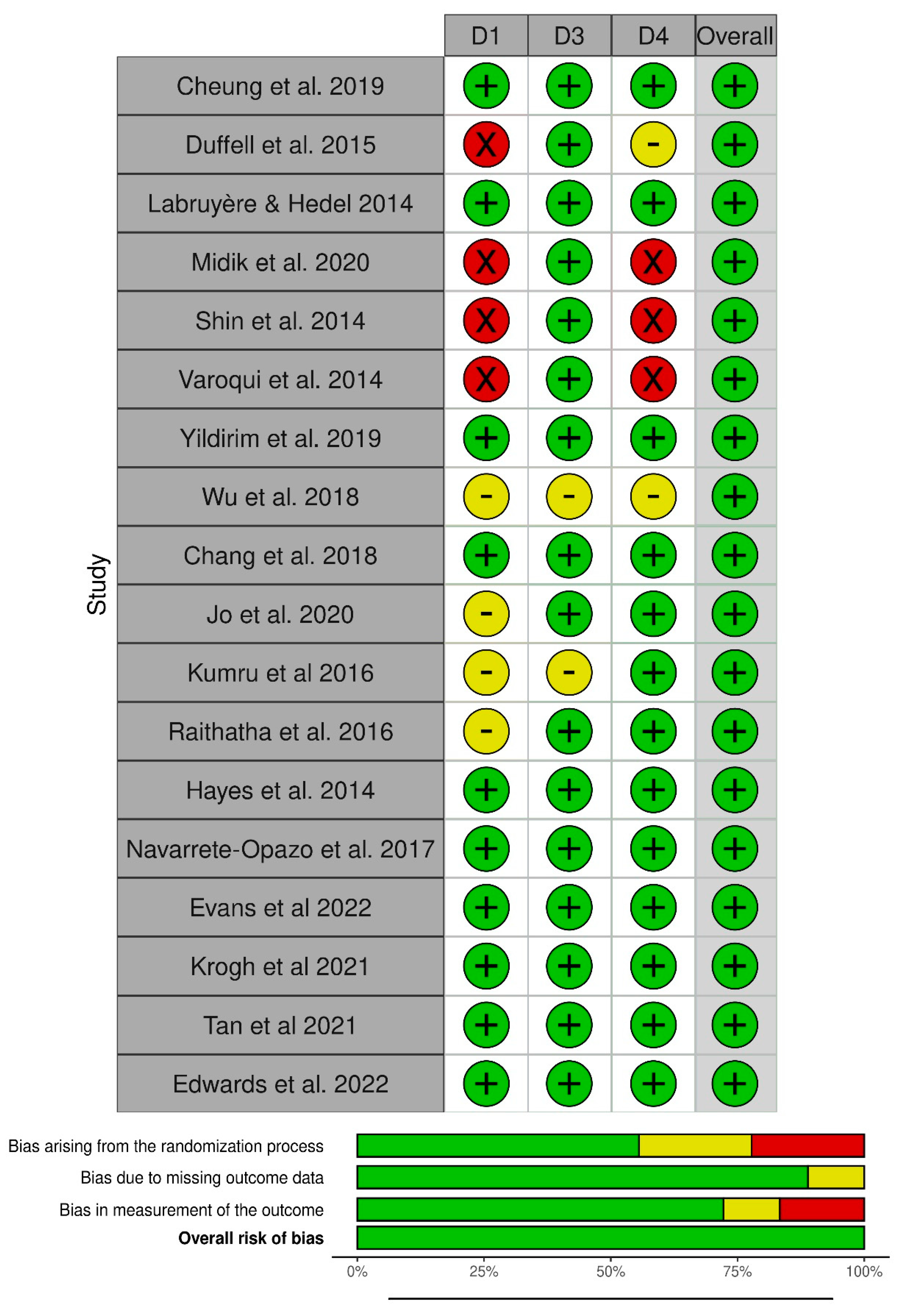
2.4. Risk of Bias
2.5. Data Analysis and Heterogeneity of the Studies
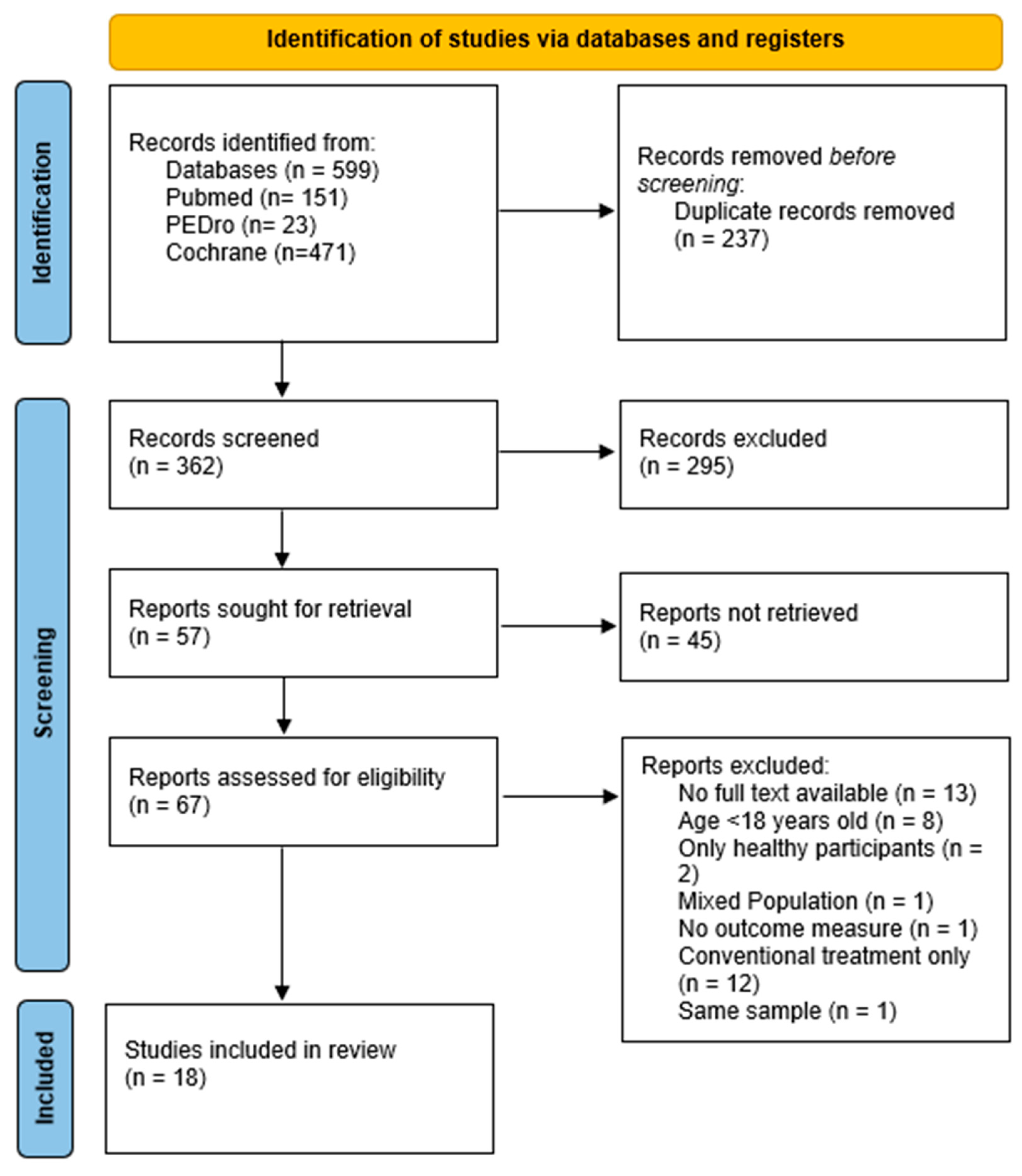
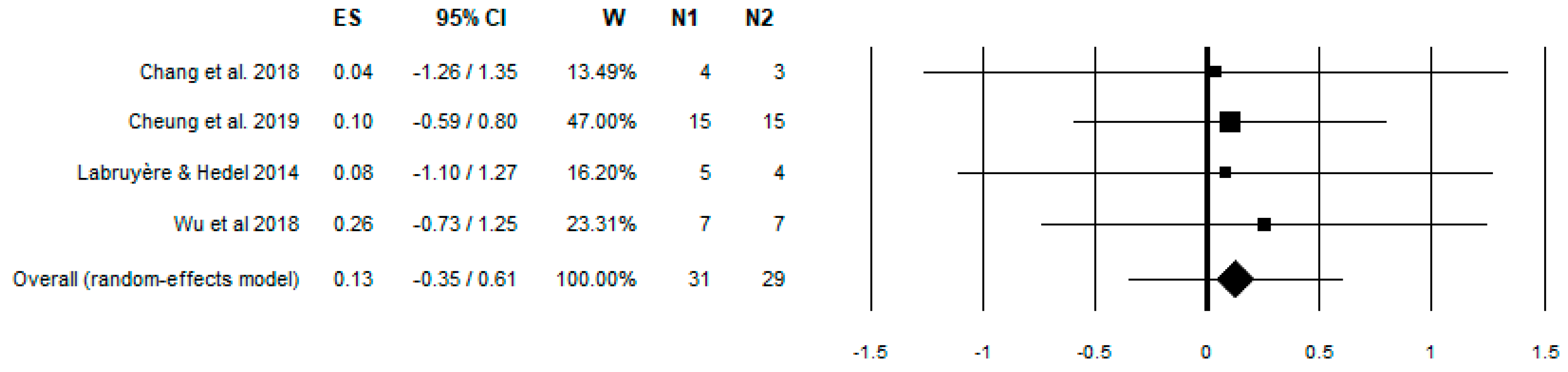
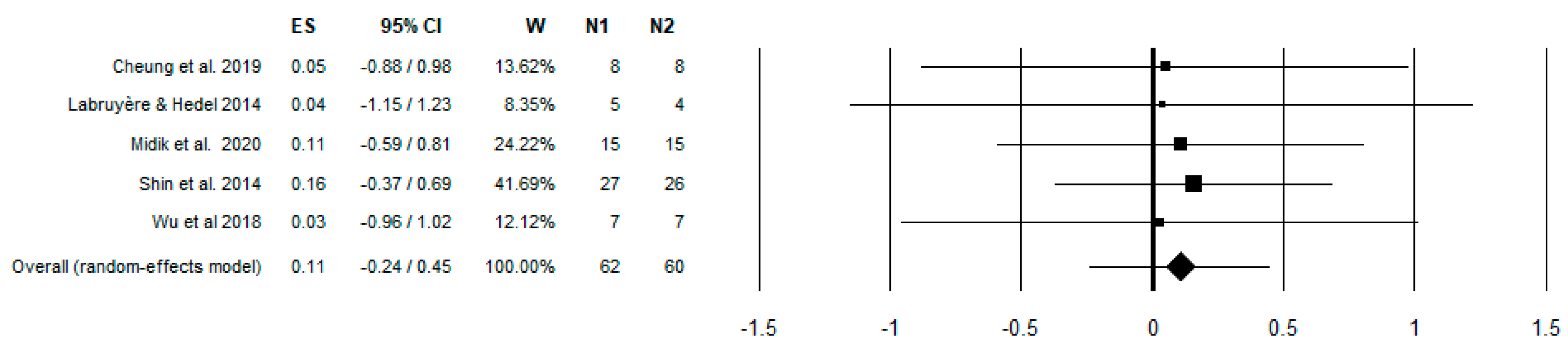
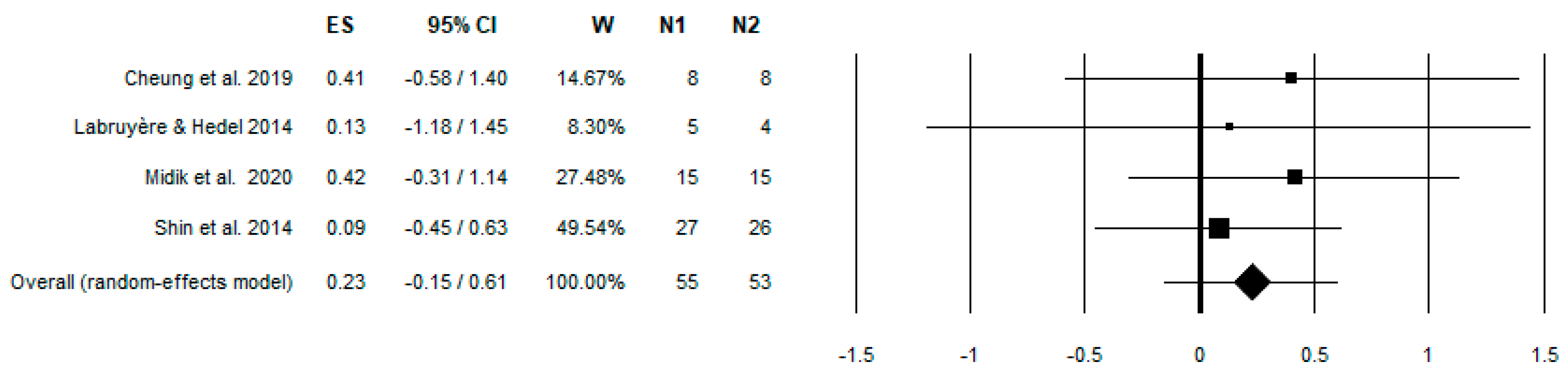
3. Results
4. Discussion
4.1. Studies on Robotics
4.2. External Stimulation (Non-Invasive Brain Stimulation)
4.3. Intermittent Hypoxia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spinal Cord Injury. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 24 September 2020).
- West, C.R.; AlYahya, A.; Laher, I.; Krassioukov, A. Peripheral vascular function in spinal cord injury: A systematic review. Spinal Cord 2013, 51, 10–19. [Google Scholar] [CrossRef]
- Raineteau, O.; Schwab, M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2001, 2, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.A.; Chancellor, M.B.; Blaivas, J.G. Bladder and Sphincter Behavior in Patients with Spinal Cord Lesions. J. Urol. 1991, 146, 113–117. [Google Scholar] [CrossRef]
- Karlsson, A.-K. Overview: Autonomic dysfunction in spinal cord injury: Clinical presentation of symptoms and signs. In Progress in Brain Research; Weaver, L.C., Polosa, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–8. [Google Scholar]
- Ditunno, P.L.; Patrick, M.; Stineman, M.; Ditunno, J.F. Who wants to walk? Preferences for recovery after SCI: A longitudinal and cross-sectional study. Spinal Cord 2008, 46, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Bishop, L.; Stein, J.; Wong, C.K. Robot-aided gait training in an individual with chronic spinal cord injury: A case study. J. Neurol. Phys. Ther. 2012, 36, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Burnfield, J.M. Gait Analysis: Normal and Pathological Function, 2nd ed.; SLACK: Thorofare, NJ, USA, 2010. [Google Scholar]
- Brooks, V.B. The Neural Basis of Motor Control; 3. [print.]; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Takakusaki, K. Neurophysiology of gait: From the spinal cord to the frontal lobe: Neurophysiology of Gait. Mov. Disord. 2013, 28, 1483–1491. [Google Scholar] [CrossRef]
- Côté, M.-P.; Murray, M.; Lemay, M.A. Rehabilitation Strategies after Spinal Cord Injury: Inquiry into the Mechanisms of Success and Failure. J. Neurotrauma 2017, 34, 1841–1857. [Google Scholar] [CrossRef]
- Klarner, T.; Zehr, E.P. Sherlock Holmes and the curious case of the human locomotor central pattern generator. J. Neurophysiol. 2018, 120, 53–77. [Google Scholar] [CrossRef]
- Scivoletto, G.; Ivanenko, Y.; Morganti, B.; Grasso, R.; Zago, M.; Lacquaniti, F.; Ditunno, J.; Molinari, M. Review Article: Plasticity of Spinal Centers in Spinal Cord Injury Patients: New Concepts for Gait Evaluation and Training. Neurorehabil. Neural Repair 2007, 21, 358–365. [Google Scholar] [CrossRef]
- Harvey, L.A.; Glinsky, J.V.; Bowden, J.L. The effectiveness of 22 commonly administered physiotherapy interventions for people with spinal cord injury: A systematic review. Spinal Cord 2016, 54, 914–923. [Google Scholar] [CrossRef]
- Fakhoury, M. Spinal cord injury: Overview of experimental approaches used to restore locomotor activity. Rev. Neurosci. 2015, 26, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, E.; Duerinck, S.; Baeyens, J.-P.; Meeusen, R.; Kerckhofs, E. Effectiveness of robot-assisted gait training in persons with spinal cord injury: A systematic review. J. Rehabil. Med. 2010, 42, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Robotic Gait Rehabilitation and Substitution Devices in Neurological Disorders: Where Are We Now? Available online: https://scholar.google.it/citations?view_op=view_citation&hl=it&user=TGgSNGUAAAAJ&citation_for_view=TGgSNGUAAAAJ:8xutWZnSdmoC (accessed on 19 March 2022).
- Chang, S.-H.; Afzal, T.; TIRR SCI Clinical Exoskeleton Group; Berliner, J.; Francisco, G.E. Exoskeleton-assisted gait training to improve gait in individuals with spinal cord injury: A pilot randomized study. Pilot Feasibility Stud. 2018, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, A.; Miller, S.; Rothwell, J.; Tallis, R.C.; Pomeroy, V. Informing Dose-Finding Studies of Repetitive Transcranial Magnetic Stimulation to Enhance Motor Function: A Qualitative Systematic Review. Neurorehabil. Neural Repair 2008, 22, 228–249. [Google Scholar] [CrossRef]
- Butler, A.J.; Wolf, S.L. Putting the brain on the map: Use of transcranial magnetic stimulation to assess and induce cortical plasticity of upper-extremity movement. Phys. Ther. 2007, 87, 719–736. [Google Scholar] [CrossRef]
- Astorino, T.A.; Harness, E.T.; White, A.C. Efficacy of Acute Intermittent Hypoxia on Physical Function and Health Status in Humans with Spinal Cord Injury: A Brief Review. Neural Plast. 2015, 2015, 409625. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Liberati, M.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Miller, S.A.; Forrest, J.L. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J. Evid. Based Dent. Pract. 2001, 1, 136–141. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Midik, M.; Paker, N.; Bugdayci, D.; Midik, A.C. Effects of robot-assisted gait training on lower extremity strength, functional independence, and walking function in men with incomplete traumatic spinal cord injury. Turk. Fiz. Tip Rehabil. Derg. [Turk. J. Phys. Med. Rehabil.] 2020, 66, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Varoqui, D.; Niu, X.; Mirbagheri, M.M. Ankle voluntary movement enhancement following robotic-assisted locomotor training in spinal cord injury. J. Neuroeng. Rehabil. 2014, 11, 46. [Google Scholar] [CrossRef]
- Yildirim, M.A.; Öneş, K.; Gökşenoğlu, G. Early term effects of robotic assisted gait training on ambulation and functional capacity in patients with spinal cord injury. Turk. J. Med. Sci. 2019, 49, 838–848. [Google Scholar] [CrossRef]
- Cheung, E.Y.Y.; Yu, K.K.K.; Kwan, R.L.C.; Ng, C.K.M.; Chau, R.M.W.; Cheing, G.L.Y. Effect of EMG-biofeedback robotic-assisted body weight supported treadmill training on walking ability and cardiopulmonary function on people with subacute spinal cord injuries—A randomized controlled trial. BMC Neurol. 2019, 19, 140. [Google Scholar] [CrossRef]
- Shin, J.C.; Kim, J.Y.; Park, H.K.; Kim, N.Y. Effect of Robotic-Assisted Gait Training in Patients with Incomplete Spinal Cord Injury. Ann. Rehabil. Med. 2014, 38, 719. [Google Scholar] [CrossRef]
- Duffell, L.D.; Brown, G.L.; Mirbagheri, M.M. Interventions to Reduce Spasticity and Improve Function in People with Chronic Incomplete Spinal Cord Injury: Distinctions Revealed by Different Analytical Methods. Neurorehabil. Neural Repair 2015, 29, 566–576. [Google Scholar] [CrossRef]
- Labruyère, R.; van Hedel, H.J.A. Strength training versus robot-assisted gait training after incomplete spinal cord injury: A randomized pilot study in patients depending on walking assistance. J. Neuroeng. Rehabil. 2014, 11, 4. [Google Scholar] [CrossRef]
- Wu, M.; Kim, J.; Wei, F. Facilitating Weight Shifting During Treadmill Training Improves Walking Function in Humans with Spinal Cord Injury: A Randomized Controlled Pilot Study. Am. J. Phys. Med. Rehabil. 2018, 97, 585–592. [Google Scholar] [CrossRef]
- Edwards, D.J.; Forrest, G.; Cortes, M.; Weightman, M.M.; Sadowsky, C.; Chang, S.-H.; Furman, K.; Bialek, A.; Prokup, S.; Carlow, J.; et al. Walking improvement in chronic incomplete spinal cord injury with exoskeleton robotic training (WISE): A randomized controlled trial. Spinal Cord 2022, 60, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Hayes, H.B.; Jayaraman, A.; Herrmann, M.; Mitchell, G.S.; Rymer, W.Z.; Trumbower, R.D. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: A randomized trial. Neurology 2014, 82, 104–113. [Google Scholar] [CrossRef]
- Tan, A.Q.; Sohn, W.J.; Naidu, A.; Trumbower, R.D. Daily acute intermittent hypoxia combined with walking practice enhances walking performance but not intralimb motor coordination in persons with chronic incomplete spinal cord injury. Exp. Neurol. 2021, 340, 113669. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Alcayaga, J.; Sepúlveda, O.; Rojas, E.; Astudillo, C. Repetitive Intermittent Hypoxia and Locomotor Training Enhances Walking Function in Incomplete Spinal Cord Injury Subjects: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J. Neurotrauma 2017, 34, 1803–1812. [Google Scholar] [CrossRef]
- Kumru, H.; Benito-Penalva, J.; Valls-Sole, J.; Murillo, N.; Tormos, J.M.; Flores, C.; Vidal, J. Placebo-controlled study of rTMS combined with Lokomat® gait training for treatment in subjects with motor incomplete spinal cord injury. Exp. Brain Res. 2016, 234, 3447–3455. [Google Scholar] [CrossRef] [PubMed]
- Corticospinal-Motor Neuronal Plasticity Promotes Exercise-Mediated Recovery in Humans with Spinal Cord Injury|Brain|Oxford Academic. Available online: https://academic.oup.com/brain/article-abstract/143/5/1368/5827586 (accessed on 20 September 2020).
- Krogh, S.; Aagaard, P.; Jønsson, A.B.; Figlewski, K.; Kasch, H. Effects of repetitive transcranial magnetic stimulation on recovery in lower limb muscle strength and gait function following spinal cord injury: A randomized controlled trial. Spinal Cord 2022, 60, 135–141. [Google Scholar] [CrossRef]
- Raithatha, R.; Carrico, C.; Powell, E.S.; Westgate, P.M.; Ii, K.C.C.; Lee, K.; Dunsmore, L.; Salles, S.; Sawaki, L. Non-invasive brain stimulation and robot-assisted gait training after incomplete spinal cord injury: A randomized pilot study. NeuroRehabilitation 2016, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.H.; Suri, C.; Field-Fote, E.C. Walking and Balance Outcomes Are Improved Following Brief Intensive Locomotor Skill Training but Are Not Augmented by Transcranial Direct Current Stimulation in Persons with Chronic Spinal Cord Injury. Front. Hum. Neurosci. 2022, 16, 849297. [Google Scholar] [CrossRef]
- Jackson, A.; Carnel, C.; Ditunno, J.; Read, M.S.; Boninger, M.; Schmeler, M.R.; Williams, S.R.; Donovan, W.H. Outcome measures for gait and ambulation in the spinal cord injury population. J. Spinal Cord Med. 2008, 31, 487–499. [Google Scholar] [CrossRef]
- Stauffer, E.S.; Hoffer, M.M.; Nickel, V.L. Ambulation in Thoracic Paraplegia. JBJS. 1978. Available online: https://journals.lww.com/jbjsjournal/Citation/1978/60060/Ambulation_in_thoracic_paraplegia_.18.aspx (accessed on 18 October 2020).
- Dittuno, P.L.; Dittuno, J.F., Jr. Walking index for spinal cord injury (WISCI II): Scale revision. Spinal Cord 2001, 39, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Itzkovich, M.; Gelernter, I.; Biering-Sorensen, F.; Weeks, C.; Laramee, M.T.; Craven, B.C.; Tonack, M.; Hitzig, S.L.; Glaser, E.; Zeilig, G.; et al. The Spinal Cord Independence Measure (SCIM) version III: Reliability and validity in a multi-center international study. Disabil. Rehabil. 2007, 29, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Alashram, A.R.; Annino, G.; Padua, E. Robot-assisted gait training in individuals with spinal cord injury: A systematic review for the clinical effectiveness of Lokomat. J. Clin. Neurosci. 2021, 91, 260–269. [Google Scholar] [CrossRef]
- Holanda, L.J.; Silva, P.M.M.; Amorim, T.C.; Lacerda, M.O.; Simão, C.R.; Morya, E. Robotic assisted gait as a tool for rehabilitation of individuals with spinal cord injury: A systematic review. J. NeuroEng. Rehabil. 2017, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-Y.; Tsai, J.-L.; Li, G.-S.; Lien, A.S.-Y.; Chang, Y.-J. Effects of Robot-Assisted Gait Training in Individuals with Spinal Cord Injury: A Meta-analysis. BioMed Res. Int. 2020, 2020, 2102785. [Google Scholar] [CrossRef]
- Cheung, E.Y.Y.; Ng, T.K.W.; Yu, K.K.K.; Kwan, R.; Cheing, G.L. Robot-Assisted Training for People with Spinal Cord Injury: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 2320–2331.e12. [Google Scholar] [CrossRef]
- Tan, K.; Koyama, S.; Sakurai, H.; Teranishi, T.; Kanada, Y.; Tanabe, S. Wearable robotic exoskeleton for gait reconstruction in patients with spinal cord injury: A literature review. J. Orthop. Transl. 2021, 28, 55–64. [Google Scholar] [CrossRef]
- Spinal Cord Injury Research Evidence (SCIRE) Research Team; Louie, D.R.; Eng, J.J.; Lam, T. Gait speed using powered robotic exoskeletons after spinal cord injury: A systematic review and correlational study. J. NeuroEng. Rehabil. 2015, 12, 82. [Google Scholar] [CrossRef]
- Stampacchia, G.; Gazzotti, V.; Olivieri, M.; Andrenelli, E.; Bonaiuti, D.; Calabro, R.S.; Carmignano, S.M.; Cassio, A.; Fundaro, C.; Companini, I.; et al. Gait robot-assisted rehabilitation in persons with spinal cord injury: A scoping review. NRE 2022, 51, 609–647. [Google Scholar] [CrossRef]
- Bertani, R.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Wasserman, S.; Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis. J. Educ. Stat. 1988, 13, 75. [Google Scholar] [CrossRef]
- Glowinskj, S.; Ptak, M. A kinematic model of a humanoid lower limb exoskeleton with pneumatic actuators. Acta Bioeng. Biomech. 2022, 24, 145–157. [Google Scholar]
- Bunday, K.L.; Perez, M.A. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr. Biol. 2012, 22, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Bunday, K.L.; Urbin, M.A.; Perez, M.A. Potentiating paired corticospinal-motoneuronal plasticity after spinal cord injury. Brain Stimul. 2018, 11, 1083–1092. [Google Scholar] [CrossRef]
- Urbin, M.A.; Ozdemir, R.A.; Tazoe, T.; Perez, M.A. Spike-timing-dependent plasticity in lower-limb motoneurons after human spinal cord injury. J. Neurophysiol. 2017, 118, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Bastani, A.; Jaberzadeh, S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis. Clin. Neurophysiol. 2012, 123, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Wittkopf, P.G.; Larsen, D.B.; Graven-Nielsen, T. Protocols for inducing homeostatic plasticity reflected in the corticospinal excitability in healthy human participants: A systematic review and meta-analysis. Eur. J. Neurosci. 2021, 54, 5444–5461. [Google Scholar] [CrossRef]
- Jonker, Z.D.; Gaiser, C.; Tulen, J.H.M.; Ribbers, G.M.; Frens, M.A.; Selles, R.W. No effect of anodal tDCS on motor cortical excitability and no evidence for responders in a large double-blind placebo-controlled trial. Brain Stimul. 2021, 14, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Trumbower, R.D.; Jayaraman, A.; Mitchell, G.S.; Rymer, W.Z. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabilit. Neural Repair 2012, 26, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.D.; Bach, K.B.; Baker, T.L.; Kinkead, R.; Mitchell, G.S. Long term facilitation of phrenic motor output. Respir. Physiol. 2000, 121, 135–146. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, P.M.; Mitchell, G.S. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J. Physiol. 2009, 587, 5469–5481. [Google Scholar] [CrossRef] [PubMed]
- Baker-Herman, T.L.; Fuller, D.D.; Bavis, R.W.; Zabka, A.G.; Golder, F.J.; Doperalski, N.J.; Johnson, R.A.; Watters, J.J.; Mitchell, G.S. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci. 2004, 7, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.S.; Nichols, N.L.; MacFarlane, P.M.; Mitchell, G.S. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J. Appl. Physiol. 2012, 113, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
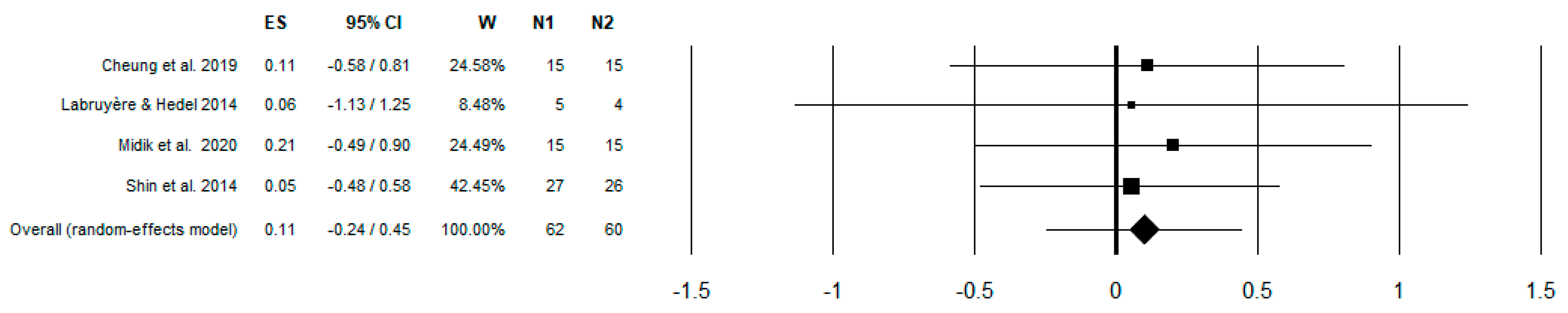
| Population | Adult people with stabilized spinal cord, tetraplegic, or paraplegic injuries with gait impairment. |
| Intervention | Rehabilitation with innovative methods |
| Comparison | New rehabilitation methods vs. conventional methods |
| Outcome | Improvement in gait parameters |
| Study | Intervention | Intervention Time | Case/Control | Outcome Measures | Mean Age ± SD Case/Control | Lesion Level | AIS | Time since Lesion | Results/Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Cheung et al. 2019 [32] | Lokomat + EMG feedback | 8 weeks | 15/15 | 10MWT 6MWT TUG WISCI-II MAS | 55.6 ± 4.98/53.0 ± 12.94 | <T10 | C, D | <1 year | Use of EMG-biofeedback RAGT enhanced the walking performance for SCI subjects and improved cardiopulmonary function |
| Duffell et al. 2015 [34] | Lokomat | 4 weeks | 27/29 | 10MWT 6MWT TUG LEMS | 46.6 ± 12.6/47.8 ± 13.1 | <T10 | C, D | >1 year | Overall, walking speed and endurance improved, with no difference between interventions. Improvements in function were achieved in a limited number of people with SCI |
| Labruyère & Hedel 2014 [35] | Lokomat | 4 weeks | 5/4 | 10 MWT LEMS WISCI-II FET SCIM BERG PCI | 59 ± 11 * | C4-T11 | C, D | >1 year | No significant differences in changes in scores between the 2 interventions, except for maximal walking speed (10MWT), which improved significantly more after strength training than after RAGT |
| Midik et al. 2020 [29] | Lokomat | 5 days | 15/15 | LEMS WISCI-II SCIM-III | 35.4 ± 12.1/37.9 ± 10.0 | T12-L3 | C, D | >3 months | Conventional rehabilitation is useful in terms of the improvement in the lower extremity motor function, walking, and functional status in men with incomplete SCI. RAGT provides greater improvement in the lower extremity motor function and functional independence. |
| Shin et al. 2014 [33] | Lokomat | 4 weeks | 27/26 | LEMS AMI SCIM-III WISCI-II | 43.15 ± 14.37/48.15 ± 11.49 | C1-L4 | D | <6 months | RAGT combined with conventional physiotherapy could yield more improvement in ambulatory function than conventional therapy alone |
| Varoqui et al. 2014 [30] | Lokomat | 4 weeks | 15/15 | Distance walked in 2 min 10MWT 6MWT TUG | 50.80 ± 2.12/44.65 ± 2.66 | <T10 | C, D | <1 year | The improvements in the kinematic and kinetic parameters of the ankle voluntary movement, and their correlation with the functional assessments, support the therapeutic effect of robotic-assisted locomotor training on motor impairment in chronic iSCI |
| Yildirim et al. 2019 [31] | Lokomat | 8 weeks | 44/44 | WISCI-II FIM | 32 ± 23/36.5 ± 24 | C1-L4 | A, B, C, D | <6 months | Robotic-assisted gait training combined with conventional therapy was found to be superior to the conventional therapy in terms of gait function and level of disability |
| Wu et al. 2018 [36] | 3DCaLT | 6 weeks | 7/7 | 6MWT speed MAS BERG SF-36 | 48.4 ± 13.5/48.1 ± 4.9 | C2-T10 | C, D | >1 year | A greater improvement in 6-min walking distance was observed after robotic training than that after treadmill-only training |
| Chang et al. 2018 [18] | Ekso | 3 weeks | 4/3 | 10MWT 6WT TUG LEMS spacial-temporal parameters | 56 ± 17/60 ± 2 | <T12 | C, D | <6 months | Improvement was observed in the 6MWT for the exoskeleton (EGT) group. Both the EGT and the conventional groups showed significant increases in right step length. The EGT group also showed improvement in stride length. |
| Jo et al. 2020 [42] | TMS + exercise | 3 weeks | 13/12 + 13 ** | 10MWT GRASSP MEP MVC | 44.2 ± 14.8 * | C2-L3 | A, C, D | >1 year | Stimulation contributed to preserving exercise gains. Our findings indicate that targeted non-invasive stimulation of spinal synapses might represent an effective strategy to facilitate exercise-mediated recovery |
| Kumru et al. 2016 [41] | TMS + Lokomat | 8 weeks | 17/17 | 10MWT WISCI-II LEMS | 46.4 ± 15.5/48.7 ± 16.5 | <T12 | C, D | <6 months | A total of 20 sessions of daily high-frequency TMS combined with Lokomat gait training can lead to clinical improvement in gait in motor-incomplete SCI |
| Raithatha et al. 2016 [44] | tDCS + Lokomat | 3 weeks | 9/6 | 10MWT 6MWT TUG BERG SCIM-III MMT | 47.5 ± 13.2 | C4-L1 | B, C, D | >1 year | Pairing tDCS with Lokomat can improve lower extremity motor function more than Lokomat alone |
| Hayes et al. 2014 [38] | IH | 5 weeks | 4/6 *** | 10MWT 6MWT | 43 ± 4 * | <T12 | C, D | >1 year | IH ± walking improved walking speed and distance in patients with chronic iSCI. The impact of IH is enhanced by combination with walking, demonstrating that combinatorial therapies may promote greater functional benefits in patients with iSCI |
| Navarrete-Opazo et al. 2017 [40] | IH + BWSTT | 4 weeks | 17/16 | 10MWT 6MWT TUG | 41 ± 17/42 ± 17 | >C5 | C, C | >6 months | Moderate IH (daily IH) combined with locomotor training improved walking speed and endurance in subjects with iSCI |
| Edwards et al. 2022 [37] | Ekso | 12 weeks | 9/10/6 | 10MWT, 6MWT, TUG, WISCI-II, NASA-Task Load Index | 41 ± 10/50 ± 15 | C3-L1 | C, D | >1 year | Chronic SCI participants with independent stepping ability at baseline can improve clinical ambulatory status |
| Tan et al. 2021 [39] | IH + WALK | 4 weeks | 5/5 *** | 10MWT, 6MWT, kinematics parameters | 46 ± 18 | C4-T9 | C, D | >1 years | Daily AIH combined with walking practice (AIH + WALK) improved overground walking performance and intralimb motor coordination in patients with chronic iSCI |
| Krogh et al. 2021 [43] | TMS | 4weeks | 10/9 | LEMS, 10MWT, 6MWT) | 57 ± 8/52 ± 12 | C2-L1 | C, D | >3 months | High-frequency TMS may increase long-term-training-induced recovery of lower limb muscle strength following SCI. |
| Evans et al. 2022 [45] | tDCS + Motor Skill Training | 3 days | 14/11 | 10MWT, kinematics parameters, BBS | 50 ± 10/46 ± 15 | CD-T8 | D | >1 year | High-frequency TMS may increase long-term-training-induced recovery of lower limb muscle strength following SCI. |
| Study | 10 MWT Self-Selected Speed | 10 MWT Fast | 6MWT | TUG | WISCI-II | SCIM-3 | LEMS |
|---|---|---|---|---|---|---|---|
| Cheung et al. 2019 [32] | 0.44 ± 0.24/0.45 ± 0.24 0.44 ± 0.29/0.48 ± 0.34 | 14.6 ± 4.27/16.3 ± 4.95 17.0 ± 2.78/17.1 ± 2.59 | 73.3 ± 19.73/71.0 ± 26.32 80.0 ± 17.44/80.3 ± 17.69 | 35.5 ± 4.50/36.5 ± 6.16 39.4 ± 9.07/40 ± 8.89 | |||
| Duffell et al. 2015 [34] | No comparable | No comparable | No comparable | 35.0 ± 13.9/34.6 ± 12.3 42.6 ± 4.6/41.9 ± 5.3 | |||
| Labruyère & Hedel 2014 [35] | 0.62 ± 0.23/0.66 ± 0.29 0.58 ± 0.19/0.64 ± 0.23 | 0.79 ± 0.31/0.80 ± 0.35 0.66 ± 022/0.80 ± 0.28 | 14.1 ± 2.5/14.9 ± 3.1 14.4 ± 2.6/14.8 ± 2.9 | 88.4 ± 7.9/89.2 ± 7.6 87.9 ± 8.1/89.2 ± 7.9 | 40.9 ± 7.5/41.6 ± 7.3 40.4 ± 6.6/41.4 ± 6.9 | ||
| Midik et al. 2020 [29] | 9.8 ± 5.42 /13.7 ± 4.26 11 ± 4.26/13.6 ± 3.87 | 69.1 ± 18.98/79.1 ± 17.81 69.2 ± 11.62/76.2 ± 9.29 | 27.1 ± 12.78/28.9 ± 13.94 23.8 ± 8.91/24.4 ± 8.52 | ||||
| Shin et al. 2014 [33] | 5.67 ± 10.97/10 ± 14.87 * 6.67 ± 12.55/9.67 ± 15.68 * | 5 ± 8.61/12 ± 20.35 * 8 ± 14.12/14 ± 25.88 * | 27.67 ± 21.91/35.33 ± 22.7 * 31 ± 15.69/35 ± 21.96 * | ||||
| Varoqui et al. 2014 [30] | 0.56 ± 0.09/0.64 ± 0.10 No available data | 206.96 ± 29.57/208.87 ± 28.36 No available data | 34.15 ± 9.61/27.83 ± 7.32 No available data | ||||
| Yildirim et al. 2019 [31] | 5 (9)/9 (7) ** 5 (6.7)/6.5 (5) ** | ||||||
| Wu et al. 2018 [36] | 0.33 ± 0.15/0.39 ± 0.20 0.56 ± 0.24/0.56 ± 0.24 | 0.48 ± 0.22/0.54 ± 0.29 0.80 ± 0.34/0.79 ± 0.35 | 120 ± 37/157 ± 59 control was 218 ± 92 m and 225 ± 96 | ||||
| Chang et al. 2018 [18] | 0.17 ± 0.01/0.22 ± 0.03 0.51 ± 0.0.28/0.55 ± 0.31 | 50 ± 23/67 ± 25 147 ± 87/154 ± 94 | 71 ± 23/55 ± 8 37 ± 17/36 ± 17 | ||||
| Edwards et al. 2022 [37] | 0.18 ± 0.23/0.07 ± 0.11/0.03 ±0.03 | 0.20 ±0.24/ 0.14 ±0.18/0.03 ± 0.13 | No comparable | No comparable | No comparable |
| Study | 10 MWT Self-Selected Speed | 10 MWT Fast | 6MWT | TUG | WISCI-II | SCIM-3 | LEMS | MMT |
|---|---|---|---|---|---|---|---|---|
| Jo et al. 2020 [42] | 12.4% * 16.5% */24.5% * | |||||||
| Kumru et al. 2016 [41] | 2 of 15/6 of 15 # 2 of 16/4 of 16 # | Comparable between the two groups | +8.2 +3.4 | |||||
| Raithatha et al. 2016 [44] | 0.18 ± 0.15/+0.04 ± 0.07 ## 0.16 ± 0.07/+0.14 ± 0.07 ## | 188 ± 212/+21.8 ± 51.4 4 ## 184 ± 88/+132.5 ± 64.35 ## | 38.7 ± 12.9/+ 0.6 ± 10.53 ## 77.5 ± 7.0/–18.5 ± 1.98 ## | 59.7 ± 19.5/1.2 ± 1.47 ## 44.2 ± 26.5/2.7 ± 1.13 ## | L 4.3 ± 2.1 ** R 9.1 ± 3.8 ** | |||
| Hayes et al. 2014 [38] | Not significative | +269 m +173 m | ||||||
| Navarrete-Opazo et al. 2017 [40] | −20.3 ± 6.9 s −15.5 ± 4.8 s | +70.5 ± 13.2 m + 43.1 ± 10.7 m | −23.7 ± 11.1 s −22.8 ±11.5 s | |||||
| Evans et al. 2022 [45] | 0.69 ± 0.51/0.83 ± 0.51 | |||||||
| Krogh et al. 2021 [43] | 18.5 ± 30.5 /2.5 ±2.1 | 77.7 ± 65.5/75.6 ± 56.9 | 4.3 ± 3.0/3.7 ± 3.8 | |||||
| Tan et al. 2022 [39] | No comparable | No comparable | No comparable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rosa, G.; Avola, M.; Di Gregorio, T.; Calabrò, R.S.; Onesta, M.P. Gait Recovery in Spinal Cord Injury: A Systematic Review with Metanalysis Involving New Rehabilitative Technologies. Brain Sci. 2023, 13, 703. https://doi.org/10.3390/brainsci13050703
La Rosa G, Avola M, Di Gregorio T, Calabrò RS, Onesta MP. Gait Recovery in Spinal Cord Injury: A Systematic Review with Metanalysis Involving New Rehabilitative Technologies. Brain Sciences. 2023; 13(5):703. https://doi.org/10.3390/brainsci13050703
Chicago/Turabian StyleLa Rosa, Giuseppe, Marianna Avola, Tiziana Di Gregorio, Rocco Salvatore Calabrò, and Maria Pia Onesta. 2023. "Gait Recovery in Spinal Cord Injury: A Systematic Review with Metanalysis Involving New Rehabilitative Technologies" Brain Sciences 13, no. 5: 703. https://doi.org/10.3390/brainsci13050703
APA StyleLa Rosa, G., Avola, M., Di Gregorio, T., Calabrò, R. S., & Onesta, M. P. (2023). Gait Recovery in Spinal Cord Injury: A Systematic Review with Metanalysis Involving New Rehabilitative Technologies. Brain Sciences, 13(5), 703. https://doi.org/10.3390/brainsci13050703







