TTFields Prolonged the PFS of Epithelioid Glioblastoma Patient: A Case Report
Abstract
1. Introduction
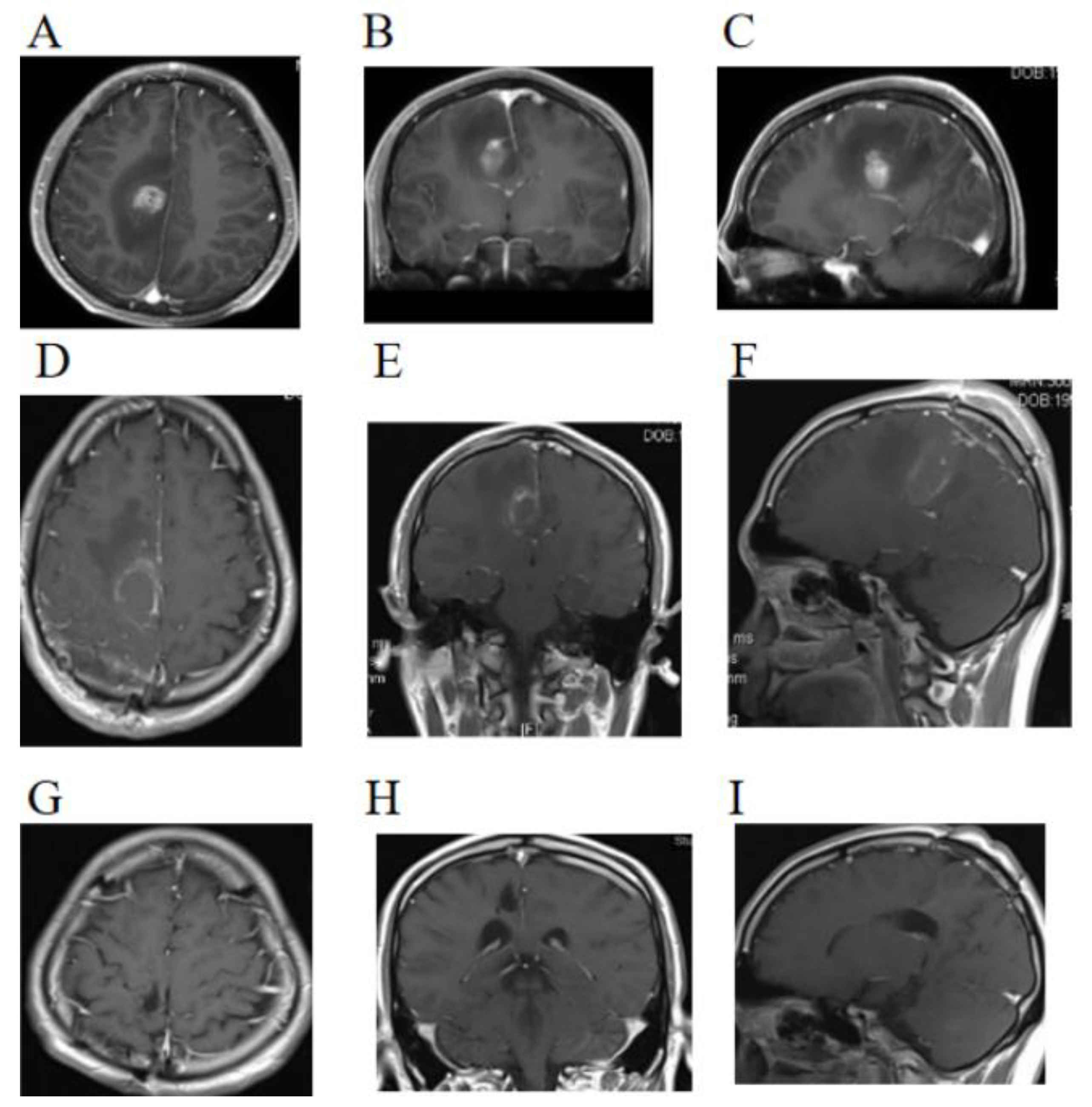
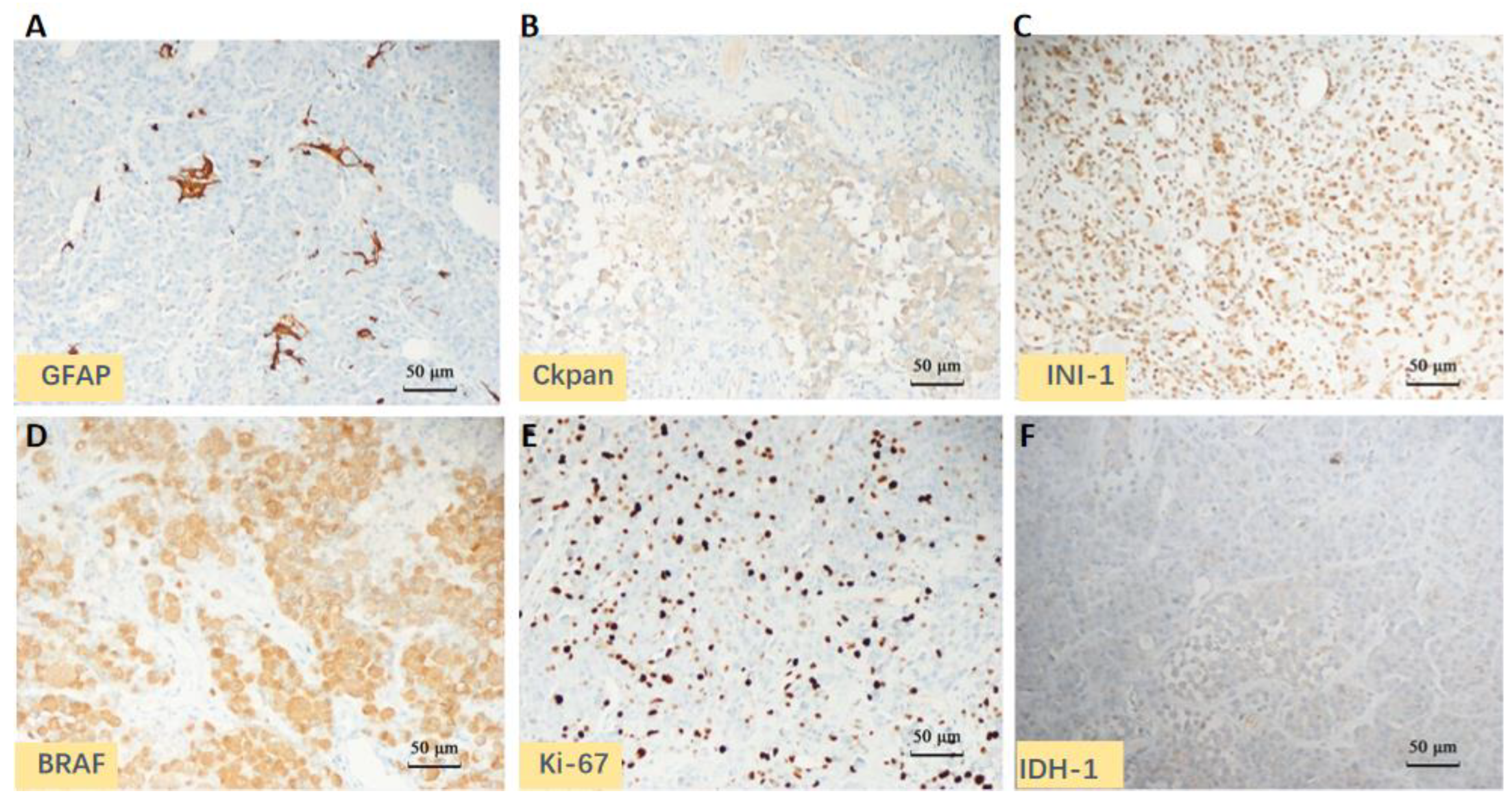
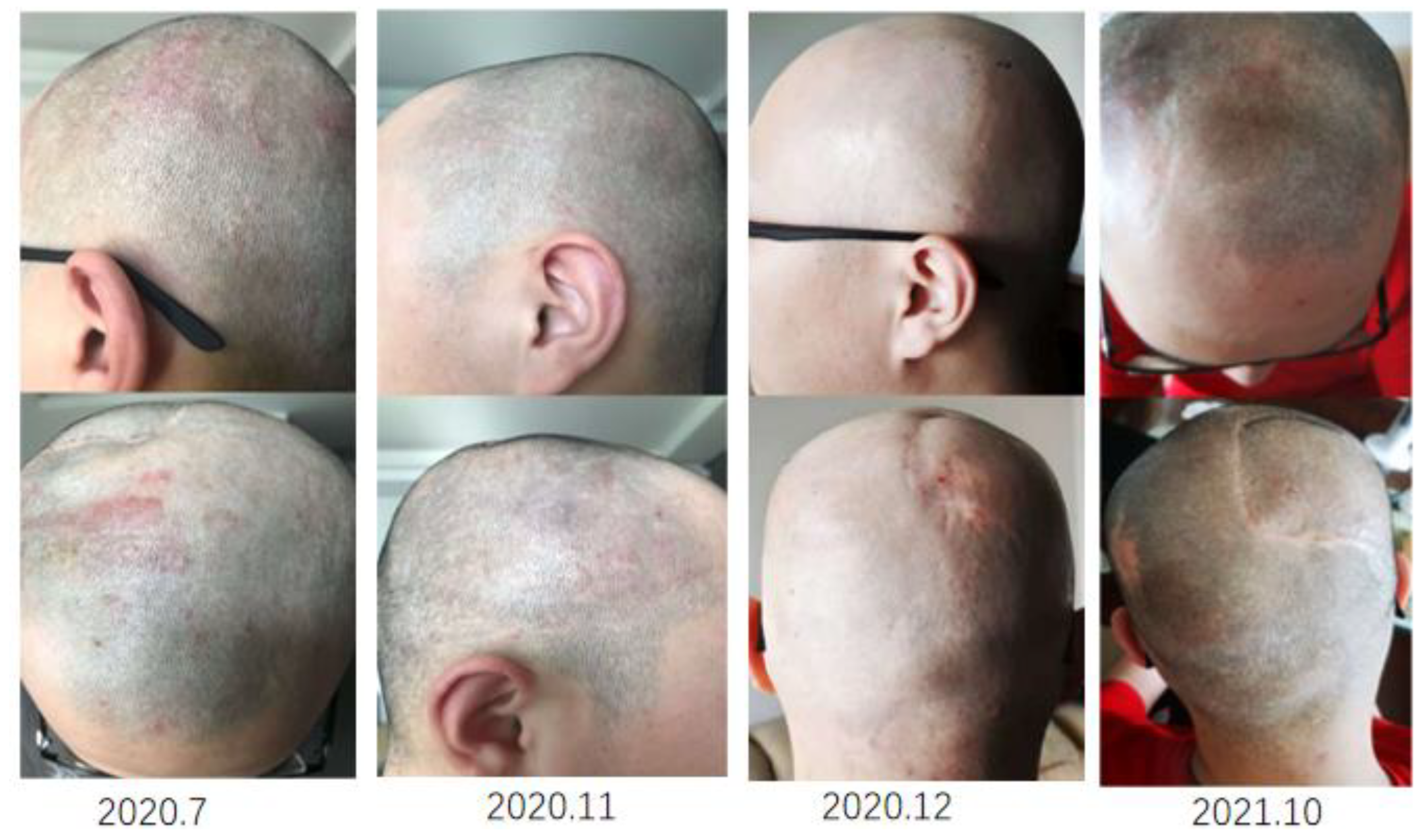
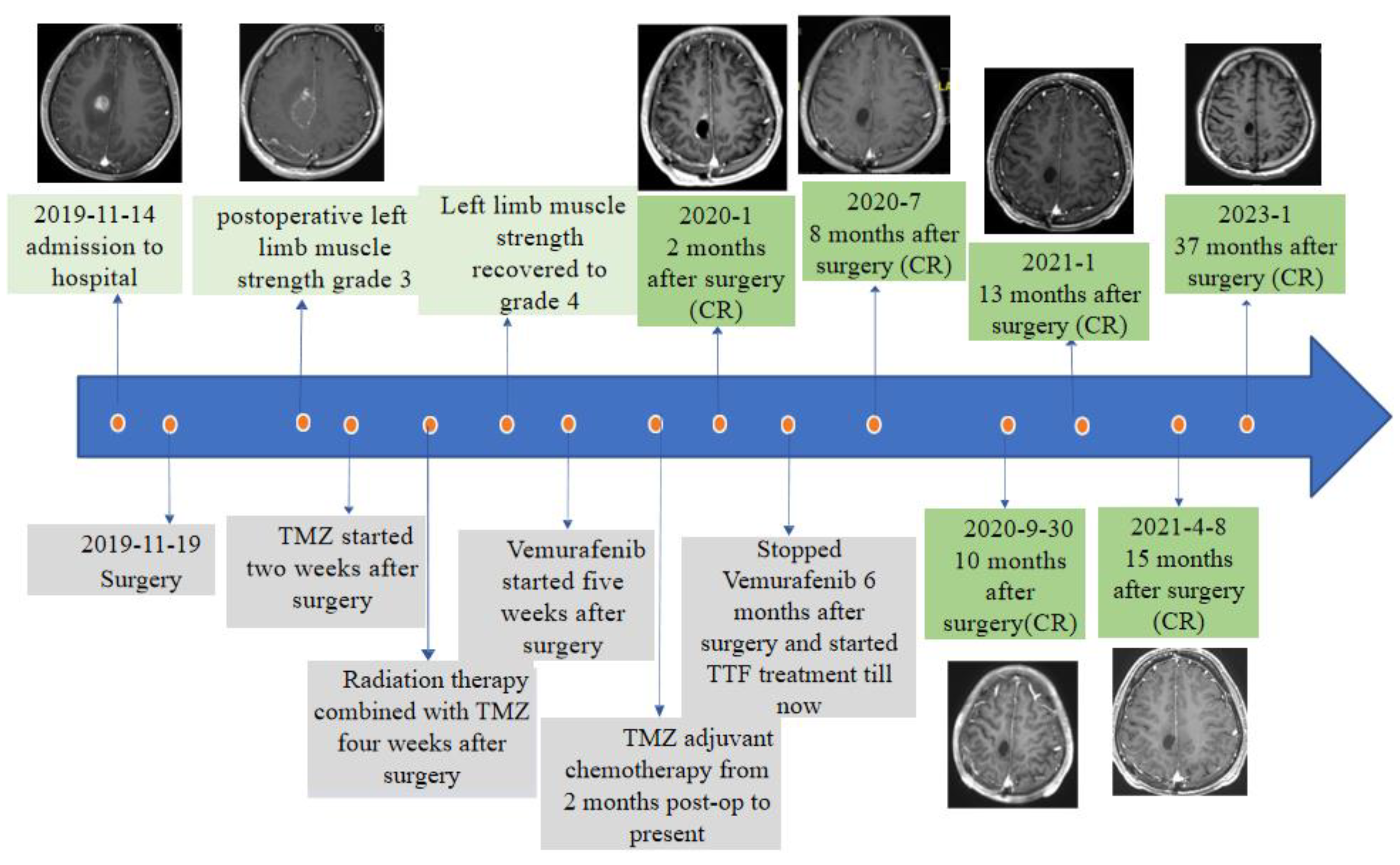
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, V.M.; George, N.D.; Brown, D.A.; Akinduro, O.O.; Raghunathan, A.; Jentoft, M.; Quinones-Hinojosa, A.; Chaichana, K.L. Confirming Diagnosis and Effective Treatment for Rare Epithelioid Glioblastoma Variant: An Integrated Survival Analysis of the Literature. World Neurosurg. 2019, 131, 243–251.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, Q.; Zhang, Q.; Guan, B.; Zhou, X. Clinicopathologic features and prognosis of epithelioid glioblastoma. Int. J. Clin. Exp. Pathol. 2020, 13, 1529–1539. [Google Scholar] [PubMed]
- Zeng, Y.; Zhu, X.; Wang, Y.; Liu, B.; Yang, X.; Wang, Q.; Du, J.; Ma, Y.; Lin, L.; Fu, P.; et al. Clinicopathological, Immunohistochemical and Molecular Genetic Study on Epithelioid Glioblastoma: A Series of Fifteen Cases with Literature Review. OncoTargets Ther. 2020, 13, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Cui, Y.; Liu, Y.; Fang, J.; Xu, L.; He, Y.; Du, J.; Su, Y.; Zou, W.; et al. Evaluation of EZH2 expression, BRAF V600E mutation, and CDKN2A/B deletions in epithelioid glioblastoma and anaplastic pleomorphic xanthoastrocytoma. J. Neurooncol. 2019, 144, 137–146. [Google Scholar] [CrossRef]
- Nakagomi, N.; Sakamoto, D.; Hirose, T.; Takagi, T.; Murase, M.; Nakagomi, T.; Yoshimura, S.; Hirota, S. Epithelioid glioblastoma with microglia features: Potential for novel therapy. Brain Pathol. 2020, 30, 1119–1133. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Taphoorn, M.J.; Dirven, L.; Kanner, A.A.; Lavy-Shahaf, G.; Weinberg, U.; Taillibert, S.; Toms, S.A.; Honnorat, J.; Chen, T.C.; Sroubek, J.; et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018, 4, 495–504. [Google Scholar] [CrossRef]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef]
- Sun, K.; Zhou, X.; Li, T.; Zuo, M.; Li, J.; Liu, Y. Clinicopathological characteristics and treatment outcomes of epithelioid glioblastoma. Neurosurg. Rev. 2021, 44, 3335–3348. [Google Scholar] [CrossRef]
- Zhao, J.P.; Cui, C.X.; Wang, J.C.; Su, H.W.; Duan, C.F.; Liu, X.J. Multimodal MR Features of 8 Cases of Epithelioid Glioblastoma. Biomed. Res. Int. 2020, 2020, 9586806. [Google Scholar] [CrossRef]
- Huang, Q.L.; Cao, X.; Chai, X.; Wang, X.; Xiao, C.; Wang, J. The Radiological Imaging Features of Easily Misdiagnosed Epithelioid Glioblastoma in Seven Patients. World Neurosurg. 2019, 124, e527–e532. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyoshi, H.; Komaki, S.; Arakawa, F.; Morioka, M.; Ohshima, K.; Nakada, M.; Sugita, Y. Clinicopathological and genetic association between epithelioid glioblastoma and pleomorphic xanthoastrocytoma. Neuropathology 2018, 38, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, S.; Korshunov, A.; Lai, S.H.; Dabiri, S.; Patil, S.; Li, R.; Shih, C.S.; Bonnin, J.M.; Baker, J.A.; Du, E.; et al. Epithelioid Glioblastomas and Anaplastic Epithelioid Pleomorphic Xanthoastrocytomas--Same Entity or First Cousins? Brain Pathol. 2016, 26, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Nobusawa, S.; Nakata, S.; Nakada, M.; Yamazaki, T.; Matsumura, N.; Harada, K.; Matsuda, H.; Funata, N.; Nagai, S.; et al. BRAF V600E, TERT promoter mutations and CDKN2A/B homozygous deletions are frequent in epithelioid glioblastomas: A histological and molecular analysis focusing on intratumoral heterogeneity. Brain Pathol. 2017, 28, 663–673. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Scheithauer, B.W.; Giannini, C.; Bryant, S.C.; Jenkins, R.B. Epithelial and Pseudoepithelial Differentiation in Glioblastoma and Gliosarcoma: A Comparative Morphologic and Molecular Genetic Study. Cancer 2008, 113, 2779–2789. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Blumenthal, D.T.; Gorlia, T.; Gilbert, M.R.; Kim, M.M.; Nabors, L.B.; Mason, W.P.; Hegi, M.; Zhang, P.; Gkolfinopoulos, V.; Perry, J.R.; et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: A secondary analysis of EORTC and NRG Oncology/RTOG. Neuro-Oncology 2017, 19, 1119–1126. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Zhao, F.; Chen, J.; He, L.; Liu, Z.; Pei, Y.; Wei, Z.; Li, R.; Ai, P.; et al. Standard or extended STUPP? Optimal duration of temozolomide for patients with high-grade gliomas: A retrospective analysis. J. Neuro-Oncol. 2022, 160, 433–443. [Google Scholar] [CrossRef]
- Freyschlag, C.F.; Smolczyk, D.R.; Janzen, E.; Schmieder, K.; Thomé, C.; Lohr, F.; Wenz, F.; Weiss, C.; Tuettenberg, J.; Seiz, M. Prolonged administration of temozolomide in adult patients with anaplastic glioma. Anticancer Res. 2011, 31, 3873–3877. [Google Scholar]
- Barbagallo, G.M.V.; Paratore, S.; Caltabiano, R.; Palmucci, S.; Parra, H.S.; Privitera, G.; Motta, F.; Lanzafame, S.; Scaglione, G.; Longo, A.; et al. Long-term therapy with temozolomide is a feasible option for newly diagnosed glioblastoma: A single-institution experience with as many as 101 temozolomide cycles. Neurosurg. Focus 2014, 37, E4. [Google Scholar] [CrossRef] [PubMed]
- Kaley, T.; Touat, M.; Subbiah, V.; Hollebecque, A.; Rodon, J.; Lockhart, A.C.; Keedy, V.; Bielle, F.; Hofheinz, R.D.; Joly, F.; et al. BRAF Inhibition in BRAFV600-Mutant Gliomas: Results From the VE-BASKET Study. J. Clin. Oncol. 2018, 36, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, D.R.; Bouffet, E.; Tabori, U.; Broniscer, A.; Cohen, K.J.; Hansford, J.R.; Geoerger, B.; Hingorani, P.; Dunkel, I.J.; Russo, M.W.; et al. Efficacy and Safety of Dabrafenib in Pediatric Patients with BRAF V600 Mutation–Positive Relapsed or Refractory Low-Grade Glioma: Results from a Phase I/IIa Study. Clin. Cancer Res. 2019, 25, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Stein, A.; Bent, M.V.D.; De Greve, J.; Wick, A.; de Vos, F.Y.F.L.; von Bubnoff, N.; van Linde, M.E.; Lai, A.; Prager, G.W.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): A multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2021, 23, 53–64. [Google Scholar] [CrossRef]
- Brown, N.F.; Carter, T.; Kitchen, N.; Mulholland, P.; Kong, B.Y.; Carlino, M.S.; Menzies, A.M.; Roque, A.; Odia, Y.; Martin-Liberal, J.; et al. Dabrafenib and trametinib in BRAFV600E mutated glioma. CNS Oncol. 2017, 6, 291–296. [Google Scholar] [CrossRef]
- Kanemaru, Y.; Natsumeda, M.; Okada, M.; Saito, R.; Kobayashi, D.; Eda, T.; Watanabe, J.; Saito, S.; Tsukamoto, Y.; Oishi, M.; et al. Dramatic response of BRAF V600E-mutant epithelioid glioblastoma to combination therapy with BRAF and MEK inhibitor: Establishment and xenograft of a cell line to predict clinical efficacy. Acta Neuropathol. Commun. 2019, 7, 119. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, H.; Yang, R.; Li, Z.; Zheng, H.; Zhang, Z.; Peng, J.; Zhang, X.; Qi, S.; Liu, Y.; et al. Effective treatment of a BRAF V600E-mutant epithelioid glioblastoma patient by vemurafenib: A case report. Anti-Cancer Drugs 2021, 33, 100–104. [Google Scholar] [CrossRef]
- Salvador, E.; Köppl, T.; Hörmann, J.; Schönhärl, S.; Bugaeva, P.; Kessler, A.F.; Burek, M.; Ernestus, R.I.; Löhr, M.; Hagemann, C. Tumor Treating Fields (TTFields) Induce Cell Junction Alterations in a Human 3D In Vitro Model of the Blood-Brain Barrier. Pharmaceutics 2023, 15, 185. [Google Scholar] [CrossRef]
- Kirson, E.D.; Schneiderman, R.S.; Dbalý, V.; Tovaryš, F.; Vymazal, J.; Itzhaki, A.; Mordechovich, D.; Gurvich, Z.; Shmueli, E.; Goldsher, D.; et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med. Phys. 2009, 9, 1–13. [Google Scholar] [CrossRef]
- Hottinger, A.F.; Pacheco, P.; Stupp, R. Tumor treating fields: A novel treatment modality and its use in brain tumors. Neuro Oncol. 2016, 18, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xu, S.; Dai, G.; Xiao, Z.; Chen, L.; Liu, Z. Tumor treating fields for high-grade gliomas. Biomed. Pharmacother. 2020, 127, 110193. [Google Scholar] [CrossRef] [PubMed]
- Dono, A.; Mitra, S.; Shah, M.; Takayasu, T.; Zhu, J.-J.; Tandon, N.; Patel, C.B.; Esquenazi, Y.; Ballester, L.Y. PTEN mutations predict benefit from tumor treating fields (TTFields) therapy in patients with recurrent glioblastoma. J. Neuro-Oncol. 2021, 153, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Lassman, A.B.; Joanta-Gomez, A.E.; Pan, P.C.; Wick, W. Current usage of tumor treating fields for glioblastoma. Neuro-Oncol. Adv. 2020, 2, vdaa069. [Google Scholar] [CrossRef] [PubMed]

| IHC | |
|---|---|
| Positive | Braf V600E, Ckpan (partial), GFAP (partial), Olig-2 (scattered), Vimentin, INI1, BRG1, S-100, Syn, P53, and Ki-67 (40%) |
| Negative | IDH1, CK7, CK20, Villin, H3-K27M, Neu-N, EMA, PR, SSTR2, PLAP, HMB-45, SMA, and Myogenin |
| GENETIC TEST | |
| 1p/19q | intact |
| ATRX | not mutated |
| BRAFV600E | mutated |
| TERT | mutated |
| TP53 | not mutated |
| MGMT promoter | not methylated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Wang, Q.; Wang, F.; Wu, N.; Li, J.; He, X.; Pan, H.; Wang, L. TTFields Prolonged the PFS of Epithelioid Glioblastoma Patient: A Case Report. Brain Sci. 2023, 13, 633. https://doi.org/10.3390/brainsci13040633
Ding Y, Wang Q, Wang F, Wu N, Li J, He X, Pan H, Wang L. TTFields Prolonged the PFS of Epithelioid Glioblastoma Patient: A Case Report. Brain Sciences. 2023; 13(4):633. https://doi.org/10.3390/brainsci13040633
Chicago/Turabian StyleDing, Yuxuan, Qiang Wang, Feijiang Wang, Nan Wu, Jianrui Li, Xia He, Hao Pan, and Lijun Wang. 2023. "TTFields Prolonged the PFS of Epithelioid Glioblastoma Patient: A Case Report" Brain Sciences 13, no. 4: 633. https://doi.org/10.3390/brainsci13040633
APA StyleDing, Y., Wang, Q., Wang, F., Wu, N., Li, J., He, X., Pan, H., & Wang, L. (2023). TTFields Prolonged the PFS of Epithelioid Glioblastoma Patient: A Case Report. Brain Sciences, 13(4), 633. https://doi.org/10.3390/brainsci13040633






