Color-Coding Method Reveals Enhancement of Stereotypic Locomotion by Phenazepam in Rat Open Field Test
Abstract
1. Introduction
2. Materials and Methods
- Mobility;
- Anxiety;
- Orientation-exploratory activity.
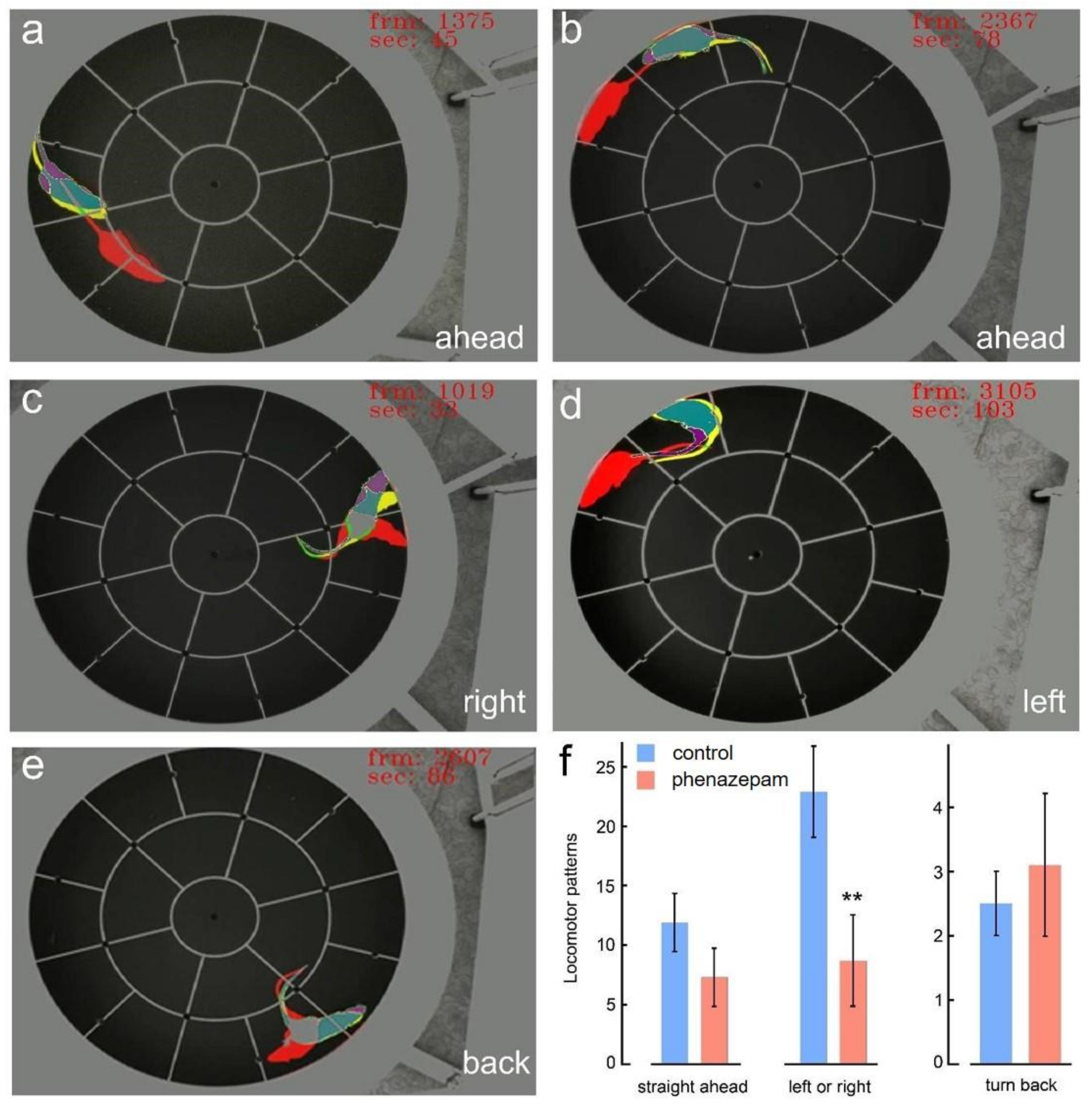
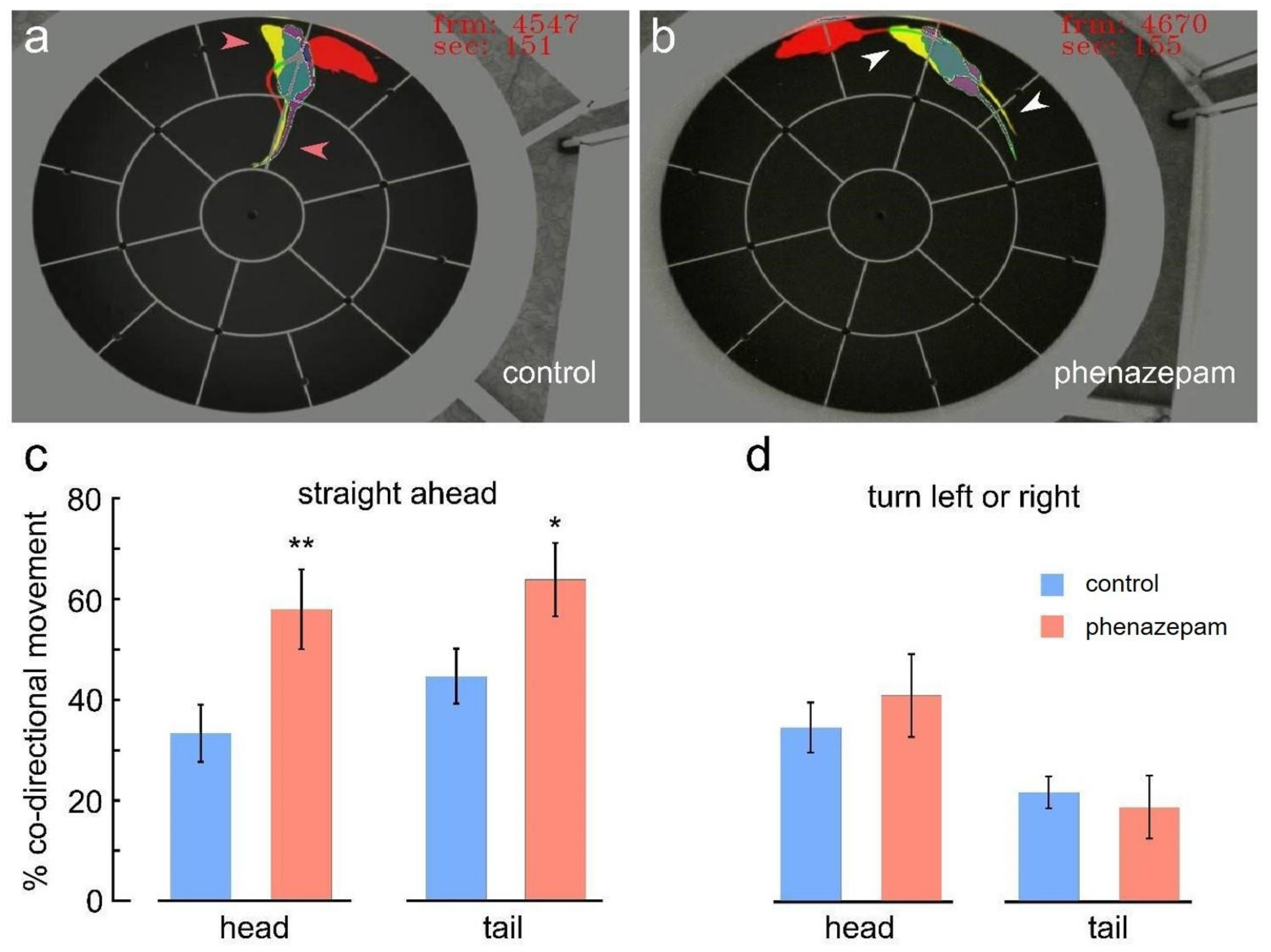
3. Results
4. Discussion
- Movements associated with orientation in space;
- Movements associated with the study of individual elements of space;
- The beginning of movement and the movement itself.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andreev, A.; Ahremenko, E.; Apushkin, D.; Kuznetsov, I.; Kovalenko, I.; Korkotian, E.; Kalchenko, V. New Approaches to Studying Rodent Behavior Using Deep Machine Learning. In Proceedings of the International Conference on Advances in Digital Science, Salvador, Brazil, 19–21 February 2021; pp. 365–374. [Google Scholar] [CrossRef]
- Godec, P.; Pančur, M.; Ilenič, N.; Čopar, A.; Stražar, M.; Erjavec, A.; Pretnar, A.; Demšar, J.; Starič, A.; Toplak, M.; et al. Democratized image analytics by visual programming through integration of deep models and small-scale machine learning. Nat. Commun. 2019, 10, 4551. [Google Scholar] [CrossRef]
- Komura, D.; Ishikawa, S. Machine learning approaches for pathologic diagnosis. Virchows Arch. 2019, 475, 131–138. [Google Scholar] [CrossRef]
- Nichols, J.A.; Chan, H.W.H.; Baker, M.A.B. Machine learning: Applications of artificial intelligence to imaging and diagnosis. Biophys. Rev. 2018, 11, 111–118. [Google Scholar] [CrossRef]
- Thieme, A.; Belgrave, D.; Doherty, G. Machine learning in mental health: A systematic review of the HCI literature to support the development of effective and implementable ML systems. ACM Trans. Comput.-Hum. Interact. (TOCHI) 2020, 27, 1–53. [Google Scholar] [CrossRef]
- Bica, I.; Alaa, A.M.; Lambert, C.; van der Schaar, M. From Real-World Patient Data to Individualized Treatment Effects Using Machine Learning: Current and Future Methods to Address Underlying Challenges. Clin. Pharmacol. Ther. 2020, 109, 87–100. [Google Scholar] [CrossRef]
- File, S.E. Animal tests of anxiety. In Recent Advances in Neuropsycho-Pharmacology; Pergamon: Oxford, UK, 1981; pp. 241–251. [Google Scholar]
- Voikar, V.; Stanford, S.C. The Open Field Test. In Psychiatric Vulnerability, Mood, and Anxiety Disorders: Tests and Models in Mice and Rats; Springer: Berlin/Heidelberg, Germany, 2022; pp. 9–29. [Google Scholar]
- Acikgoz, B.; Dalkiran, B.; Dayi, A. An overview of the currency and usefulness of behavioral tests used from past to present to assess anxiety, social behavior and depression in rats and mice. Behav. Process. 2022, 200, 104670. [Google Scholar] [CrossRef]
- Watson, G.D.R.; Hughes, R.N.; Petter, E.A.; Fallon, I.P.; Kim, N.; Severino, F.P.U.; Yin, H.H. Thalamic projections to the subthalamic nucleus contribute to movement initiation and rescue of parkinsonian symptoms. Sci. Adv. 2021, 7, eabe9192. [Google Scholar] [CrossRef]
- Hsu Alexander, I.; Yttr Eric, A. B-SOiD, an open-source unsupervised algorithm for identification and fast prediction of behaviors. Nat. Commun. 2021, 12, 31. [Google Scholar]
- Bellomo, G.; Piscopo, P.; Corbo, M.; Pupillo, E.; Stipa, G.; Beghi, E.; Vanacore, N.; Lacorte, E. A systematic review on the risk of neurodegenerative diseases and neurocognitive disorders in professional and varsity athletes. Neurol. Sci. 2022, 43, 6667–6691. [Google Scholar] [CrossRef]
- Calne, D.B.; McGeer, E.; Eisen, A.; Spencer, P.S. Alzheimer’s disease, Parkinson’s disease, and motoneurone disease: Abiotropic interaction between ageing and environment? Lancet 1986, 328, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, H.A.; Chen, A.T. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann. Clin. Psychiatry 2017, 29, 195–202. [Google Scholar] [PubMed]
- Cornett, E.M.; Novitch, M.B.; Brunk, A.J.; Davidson, K.S.; Menard, B.L.; Urman, R.D.; Kaye, A.D. New benzodi-azepines for sedation. Best Pract. Res. Clin. Anaesthesiol. 2018, 32, 149–164. [Google Scholar] [CrossRef]
- Manchester, K.R.; Lomas, E.C.; Waters, L.; Dempsey, F.C.; Maskell, P.D. The emergence of new psychoactive substance (NPS) benzodiazepines: A review. Drug Test. Anal. 2017, 10, 37–53. [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Shits, D.D.; Puchik, M.M.; Prikhodko, V.A.; Idiyatullin, R.D.; Kotelnikova, A.A.; Okovityi, S.V. Use of Naïve Bayes Classifier to Assess the Effects of Antipsychotic Agents on Brain Electrical Activity Parameters in Rats. J. Evol. Biochem. Physiol. 2022, 58, 1130–1141. [Google Scholar] [CrossRef]
- Maskell, P.D.; De Paoli, G.; Seetohul, L.N.; Pounder, D.J. Phenazepam: The drug that came in from the cold. J. Forensic Leg. Med. 2012, 19, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Herde, A.M.; Benke, D.; Ralvenius, W.T.; Mu, L.; Schibli, R.; Zeilhofer, H.U.; Krämer, S.D. GABAA receptor subtypes in the mouse brain: Regional mapping and diazepam receptor occupancy by in vivo [18F]flumazenil PET. Neuroimage 2017, 150, 279–291. [Google Scholar] [CrossRef]
- Garcia, A.M.B.; Cardenas, F.P.; Morato, S. Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiol. Behav. 2005, 85, 265–270. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The open-field test: A critical review. Psychol. Bull. 1976, 83, 482. [Google Scholar] [CrossRef]
- Crusio, W.E. Genetic dissection of mouse exploratory behaviour. Behav. Brain Res. 2001, 125, 127–132. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. JoVE 2015, 96, e52434. [Google Scholar]
- Google Colab. 2022. Available online: https://colab.research.google.com (accessed on 24 January 2023).
- Estanislau, C.; Veloso, A.W.; Filgueiras, G.B.; Maio, T.P.; Dal-Cól, M.L.; Cunha, D.C.; Klein, R.; Carmona, L.F.; Fernández-Teruel, A. Rat self-grooming and its relationships with anxiety, dearousal and perseveration: Evidence for a self-grooming trait. Physiol. Behav. 2019, 209, 112585. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2015, 17, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Voronina, T.A.; Molodavkin, G.M.; Chernyavskaya, L.I.; Seredenin, S.B.; Burlakova, E.B. Effect of superlow doses of phenazepam on the EEG and behavior of rats in different models of anxiet. Bull. Exp. Biol. Med. 1997, 124, 308–310. [Google Scholar] [CrossRef]
- Epstein, O.I.; Voronina, T.A.; Molodavkin, G.M.; Belopol’skaya, M.V.; Kheyfets, I.A.; Dugina, J.L.; Sergeeva, S.A. Study of bipathic effect of phenazepam. Bull. Exp. Biol. Med. 2007, 144, 536–538. [Google Scholar] [CrossRef]
- Heldt, S.A.; Ressler, K.J. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur. J. Neurosci. 2007, 26, 3631–3644. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef]
- Rudolph, U.; Möhler, H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 483–507. [Google Scholar] [CrossRef]
- Marowsky, A.; Fritschy, J.M.; Vogt, K.E. Functional mapping of GABAA receptor subtypes in the amygdala. Eur. J. Neurosci. 2004, 20, 1281–1289. [Google Scholar] [CrossRef]
- Mc Reynolds, P. Exploratory behavior: A theoretical interpretation. Psychol. Rep. 1962, 11, 311–318. [Google Scholar] [CrossRef]
- Hacques, G.; Komar, J.; Dicks, M.; Seifert, L. Exploring to learn and learning to explore. Psychol. Res. 2021, 85, 1367–1379. [Google Scholar] [CrossRef]
- Noonan, M.P.; Walton, M.E.; Behrens, T.E.J.; Sallet, J.; Buckley, M.J.; Rushworth, M.F.S. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc. Natl. Acad. Sci. USA 2010, 107, 20547–20552. [Google Scholar] [CrossRef] [PubMed]
- Noonan, M.P.; Chau, B.K.; Rushworth, M.F.; Fellows, L.K. Contrasting Effects of Medial and Lateral Orbitofrontal Cortex Lesions on Credit Assignment and Decision-Making in Humans. J. Neurosci. 2017, 37, 7023–7035. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J.; Venditto, S.J.C. Multi-step planning in the brain. Curr. Opin. Behav. Sci. 2021, 38, 29–39. [Google Scholar] [CrossRef]
- Goodroe, S.C.; Spiers, H.J. Extending neural systems for navigation to hunting behavior. Curr. Opin. Neurobiol. 2022, 73, 102545. [Google Scholar] [CrossRef] [PubMed]
- Gremel, C.M.; Costa, R.M. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat. Commun. 2013, 4, 2264. [Google Scholar] [CrossRef] [PubMed]
- Ben-Azu, B.; Aderibigbe, A.O.; Omogbiya, I.A.; Ajayi, A.M.; Iwalewa, E.O. Morin pretreatment attenuates schizophrenia-like behaviors in experimental animal models. Drug Res. 2018, 68, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Hareyama, N.; Ikeda, K.; Kurokawa, T.; Nakajima, M.; Nakao, K.; Mochizuki, H.; Ichinose, H. Effects of TRK-820, a selective kappa opioid receptor agonist, on rat schizophrenia models. Eur. J. Pharmacol. 2009, 606, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Peyrache, A.; Schieferstein, N.; Buzsáki, G. Transformation of the head-direction signal into a spatial code. Nat. Commun. 2017, 8, 1752. [Google Scholar] [CrossRef]
- Weiss, S.; Talhami, G.; Gofman-Regev, X.; Rapoport, S.; Eilam, D.; Derdikman, D. Consistency of spatial represen-tations in rat entorhinal cortex predicts performance in a reorientation task. Curr. Biol. 2017, 27, 3658–3665. [Google Scholar] [CrossRef]
- Jeffery, K.J. The hippocampus: From memory, to map, to memory map. Trends Neurosci. 2018, 41, 64–66. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarov, M.; Sysoev, Y.I.; Agafonova, O.; Prikhodko, V.A.; Korkotian, E.; Okovityi, S.V. Color-Coding Method Reveals Enhancement of Stereotypic Locomotion by Phenazepam in Rat Open Field Test. Brain Sci. 2023, 13, 408. https://doi.org/10.3390/brainsci13030408
Makarov M, Sysoev YI, Agafonova O, Prikhodko VA, Korkotian E, Okovityi SV. Color-Coding Method Reveals Enhancement of Stereotypic Locomotion by Phenazepam in Rat Open Field Test. Brain Sciences. 2023; 13(3):408. https://doi.org/10.3390/brainsci13030408
Chicago/Turabian StyleMakarov, Mark, Yuri I. Sysoev, Oksana Agafonova, Veronika A. Prikhodko, Eduard Korkotian, and Sergey V. Okovityi. 2023. "Color-Coding Method Reveals Enhancement of Stereotypic Locomotion by Phenazepam in Rat Open Field Test" Brain Sciences 13, no. 3: 408. https://doi.org/10.3390/brainsci13030408
APA StyleMakarov, M., Sysoev, Y. I., Agafonova, O., Prikhodko, V. A., Korkotian, E., & Okovityi, S. V. (2023). Color-Coding Method Reveals Enhancement of Stereotypic Locomotion by Phenazepam in Rat Open Field Test. Brain Sciences, 13(3), 408. https://doi.org/10.3390/brainsci13030408





