Enhanced Targeted Delivery of Minocycline via Transferrin Conjugated Albumin Nanoparticle Improves Neuroprotection in a Blast Traumatic Brain Injury Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Instruments
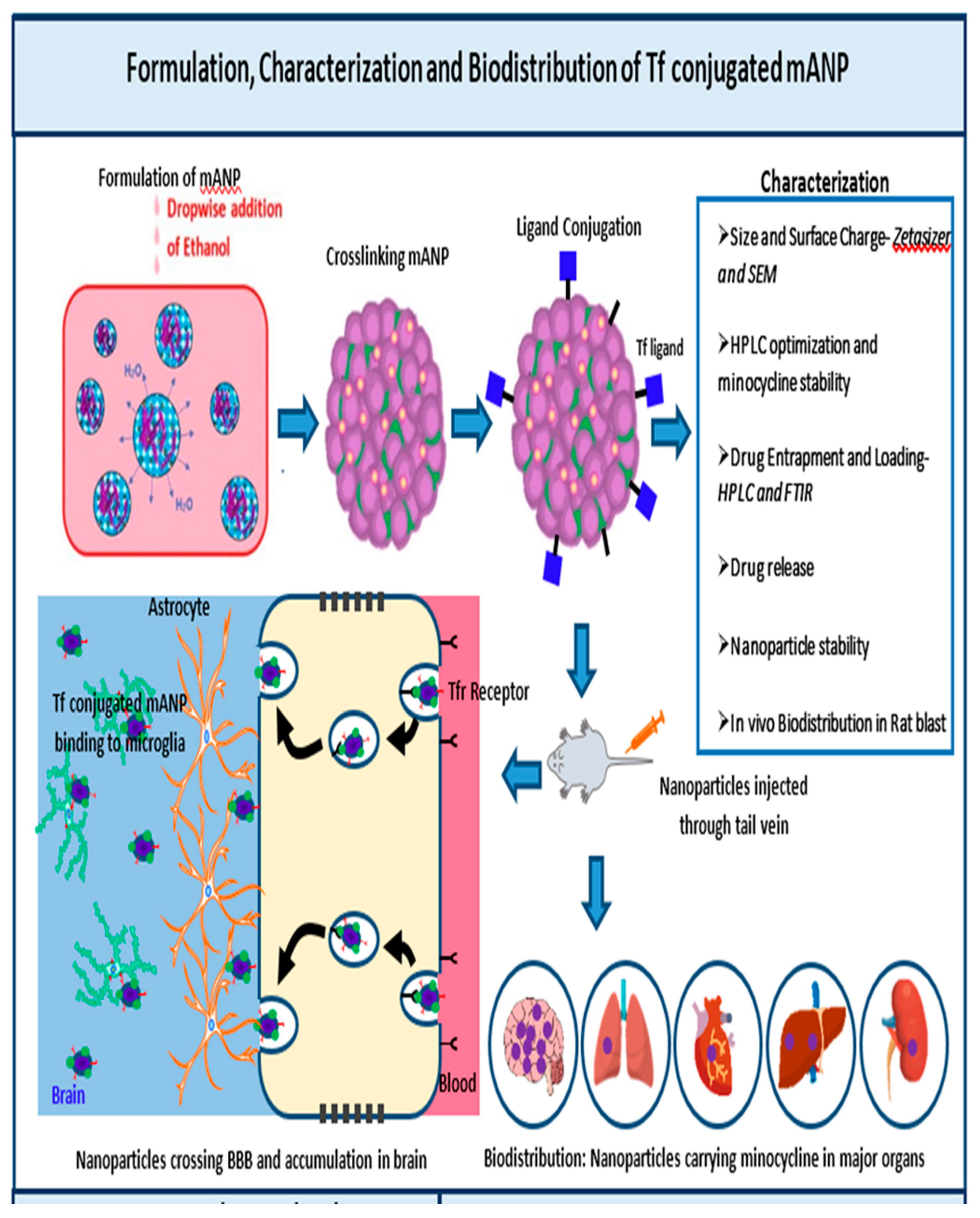
2.2. Formulation of tf Conjugated Minocycline Loaded Albumin Nanoparticle (tf-MANP)
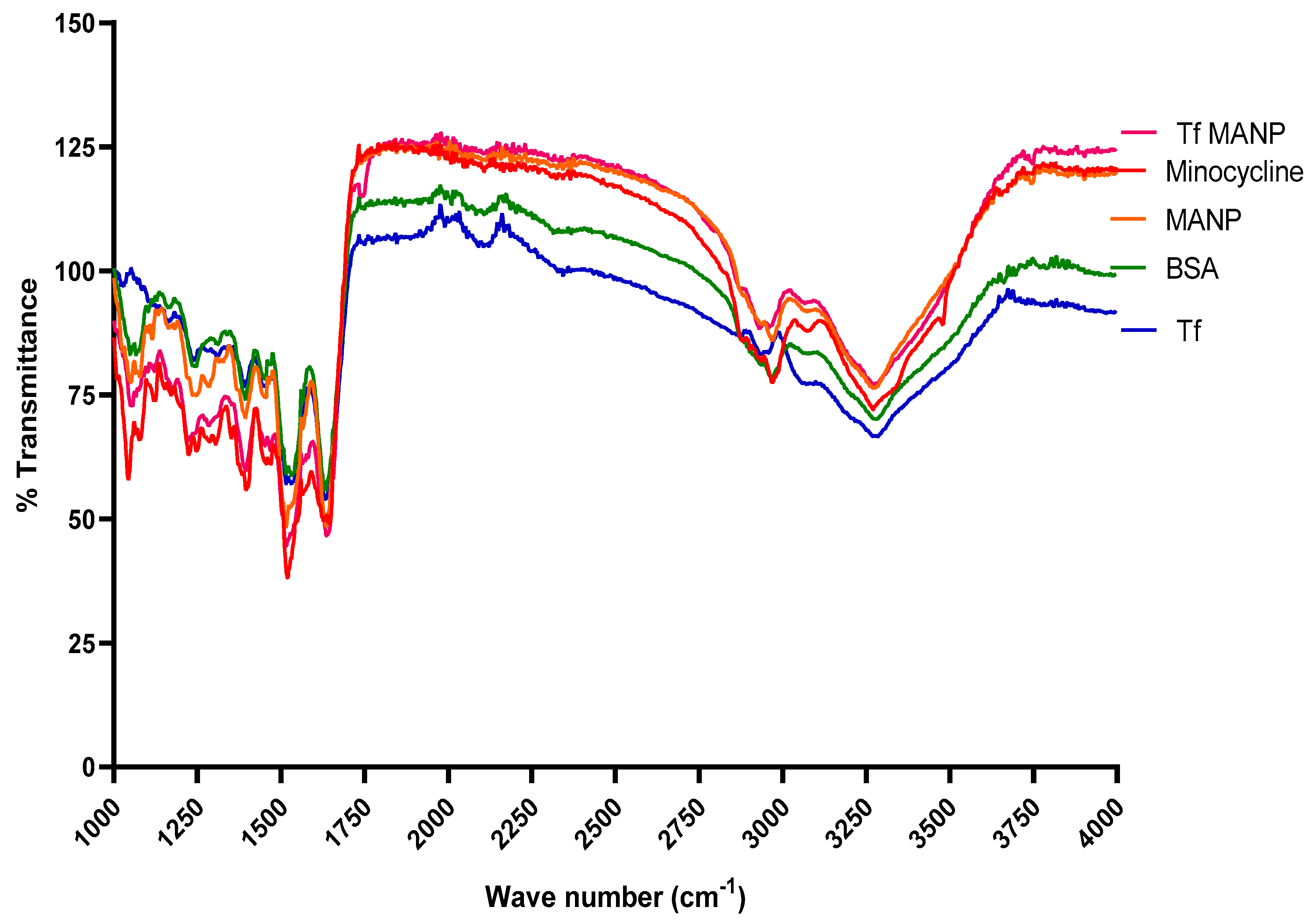
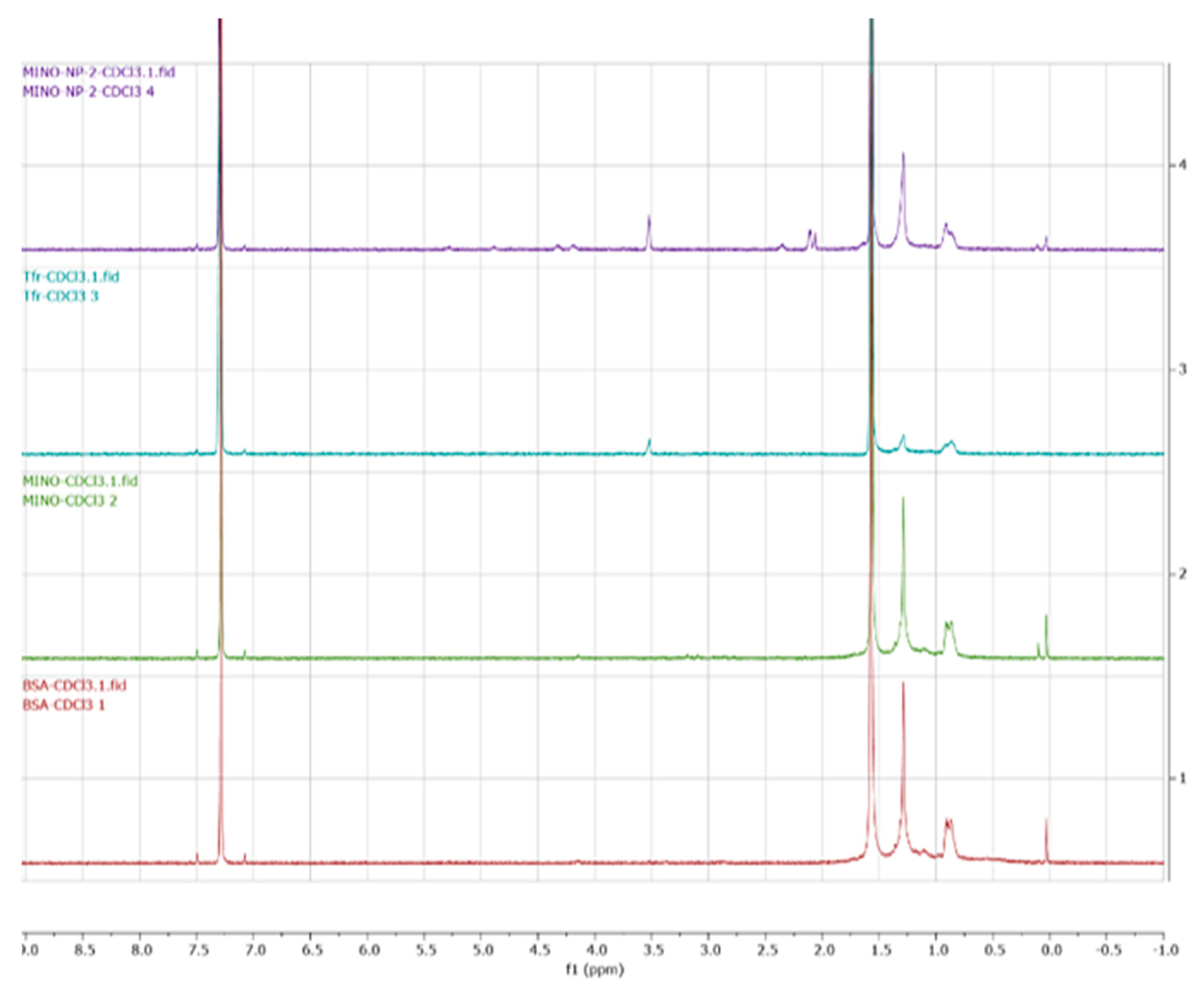
2.3. Characterization of the Nanoparticles
2.4. Entrapment Efficiency and Loading of Minocycline in tf Conjugated MANP
2.5. In Vitro Minocycline Release from Nanoparticle
2.6. Stability of tf Conjugated MANP
2.7. Neuroprotection, In Vivo Biodistribution and Toxicity of Targeted tf-MANP in Blast TBI Rat Model
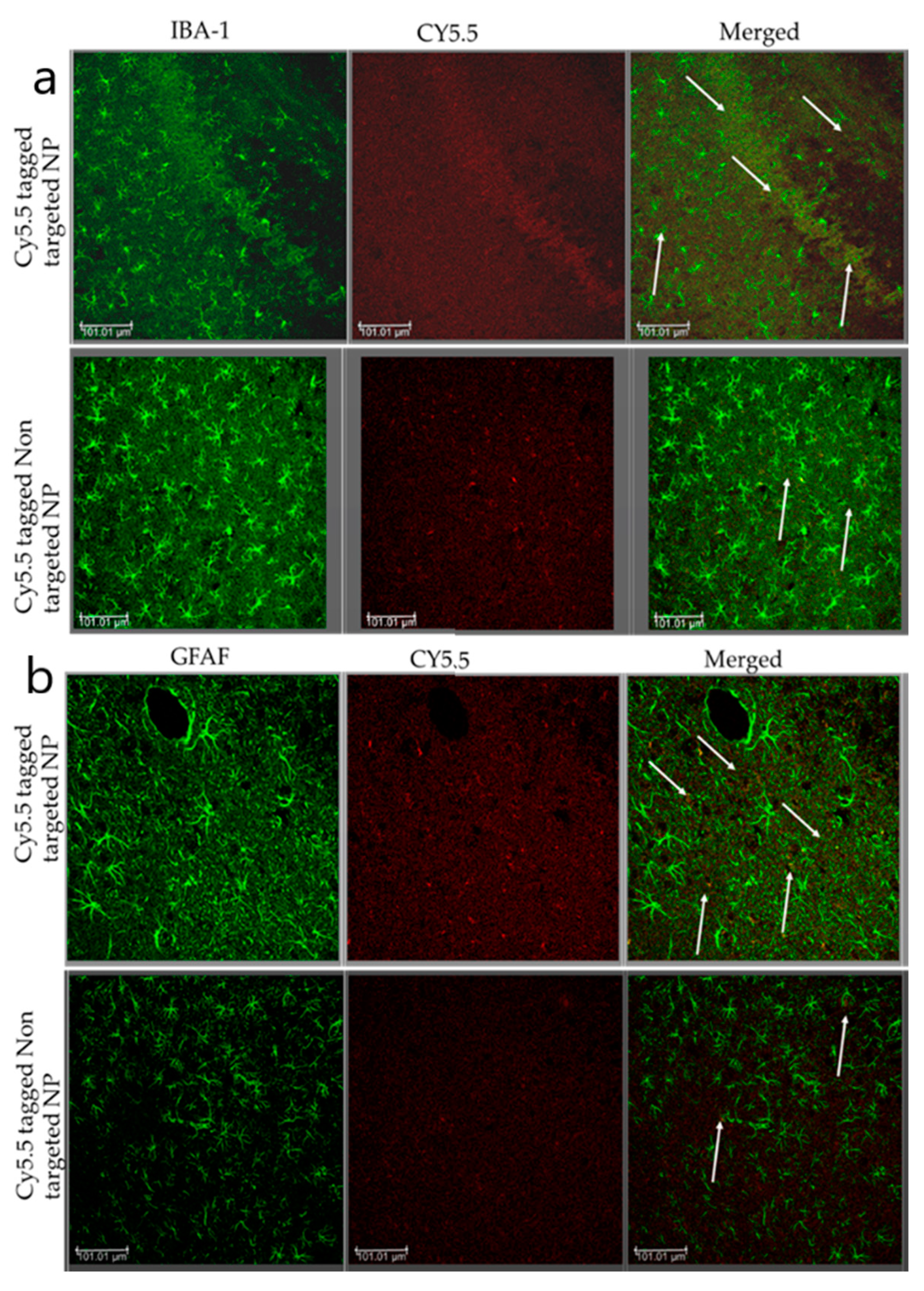
Localization of Nanoparticle: Immunofluorescence Staining
2.8. Behavioral and Neuroprotection Study
2.8.1. Novel Object Recognition (NOR) Test
2.8.2. In Vivo Toxicity of Minocycline, Non-Targeted MANP and Targeted tfMANP
2.8.3. Body Weight Examinations
2.8.4. Gross Observable Behavioral Effects
2.8.5. Hematoxylin and Eosin (H&E) Staining
2.9. Statistical Analysis
3. Results and Discussion
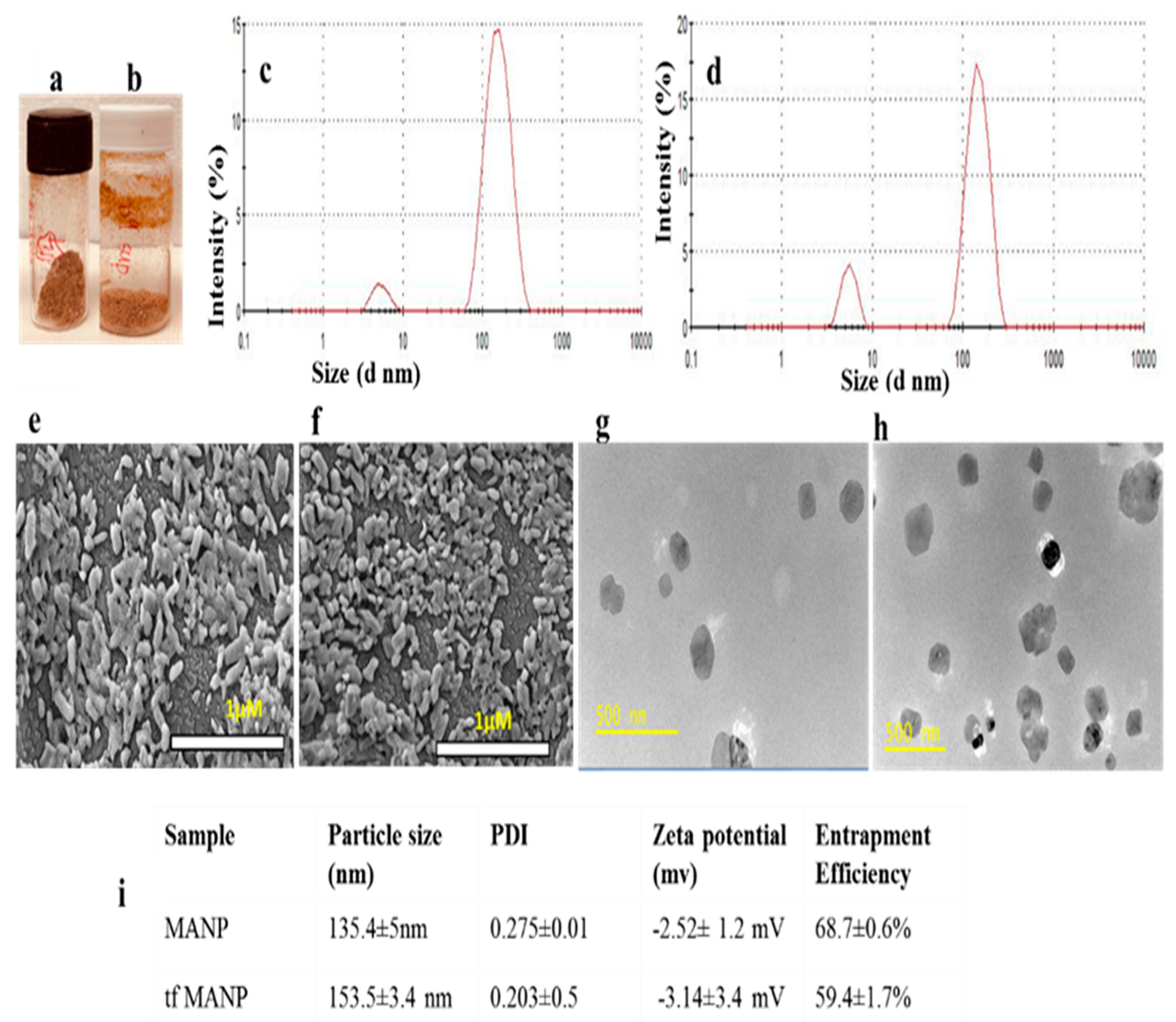
3.1. Minocycline-Loaded Albumin Nanoparticles (MANP)
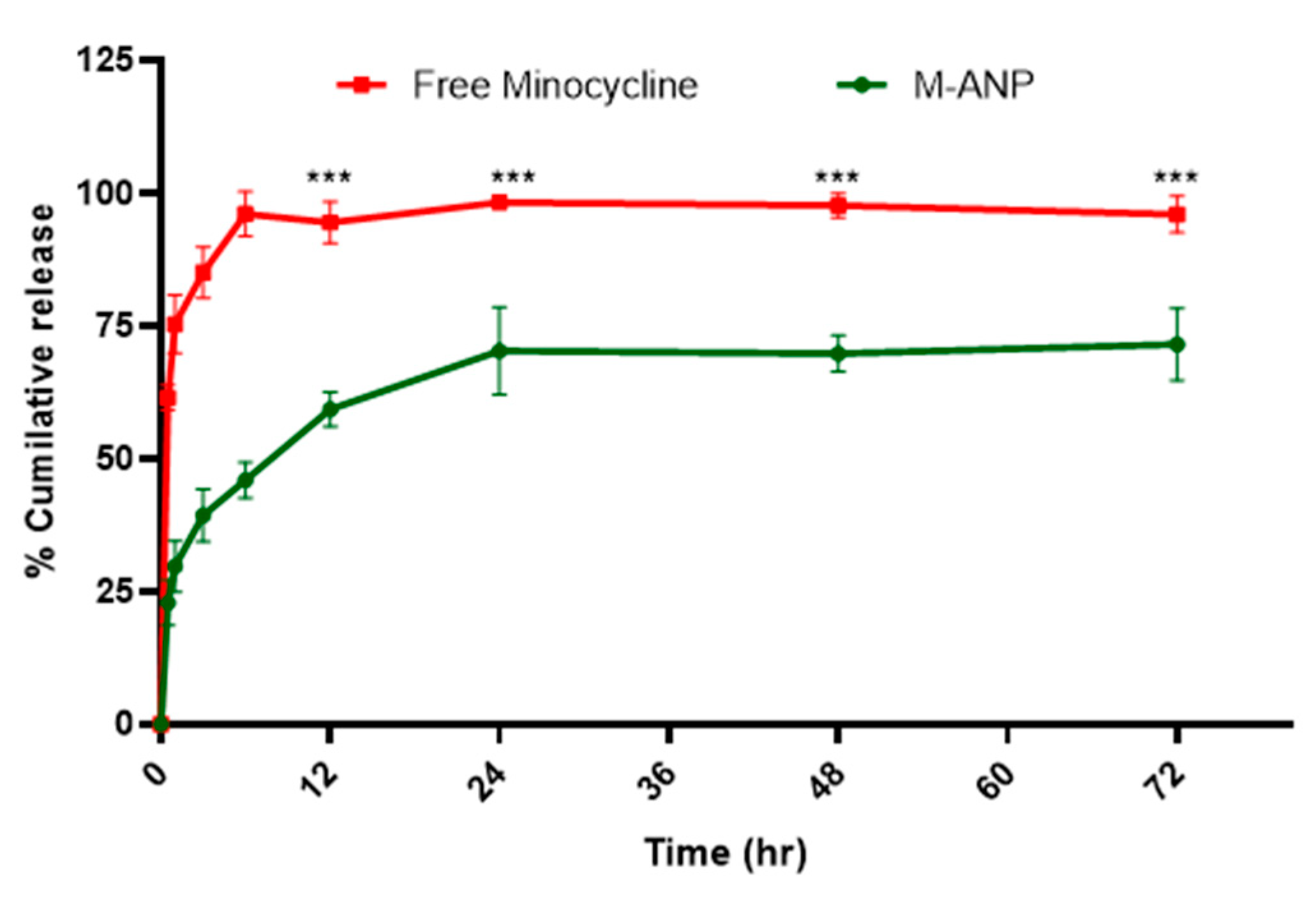
3.2. Entrapment Efficiency and In Vitro Release Study
3.3. Stability Study
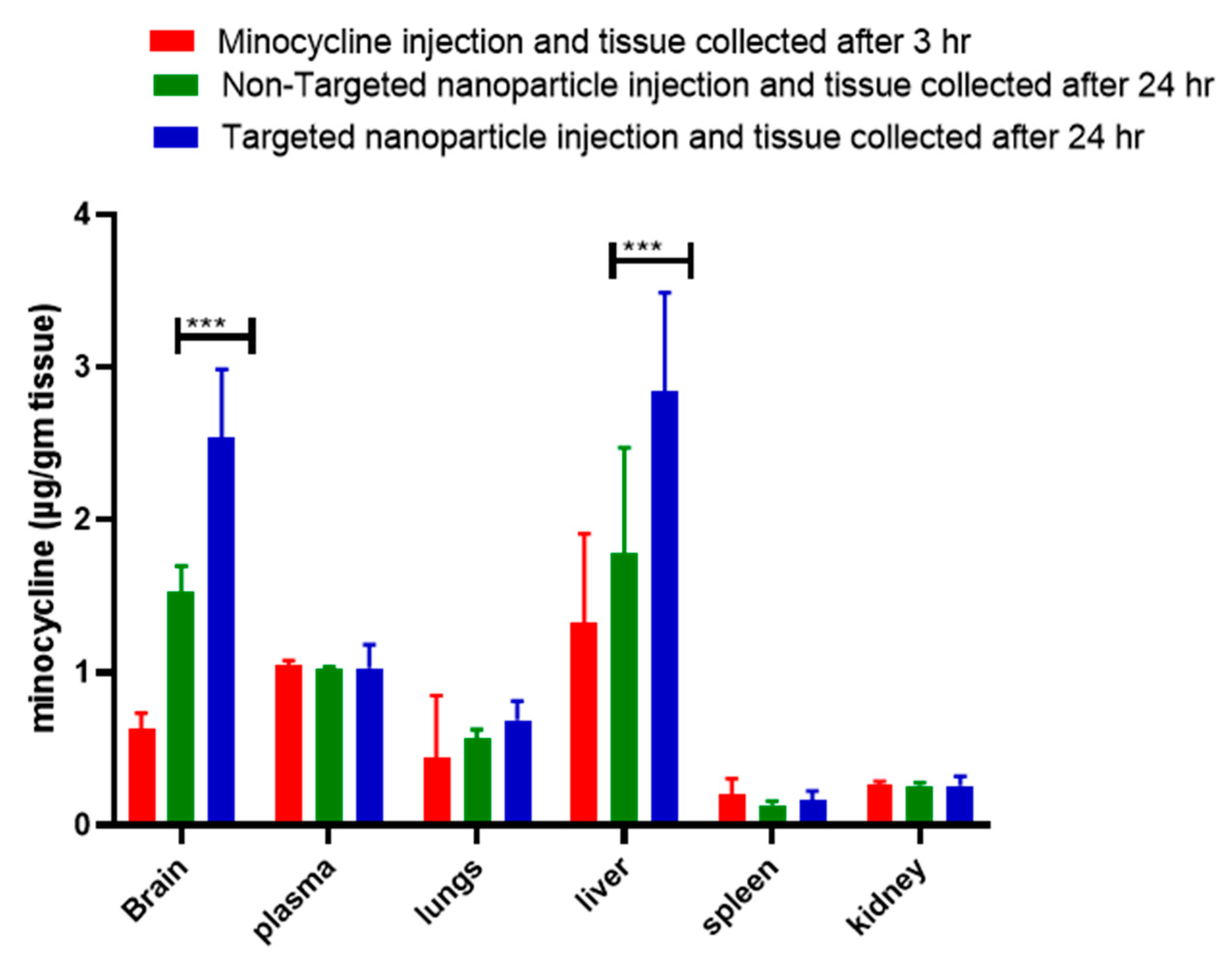
3.4. Enhanced Biodistribution of Nanoparticle/Minocycline in bTBI Rat Model
3.5. Behavior, Neuroprotection and Toxicity Analysis of Minocycline and Its Nano-Formulations
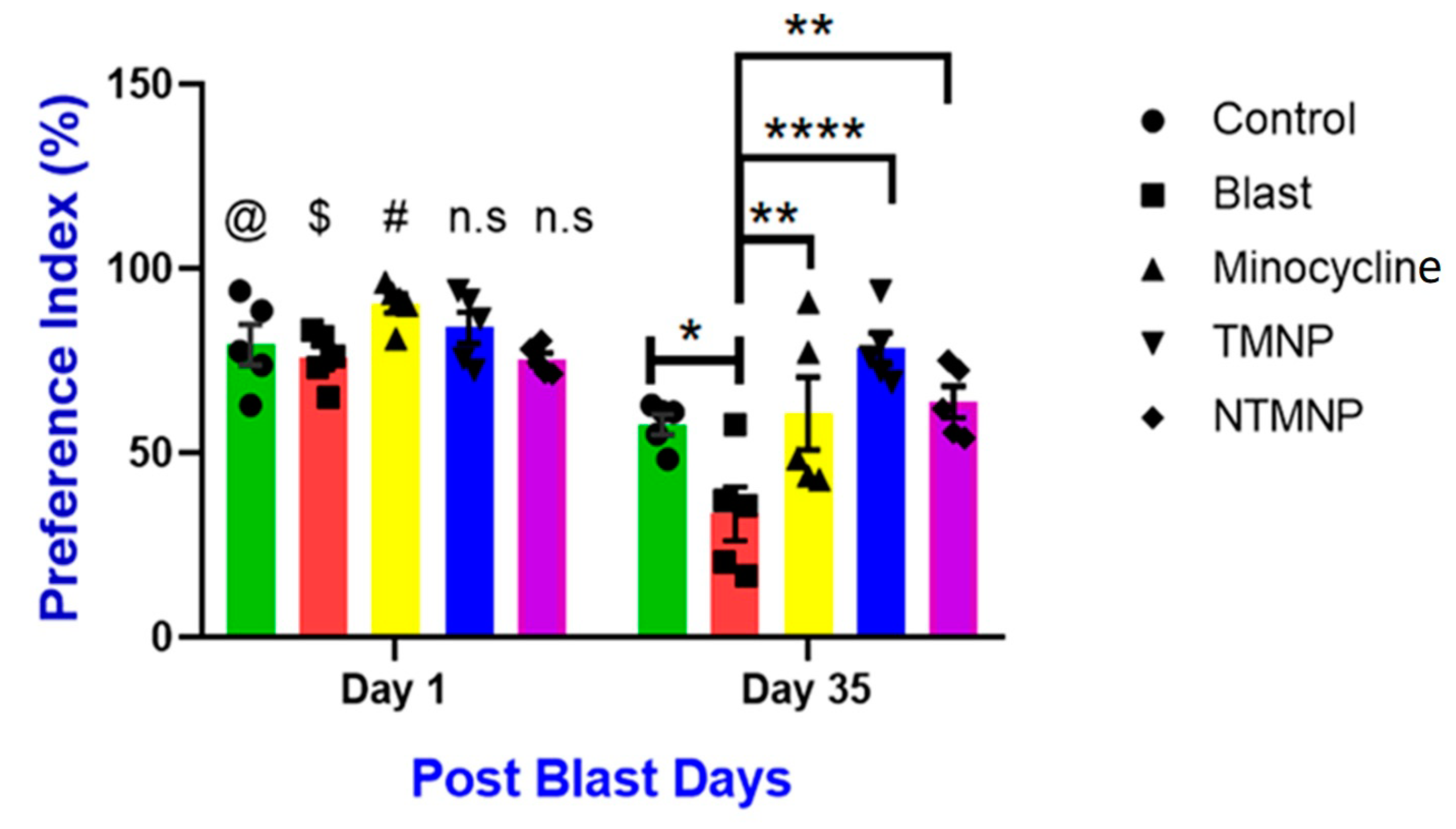
Free, Targeted and Non-Targeted Minocycline Protected against Moderate Blast-Induced Chronic Memory Impairments
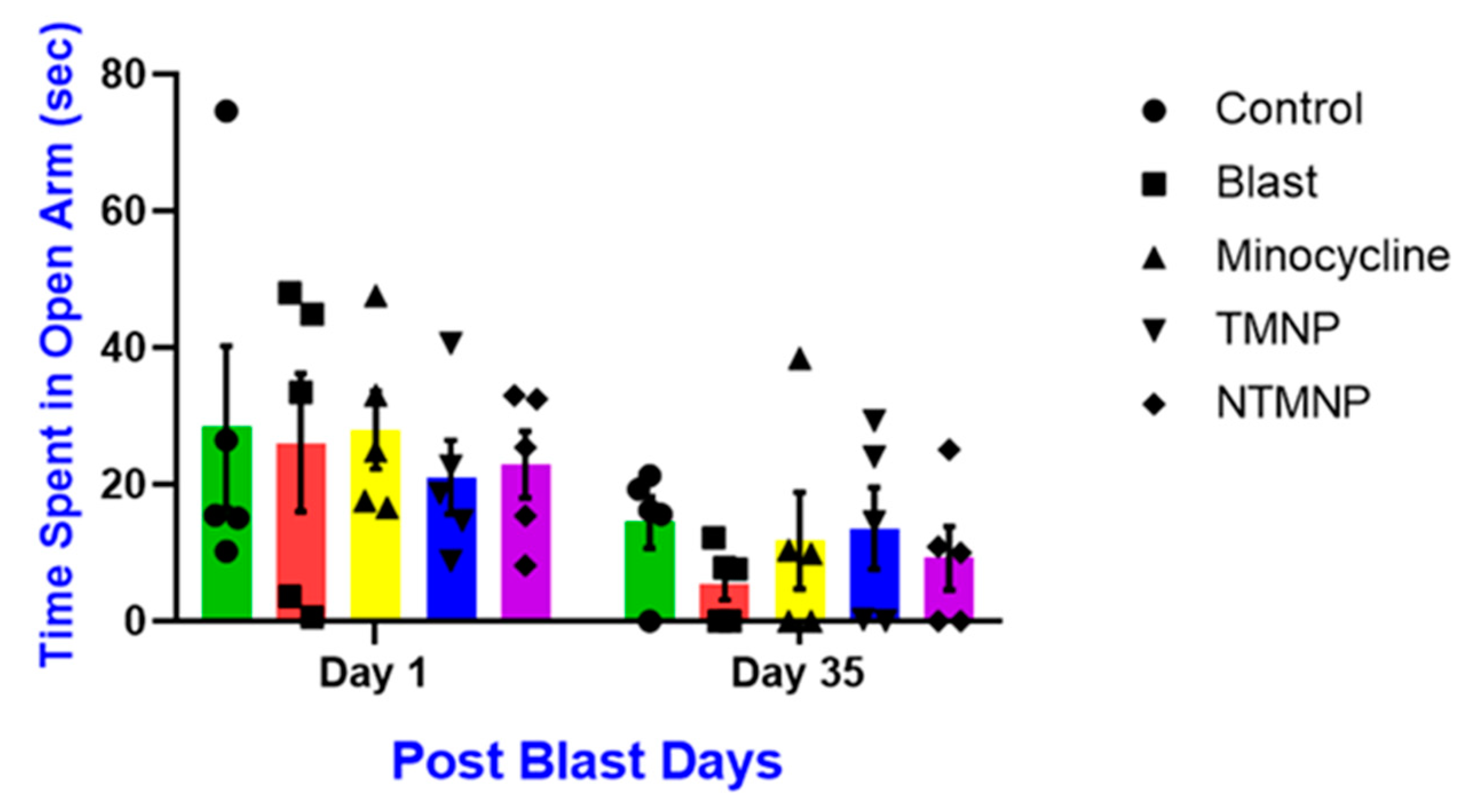
3.6. Free, Targeted and Non-Targeted Minocycline Reduced Moderate Blast-Induced Chronic Anxiety
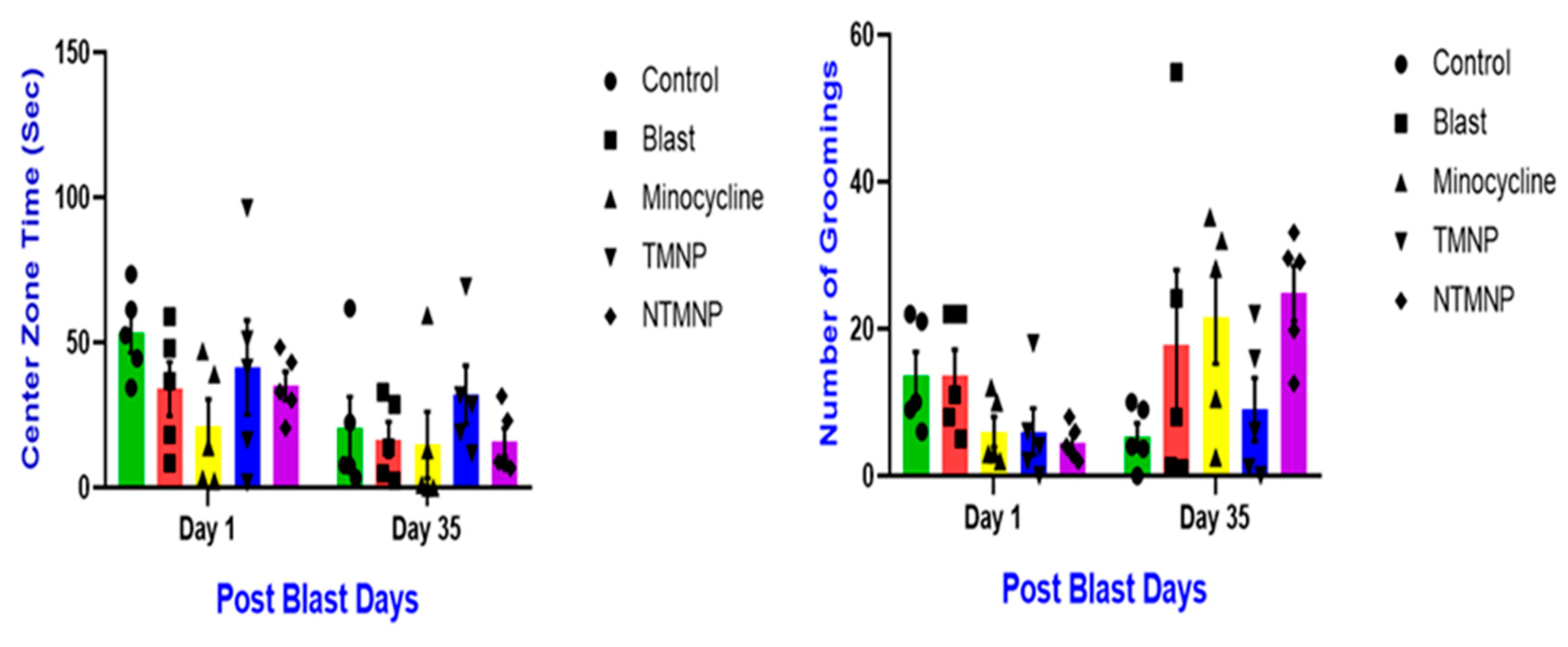
3.7. Free, Targeted and Non-Targeted Minocycline Reduced Moderate Blast Induced Gross Motor Movements, Exploratory Skills and Chronic Anxiety
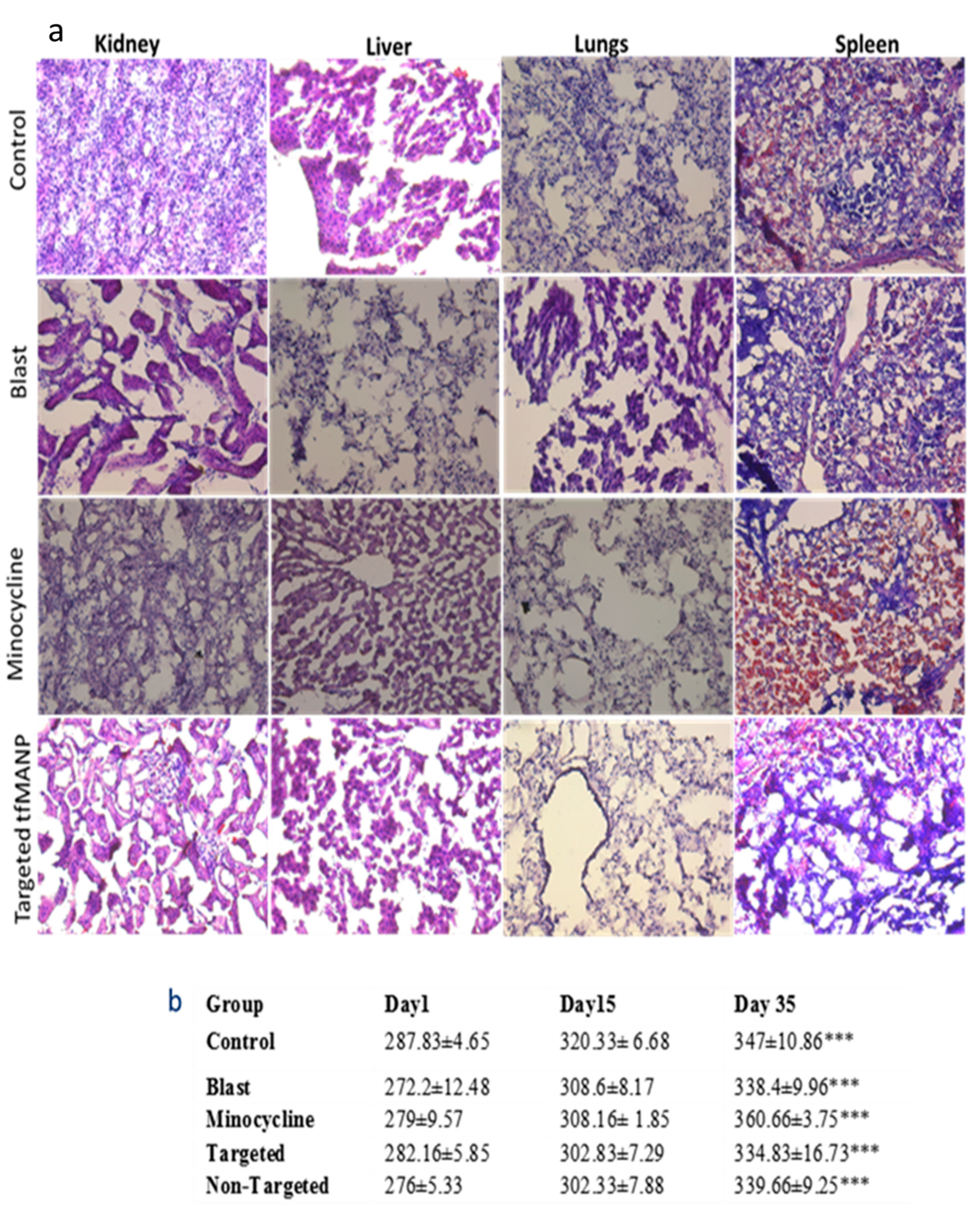
3.8. Toxicity of Minocycline and Nanoparticle
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prins, M.L.; Alexander, D.; Giza, C.C.; Hovda, D.A. Repeated mild traumatic brain injury: Mechanisms of cerebral vulnerability. J. Neurotrauma 2013, 30, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 2018, 21, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Trotter, B.B.; Robinson, M.E.; Milberg, W.P.; McGlinchey, R.E.; Salat, D.H. Military Blast Exposure, Ageing and White Matter Integrity. Brain 2015, 138, 2278–2292. [Google Scholar] [CrossRef]
- Ling, G.S.; Ecklund, J.M. Traumatic Brain Injury in Modern War. Curr. Opin. Anesthesiol. 2011, 24, 124–130. [Google Scholar] [CrossRef]
- Mathews, Z.R.; Koyfman, A. Blast Injuries. J. Emerg. Med. 2015, 49, 573–587. [Google Scholar] [CrossRef]
- Moore, B. Blast Injuries-a Prehospital Perspective. Australas. J. Paramed. 2015, 4. [Google Scholar] [CrossRef]
- Abbott, B.P.; Abbott, R.; Abbott, T.D.; Abernathy, M.R.; Acernese, F.; Ackley, K.; Adams, C.; Adams, T.; Addesso, P.; Adhikari, R.X.; et al. Tests of General Relativity with GW150914. Phys. Rev. Lett. 2016, 116, 221101. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Faden, A.I. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010, 31, 596–604. [Google Scholar] [CrossRef]
- Maas, A.I.; Roozenbeek, B.; Manley, G.T. Clinical trials in traumatic brain injury: Past experience and current developments. Neurotherapeutics 2010, 7, 115–126. [Google Scholar] [CrossRef]
- Kovesdi, E.; Kamnaksh, A.; Wingo, D.; Ahmed, F.; Grunberg, N.E.; Long, J.B.; Kasper, C.E.; Agoston, D.V. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front. Neurol. 2012, 3, 111. [Google Scholar] [CrossRef]
- Brenza, T.M.; Ghaisas, S.; Ramirez, J.E.V.; Harischandra, D.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G.; Narasimhan, B. Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy. Nanomedicine 2017, 13, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Brenza, T.M.; Schlichtmann, B.W.; Bhargavan, B.; Vela Ramirez, J.E.; Nelson, R.D.; Panthani, M.G.; McMillan, J.M.; Kalyanaraman, B.; Gendelman, H.E.; Anantharam, V.; et al. Biodegradable polyanhydride-based nanomedicines for blood to brain drug delivery. J. Biomed. Mater. Res. A 2018, 106, 2881–2890. [Google Scholar] [CrossRef]
- Utari, A.; Chonchaiya, W.; Rivera, S.M.; Schneider, A.; Hagerman, R.J.; Faradz, S.M.H.; Ethell, I.M.; Nguyen, D.V. Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am. J. Intellect. Dev. Disabil. 2010, 115, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.F.; Maganti, R.K. Neurotoxic effects associated with antibiotic use: Management considerations. Br. J. Clin. Pharmacol. 2011, 72, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Wrightson, W.R.; Myers, S.R.; Galandiuk, S. Analysis of minocycline by high-performance liquid chromatography in tissue and serum. J. Chromatogr. B Biomed. Sci. Appl. 1998, 706, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, M.A.J.; Rigdova, K.; Kyriakides, M.; Grier, S.; Lovering, A.M.; Williams, H.; Griffith, D.C.; MacGowan, A. Development, validation and application of a novel HPLC-MS/MS method for the measurement of minocycline in human plasma and urine. J. Pharm. Biomed. Anal. 2019, 169, 90–98. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Elewa, H.F.; Hilali, H.; Hess, D.C.; Machado, L.S.; Fagan, S.C. Minocycline for short-term neuroprotection. Pharmacotherapy 2006, 26, 515–521. [Google Scholar] [CrossRef]
- Cankaya, S.; Cankaya, B.; Kilic, U.; Kilic, E.; Yulug, B. The therapeutic role of minocycline in Parkinson’s disease. Drugs Context 2019, 8, 212553. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, L.; Wang, D.; Wang, Z.; Huang, Q.Y. Minocycline ameliorates hypoxia-induced blood-brain barrier damage by inhibition of HIF-1alpha through SIRT-3/PHD-2 degradation pathway. Neuroscience 2015, 304, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, L.; An, C.; Wang, R.; Yang, L.; Yu, W.; Li, P.; Gao, Y. The blood brain barrier in cerebral ischemic injury—Disruption and repair. Brain Hemorrhages 2020, 304, 250–259. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Sharma, R.; Kim, S.Y.; Sharma, A.; Zhang, Z.; Kambhampati, S.P.; Kannan, S.; Kannan, R.M. Activated Microglia Targeting Dendrimer-Minocycline Conjugate as Therapeutics for Neuroinflammation. Bioconjugate Chem. 2017, 28, 2874–2886. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Formulation, Optimization, in Vivo Pharmacokinetic, Behavioral and Biochemical Estimations of Minocycline Loaded Chitosan Nanoparticles for Enhanced Brain Uptake. Chem. Pharm. Bull. 2013, 61, 258–272. [Google Scholar] [CrossRef]
- Stirling, D.P.; Khodarahmi, K.; Liu, J.; McPhail, L.T.; McBride, C.B.; Steeves, J.D.; Ramer, M.S.; Tetzlaff, W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 2182–2190. [Google Scholar] [CrossRef]
- Smith, K.; Leyden, J. Safety of doxycycline and minocycline: A systematic review. Clin. Ther. 2005, 27, 1329–1342. [Google Scholar] [CrossRef]
- Asadi, A.; Abdi, M.; Kouhsari, E.; Panahi, P.; Sholeh, M.; Sadeghifard, N.; Amiriani, T.; Ahmadi, A.; Maleki, A.; Gholami, M. Minocycline, Focused on mechanisms of resistance, antibacterial activity, and clinical effectiveness; Back to Future. J. Glob. Antimicrob. Resist. 2020, 22, 161–174. [Google Scholar] [CrossRef]
- Maples, D.; McLean, K.; Sahoo, K.; Newhardt, R.; Venkatesan, P.; Wood, B.; Ranjan, A. Synthesis and characterisation of ultrasound imageable heat-sensitive liposomes for HIFU therapy. Int. J. Hyperth. 2015, 31, 674–685. [Google Scholar] [CrossRef]
- Perumal, V.; Banerjee, S.; Das, S.; Sen, R.K.; Mandal, M. Effect of liposomal celecoxib on proliferation of colon cancer cell and inhibition of DMBA-induced tumor in rat model. Cancer Nanotechnol. 2011, 2, 67–79. [Google Scholar] [CrossRef]
- Saravanan, M.; Mostafavi, E.; Vincent, S.; Negash, H.; Andavar, R.; Perumal, V.; Chandra, N.; Narayanasamy, S.; Kalimuthu, K.; Barabadi, H. Nanotechnology-based approaches for emerging and re-emerging viruses: Special emphasis on COVID-19. Microb. Pathog. 2021, 156, 104908. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, M.; Rama Rao, K.V.; Younger, D.; Chandra, N. Temporal and Spatial Effects of Blast Overpressure on Blood-Brain Barrier Permeability in Traumatic Brain Injury. Sci. Rep. 2018, 8, 8681. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.R.; Iring, S.; Younger, D.; Kuriakose, M.; Skotak, M.; Alay, E.; Gupta, R.K.; Chandra, N. A Single Primary Blast-Induced Traumatic Brain Injury in a Rodent Model Causes Cell-Type Dependent Increase in Nicotinamide Adenine Dinucleotide Phosphate Oxidase Isoforms in Vulnerable Brain Regions. J. Neurotrauma 2018, 35, 2077–2090. [Google Scholar] [CrossRef]
- Mizutani, T.; Ishizaka, A.; Nihei, C. Transferrin Receptor 1 Facilitates Poliovirus Permeation of Mouse Brain Capillary Endothelial Cells. J. Biol. Chem. 2016, 291, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, K.; Hoffmann, M.M.; Dreis, S.; Herbert, E.; Alyautdin, R.N.; Michaelis, M.; Kreuter, J.; Langer, K. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J. Pharmacol. Exp. Ther. 2006, 317, 1246–1253. [Google Scholar] [CrossRef]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; von Briesen, H.; Kreuter, J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood–Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- Lu, C.T.; Zhao, Y.Z.; Wong, H.L.; Cai, J.; Peng, L.; Tian, X.Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014, 9, 2241–2257. [Google Scholar] [CrossRef] [PubMed]
- Siegal, T. Which drug or drug delivery system can change clinical practice for brain tumor therapy? Neuro-Oncology 2013, 15, 656–669. [Google Scholar] [CrossRef] [PubMed]
- An, F.F.; Zhang, X.H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.R.; Weiss, C.K. Nanoparticles from renewable polymers. Front. Chem. 2014, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric nanoparticles: Potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum. Vaccin Immunother. 2014, 10, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Sivakumar, P.M.; Zarrabi, A.; Muthupandian, S.; Vijayaraghavalu, S.; Sahoo, K.; Das, A.; Das, S.; Payyappilly, S.S.; Das, S. Near infra-red polymeric nanoparticle based optical imaging in Cancer diagnosis. J. Photochem. Photobiol. B Biol. 2019, 199, 111630. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Nitta, S.K.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Prasad, M.; Lambe, U.P.; Brar, B.; Shah, I.; Manimegalai, J.; Ranjan, K.; Rao, R.; Kumar, S.; Mahant, S.; Khurana, S.K.; et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018, 97, 1521–1537. [Google Scholar] [CrossRef]
- Lee, E.; Youn, Y. Albumin-based potential drugs: Focus on half-life extension and nanoparticle preparation. J. Pharm. Investig. 2016, 46, 305–315. [Google Scholar] [CrossRef]
- Raval, N.; Mistry, T.; Acharya, N.; Acharya, S. Development of glutathione-conjugated asiatic acid-loaded bovine serum albumin nanoparticles for brain-targeted drug delivery. J. Pharm. Pharmacol. 2015, 67, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Martín, M. nab-Paclitaxel dose and schedule in breast cancer. Breast Cancer Res. 2015, 17, 81. [Google Scholar] [CrossRef]
- Kazim, S.; Malafa, M.P.; Coppola, D.; Husain, K.; Zibadi, S.; Kashyap, T.; Crochiere, M.; Landesman, Y.; Rashal, T.; Sullivan, D.M.; et al. Selective Nuclear Export Inhibitor KPT-330 Enhances the Antitumor Activity of Gemcitabine in Human Pancreatic Cancer. Mol. Cancer Ther. 2015, 14, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Drug targeting. Eur. J. Pharm. Sci. 2000, 11, 81–91. [Google Scholar] [CrossRef]
- Li, J.M.; Chen, W.; Wang, H.; Jin, C.; Yu, X.J.; Lu, W.Y.; Cui, L.; Fu, D.L.; Ni, Q.X.; Hou, H.M. Preparation of albumin nanospheres loaded with gemcitabine and their cytotoxicity against BXPC-3 cells in vitro. Acta Pharmacol. Sin. 2009, 30, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Motevalli, S.M.; Eltahan, A.S.; Liu, L.; Magrini, A.; Rosato, N.; Guo, W.; Bottini, M.; Liang, X.-J. Co-encapsulation of curcumin and doxorubicin in albumin nanoparticles blocks the adaptive treatment tolerance of cancer cells. Biophys. Rep. 2019, 5, 19–30. [Google Scholar] [CrossRef]
- Mahobia, S.; Bajpai, J.; Bajpai, A.K. An In-vitro Investigation of Swelling Controlled Delivery of Insulin from Egg Albumin Nanocarriers. Iran. J. Pharm. Res. 2016, 15, 695–711. [Google Scholar]
- Bronze-Uhle, E.S.; Costa, B.C.; Ximenes, V.F.; Lisboa-Filho, P.N. Synthetic nanoparticles of bovine serum albumin with entrapped salicylic acid. Nanotechnol. Sci. Appl. 2016, 10, 11–21. [Google Scholar] [CrossRef]
- Kuriakose, M.; Younger, D.; Ravula, A.R.; Alay, E.; Rama Rao, K.V.; Chandra, N. Synergistic Role of Oxidative Stress and Blood-Brain Barrier Permeability as Injury Mechanisms in the Acute Pathophysiology of Blast-induced Neurotrauma. Sci. Rep. 2019, 9, 7717. [Google Scholar] [CrossRef]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm 2018, 2018, 9285854. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Di, Y.; Jin, C.; Fu, D.; Yang, F.; Jiang, Y.; Yao, L.; Hao, S.; Wang, X.; Subedi, S.; et al. Gemcitabine-loaded albumin nanospheres (GEM-ANPs) inhibit PANC-1 cells in vitro and in vivo. Nanoscale Res. Lett. 2013, 8, 176. [Google Scholar] [CrossRef]
- Yadav, K.S.; Sawant, K.K. Modified nanoprecipitation method for preparation of cytarabine-loaded PLGA nanoparticles. AAPS PharmSciTech 2010, 11, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, Y.H.; Kim, Y.J.; Kwon, S.H.; Bang, J.K.; Lee, S.M.; Song, Y.S.; Hahm, D.H.; Shim, I.; Han, D.; et al. Pharmacokinetics and biodistribution of human serum albumin-TIMP-2 fusion protein using near-infrared optical imaging. J. Pharm. Pharm. Sci. 2011, 14, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Wu, M.; Feng, Z.; Deng, Y.; Zhong, C.; Zhao, X. Folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles: Preparation, characterization, bioavailability and targeting of liver tumors. Artif. Cells Nanomed. Biotechnol. 2019, 47, 154–165. [Google Scholar] [CrossRef]
- Bunschoten, A.; Buckle, T.; Kuil, J.; Luker, G.D.; Luker, K.E.; Nieweg, O.E.; van Leeuwen, F.W. Targeted non-covalent self-assembled nanoparticles based on human serum albumin. Biomaterials 2012, 33, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K.; Gao, W.; Patel, S.D.; Siddiqui, Z.; Weiner, S.; Shimizu, E.; Sarkar, B.; Kumar, V.A. Self-Assembly of a Dentinogenic Peptide Hydrogel. ACS Omega 2018, 3, 5980–5987. [Google Scholar] [CrossRef]
- Zaman, R.U.; Mulla, N.S.; Braz Gomes, K.; D’Souza, C.; Murnane, K.S.; D’Souza, M.J. Nanoparticle formulations that allow for sustained delivery and brain targeting of the neuropeptide oxytocin. Int. J. Pharm. 2018, 548, 698–706. [Google Scholar] [CrossRef]
- Kim, K.K.; Siddiqui, Z.; Patel, M.; Sarkar, B.; Kumar, V.A. A self-assembled peptide hydrogel for cytokine sequestration. J. Mater. Chem. B 2020, 8, 945–950. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, H.K.; Kim, H.; Lee, W.T.; Lee, E.S.; Oh, K.T.; Choi, H.G.; Youn, Y.S. Hyperthermal paclitaxel-bound albumin nanoparticles co-loaded with indocyanine green and hyaluronidase for treating pancreatic cancers. Arch. Pharm Res. 2021, 44, 182–193. [Google Scholar] [CrossRef]
- Venkatesan, P.; Puvvada, N.; Dash, R.; Prashanth Kumar, B.N.; Sarkar, D.; Azab, B.; Pathak, A.; Kundu, S.C.; Fisher, P.B.; Mandal, M. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials 2011, 32, 3794–3806. [Google Scholar] [CrossRef]
- Sebak, S.; Mirzaei, M.; Malhotra, M.; Kulamarva, A.; Prakash, S. Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: Preparation and in vitro analysis. Int. J. Nanomed. 2010, 5, 525–532. [Google Scholar]
- Howard, M.D.; Lu, X.; Jay, M.; Dziubla, T.D. Optimization of the lyophilization process for long-term stability of solid-lipid nanoparticles. Drug Dev. Ind. Pharm. 2012, 38, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Murugan, M.; Ravula, A.; Gandhi, A.; Vegunta, G.; Mukkamalla, S.; Mujib, W.; Chandra, N. Chemokine signaling mediated monocyte infiltration affects anxiety-like behavior following blast injury. Brain Behav. Immun. 2020, 88, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Ravula, A.R.; Shao, N.; Chandra, N. Effect of minocycline and its nano-formulation on central auditory system in blast-induced hearing loss rat model. J. Otol. 2022, 18, 38–48. [Google Scholar] [CrossRef]
- Kay, G.W.; Palmer, D.N. Chronic oral administration of minocycline to sheep with ovine CLN6 neuronal ceroid lipofuscinosis maintains pharmacological concentrations in the brain but does not suppress neuroinflammation or disease progression. J. Neuroinflammation 2013, 10, 97. [Google Scholar] [CrossRef]
- Pijpers, A.; Schoevers, E.J.; Haagsma, N.; Verheijden, J.H. Plasma levels of oxytetracycline, doxycycline, and minocycline in pigs after oral administration in feed. J. Anim. Sci. 1991, 69, 4512–4522. [Google Scholar] [CrossRef]
- Baratta, J.L.; Ngo, A.; Lopez, B.; Kasabwalla, N.; Longmuir, K.J.; Robertson, R.T. Cellular organization of normal mouse liver: A histological, quantitative immunocytochemical, and fine structural analysis. Histochem. Cell Biol. 2009, 131, 713–726. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.Y.; Shen, S.; Wang, J. Protecting neurons from cerebral ischemia/reperfusion injury via nanoparticle-mediated delivery of an siRNA to inhibit microglial neurotoxicity. Biomaterials 2018, 161, 95–105. [Google Scholar] [CrossRef]
- Ravula, A.R.; Rodriguez, J.; Younger, D.; Perumal, V.; Shao, N.; Rama Rao, K.V.; Pfister, B.; Chandra, N. Animal model of repeated low-level blast traumatic brain injury displays acute and chronic neurobehavioral and neuropathological changes. Exp. Neurol. 2022, 349, 113938. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, C.; Fu, Y.; Chen, T.; Liu, X.; Zhang, Z.; Gong, T. Targeted delivery of hyaluronic acid nanomicelles to hepatic stellate cells in hepatic fibrosis rats. Acta Pharm. Sin. B 2020, 10, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D. Evaluation of safety and efficacy of brain targeted chitosan nanoparticles of minocycline. Int. J. Biol. Macromol. 2013, 59, 20–28. [Google Scholar] [CrossRef]
- Chen, T.Y.; Chen, M.R.; Liu, S.W.; Lin, J.Y.; Yang, Y.T.; Huang, H.Y.; Chen, J.K.; Yang, C.S.; Lin, K.M. Assessment of Polyethylene Glycol-Coated Gold Nanoparticle Toxicity and Inflammation In Vivo Using NF-κB Reporter Mice. Int. J. Mol. Sci. 2020, 21, 8158. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.W.; Rink, J.S.; Jiang, Q.; Kelly, M.E.; Vercammen, J.M.; Thaxton, C.S.; Kibbe, M.R. Systemically administered collagen-targeted gold nanoparticles bind to arterial injury following vascular interventions. Physiol. Rep. 2017, 5, e13128. [Google Scholar] [CrossRef]
- Li, X.; Guo, X.; Cheng, Y.; Zhao, X.; Fang, Z.; Luo, Y.; Xia, S.; Feng, Y.; Chen, J.; Yuan, W.E. pH-Responsive Cross-Linked Low Molecular Weight Polyethylenimine as an Efficient Gene Vector for Delivery of Plasmid DNA Encoding Anti-VEGF-shRNA for Tumor Treatment. Front. Oncol. 2018, 8, 354. [Google Scholar] [CrossRef]
- Nan, A.; Bai, X.; Son, S.J.; Lee, S.B.; Ghandehari, H. Cellular uptake and cytotoxicity of silica nanotubes. Nano Lett. 2008, 8, 2150–2154. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Wen, X.; Zhou, S.; Tong, X.; Su, P.; Li, H.; Shi, D. Anti-tumor activity of paclitaxel-loaded chitosan nanoparticles: An in vitro study. Mater. Sci. Eng. C 2008, 29, 2392–2397. [Google Scholar] [CrossRef]
- Katanasaka, Y.; Ida, T.; Asai, T.; Shimizu, K.; Koizumi, F.; Maeda, N.; Baba, K.; Oku, N. Antiangiogenic cancer therapy using tumor vasculature-targeted liposomes encapsulating 3-(3,5-dimethyl-1H-pyrrol-2-ylmethylene)-1,3-dihydro-indol-2-one, SU5416. Cancer Lett. 2008, 270, 260–268. [Google Scholar] [CrossRef]
- Niknejad, H.; Mahmoudzadeh, R. Comparison of Different Crosslinking Methods for Preparation of Docetaxel-loaded Albumin Nanoparticles. Iran. J. Pharm. Res. 2015, 14, 385–394. [Google Scholar]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. BioMed Res. Int. 2014, 2014, 12. [Google Scholar] [CrossRef]
- Janakiraman, K.; Krishnaswami, V.; Sethuraman, V.; Natesan, S.; Rajendran, V.; Kandasamy, R. Development of Methotrexate and Minocycline-Loaded Nanoparticles for the Effective Treatment of Rheumatoid Arthritis. AAPS PharmSciTech 2019, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Ribeiro, I.A.C.; Alves, M.M.; Gonçalves, L.; Almeida, A.J.; Grenho, L.; Fernandes, M.H.; Santos, C.F.; Gomes, P.S.; Bettencourt, A.F. Understanding intracellular trafficking and anti-inflammatory effects of minocycline chitosan-nanoparticles in human gingival fibroblasts for periodontal disease treatment. Int. J. Pharm. 2019, 572, 118821. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.C.; Pinto, R.V.; Bettencourt, A.F. Easy-Assessment of Levofloxacin and Minocycline in Relevant Biomimetic Media by HPLC-UV Analysis. J. Chromatogr. Sci. 2017, 55, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Bochkova, O.; Mustafina, A.; Fedorenko, S.; Konovalov, A. Silica nanoparticles with a substrate switchable luminescence. Proc. J. Phys. Conf. Ser. 2011, 291, 012038. [Google Scholar] [CrossRef]
- Yen, F.L.; Wu, T.H.; Tzeng, C.W.; Lin, L.T.; Lin, C.C. Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antihepatoma activities. J. Agric. Food Chem. 2010, 58, 7376–7382. [Google Scholar] [CrossRef]
- Lorenzer, C.; Dirin, M.; Winkler, A.; Baumann, V.; Winkler, J. Going Beyond the Liver: Progress and Challenges of Targeted Delivery of siRNA Therapeutics. J Control Release. 2015, 203, 1–15. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nagasaki, Y.; Kato, Y.; Sugiyama, Y.; Kataoka, K. Long-circulating poly(ethylene glycol)-poly(D,L-lactide) block copolymer micelles with modulated surface charge. J. Control. Release 2001, 77, 27–38. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.S.; Shin, K.Y.; Kim, E.M.; Kim, M.; Kim, H.S.; Park, C.H.; Jeong, Y.H.; Yoo, J.; Lee, J.P.; et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2007, 32, 2393–2404. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Xu, F.; Previti, M.L.; Davis, J.; Grande, A.M.; Robinson, J.K.; Van Nostrand, W.E. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J. Neurosci. 2007, 27, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, L.; Wang, Q.; Zhang, D.; Zhao, Q.; Zhang, J.; Xie, L.; Liu, G.; You, Z. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology 2019, 107, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Sajja, V.; Vandevord, P.J.; Lee, Y.W. Blast Induces Oxidative Stress, Inflammation, Neuronal Loss and Subsequent Short-Term Memory Impairment in Rats. Neuroscience 2013, 253, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.A.; Kim, J.H.; Situ, R.; Taylor, W.; Westmoreland, T.; Du, F.; Parks, S.; Ling, G.; Hwang, J.Y.; Rapuano, A.; et al. Neuronal and Glial Changes in The Brain Resulting From Explosive Blast In An Experimental Model. Acta Neuropathol. Commun. 2016, 4, 124. [Google Scholar] [CrossRef]
- Park, E.; Eisen, R.; Kinio, A.; Baker, A.J. Electrophysiological white matter dysfunction and association with neurobehavioral deficits following low-level primary blast trauma. Neurobiol. Dis. 2013, 52, 150–159. [Google Scholar] [CrossRef]
- Jain, D.; Basniwal, P.K. ICH guideline practice: Application of validated RP-HPLC-DAD method for determination of tapentadol hydrochloride in dosage form. J. Anal. Sci. Technol. 2013, 4, 9. [Google Scholar] [CrossRef]
- de Oliveira, J.K.; Ronik, D.F.V.; Ascari, J.; Mainardes, R.M.; Khalil, N.M. A stability-indicating high performance liquid chromatography method to determine apocynin in nanoparticles. J. Pharm. Anal 2017, 7, 129–133. [Google Scholar] [CrossRef]
- Valerie, O.; Audran, M.; Gibert, P.; Bougard, G.; Bressolle, F. High-performance liquid chromatographic assay for minocycline in human plasma and parotid saliva. J. Chromatogr. B Biomed. Sci. Appl. 2000, 738, 357–365. [Google Scholar] [CrossRef]
- Nelson, M.; Hillen, W.; Greenwald, R. Tetracyclines in Biology, Chemistry and Medicine; Birkhäuser Basel: Basel, Switzerland, 2001. [Google Scholar]
- Young, J.E.; Matyska, M.T.; Azad, A.K.; Yoc, S.E.; Pesek, J.J. Separation Differences Among Phenyl Hydride, UDC Cholesterol and Bidentate C8 Stationary Phases for Stability Indicating Methods of Tetracyclines: Journal of Liquid Chromatography & Related Technologies. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 926–942. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, V.; Ravula, A.R.; Agas, A.; Gosain, A.; Aravind, A.; Sivakumar, P.M.; I, S.S.; Sambath, K.; Vijayaraghavalu, S.; Chandra, N. Enhanced Targeted Delivery of Minocycline via Transferrin Conjugated Albumin Nanoparticle Improves Neuroprotection in a Blast Traumatic Brain Injury Model. Brain Sci. 2023, 13, 402. https://doi.org/10.3390/brainsci13030402
Perumal V, Ravula AR, Agas A, Gosain A, Aravind A, Sivakumar PM, I SS, Sambath K, Vijayaraghavalu S, Chandra N. Enhanced Targeted Delivery of Minocycline via Transferrin Conjugated Albumin Nanoparticle Improves Neuroprotection in a Blast Traumatic Brain Injury Model. Brain Sciences. 2023; 13(3):402. https://doi.org/10.3390/brainsci13030402
Chicago/Turabian StylePerumal, Venkatesan, Arun Reddy Ravula, Agnieszka Agas, Aakaash Gosain, Aswati Aravind, Ponnurengam Malliappan Sivakumar, Shanmuga Sundari I, Karthik Sambath, Sivakumar Vijayaraghavalu, and Namas Chandra. 2023. "Enhanced Targeted Delivery of Minocycline via Transferrin Conjugated Albumin Nanoparticle Improves Neuroprotection in a Blast Traumatic Brain Injury Model" Brain Sciences 13, no. 3: 402. https://doi.org/10.3390/brainsci13030402
APA StylePerumal, V., Ravula, A. R., Agas, A., Gosain, A., Aravind, A., Sivakumar, P. M., I, S. S., Sambath, K., Vijayaraghavalu, S., & Chandra, N. (2023). Enhanced Targeted Delivery of Minocycline via Transferrin Conjugated Albumin Nanoparticle Improves Neuroprotection in a Blast Traumatic Brain Injury Model. Brain Sciences, 13(3), 402. https://doi.org/10.3390/brainsci13030402








