Qualitative Evaluation of Intracranial Pressure Slopes in Patients Undergoing Brain Death Protocol
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Neuromonitoring
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Collection
2.6. Statistical Analysis
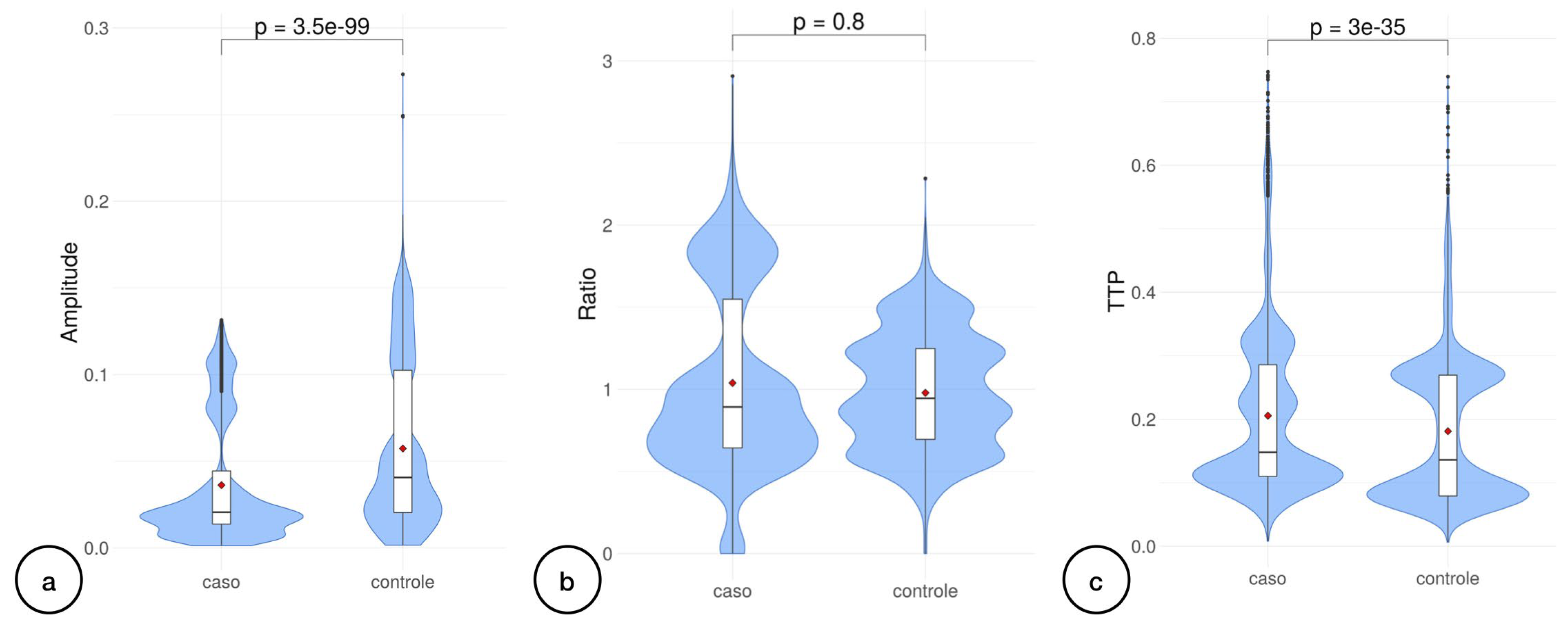
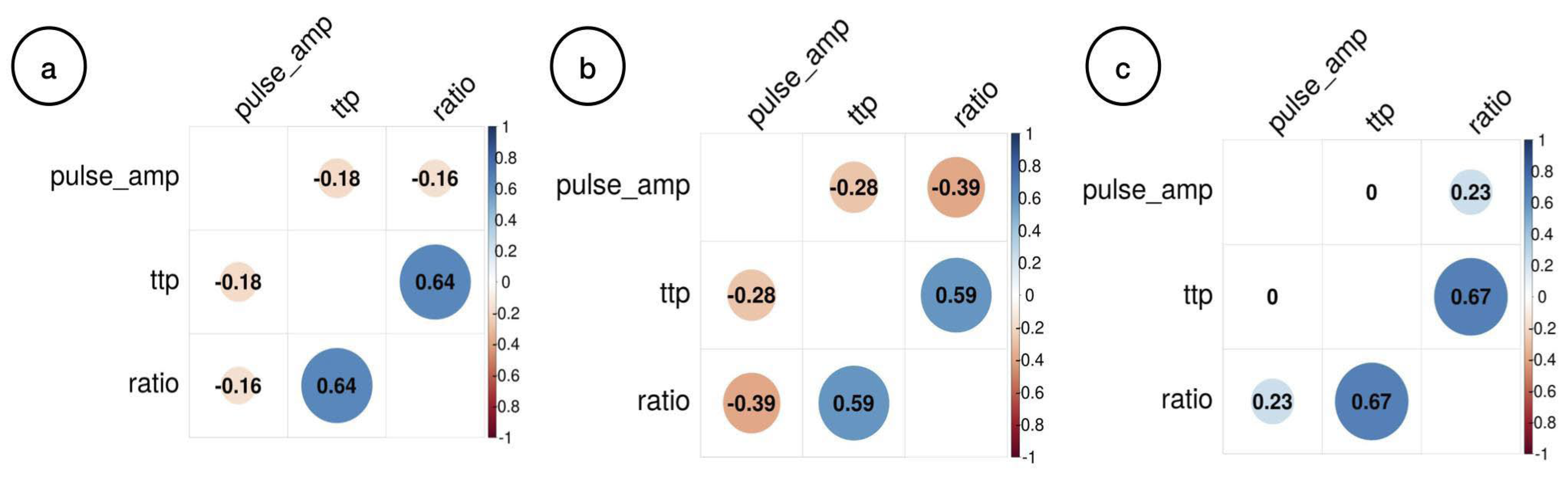
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brasil, S.; de Carvalho Nogueira, R.; de-Lima-Oliveira, M. Determination of Brain Death. JAMA 2021, 325, 493. [Google Scholar] [CrossRef]
- Brasil, S. Brain Death Diagnostic Security is over Organ Donation. Transplant. Proc. 2021, 53, 2415. [Google Scholar] [CrossRef]
- Wijdicks, E.F.; Varelas, P.N.; Gronseth, G.S.; Greer, D.M.; American Academy of Neurology. Evidence-based guideline update: Determining brain death in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010, 74, 1911–1918. [Google Scholar] [CrossRef]
- Brasil, S. Mistaken concepts on the use of ancillary testing in brain death diagnosis. Can. J. Anaesth. 2022, 69, 405–406. [Google Scholar] [CrossRef]
- Brasil, S.; Bor-Seng-Shu, E.; de-Lima-Oliveira, M.; Azevedo, M.K.; Teixeira, M.J.; Bernardo, L.; Bernardo, W.M. Role of computed tomography angiography and perfusion tomography in diagnosing brain death: A systematic review. J. Neuroradiol. 2016, 43, 133–140. [Google Scholar] [CrossRef]
- Brasil, S.; Bor-Seng-Shu, E.; de-Lima-Oliveira, M.; Taccone, F.S.; Gattas, G.; Nunes, D.M.; de Oliveira, R.A.G.; Tomazini, B.M.; Tierno, P.F.; Becker, R.A.; et al. Computed tomography angiography accuracy in brain death diagnosis. J. Neurosurg. 2019, 133, 1220–1228. [Google Scholar] [CrossRef]
- Ronconi, K.A.L.; Amorim, R.L.O.; Paschoal, F.M., Jr.; Oliveira, M.L.; Nogueira, R.C.; Paiva, W.S.; Gonçalves, D.B.; Farias, S.R.; Brasil, S.P.; Teixeira, M.J.; et al. Transcranial Doppler: A Useful Tool to Predict Brain Death Still Not Confirmed by Clinical Assessment. Transplant. Proc. 2021, 53, 1803–1807. [Google Scholar] [CrossRef]
- Powner, D.J.; Darby, J.M. Current considerations in the issue of brain death. Neurosurgery 1999, 45, 1222–1226; discussion 1226–1227. [Google Scholar] [CrossRef]
- Brasil, S.; Frigieri, G.; Taccone, F.S.; Robba, C.; Solla, D.J.F.; de Carvalho Nogueira, R.; Yoshikawa, M.H.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.S. Noninvasive intracranial pressure waveforms for estimation of intracranial hypertension and outcome prediction in acute brain-injured patients. J. Clin. Monit. Comput. 2022. [Google Scholar] [CrossRef]
- Dattilo, M. Noninvasive methods to monitor intracranial pressure. Curr. Opin. Neurol. 2023, 36, 1–9. [Google Scholar] [CrossRef]
- Robba, C.; Graziano, F.; Rebora, P.; Elli, F.; Giussani, C.; Oddo, M.; Meyfroidt, G.; Helbok, R.; Taccone, F.S.; Prisco, L.; et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): An international, prospective observational cohort study. Lancet Neurol. 2021, 20, 548–558. [Google Scholar] [CrossRef]
- Brasil, S. Intracranial pressure pulse morphology: The missing link? Intensive Care Med. 2022, 48, 1667–1669. [Google Scholar] [CrossRef]
- Brasil, S.; Solla, D.J.F.; Nogueira, R.d.C.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.D.S. A Novel Noninvasive Technique for Intracranial Pressure Waveform Monitoring in Critical Care. J. Pers. Med. 2021, 11, 1302. [Google Scholar] [CrossRef]
- de Moraes, F.M.; Rocha, E.; Barros, F.C.D.; Freitas, F.G.R.; Miranda, M.; Valiente, R.A.; de Andrade, J.B.C.; Neto, F.; Silva, G.S. Waveform Morphology as a Surrogate for ICP Monitoring: A Comparison between an Invasive and a Noninvasive Method. Neurocrit. Care 2022, 37, 219–227. [Google Scholar] [CrossRef]
- Cabella, B.; Vilela, G.H.; Mascarenhas, S.; Czosnyka, M.; Smielewski, P.; Dias, C.; Cardim, D.A.; Wang, C.C.; Mascarenhas, P.; Andrade, R.; et al. Validation of a New Noninvasive Intracranial Pressure Monitoring Method by Direct Comparison with an Invasive Technique. In Acta Neurochirurgica Supplement; Springer: Cham, Switzerland, 2016; Volume 122, pp. 93–96. [Google Scholar] [CrossRef]
- Frigieri, G.; Andrade, R.A.P.; Wang, C.C.; Spavieri, D., Jr.; Lopes, L.; Brunelli, R.; Cardim, D.A.; Verzola, R.M.M.; Mascarenhas, S. Analysis of a Minimally Invasive Intracranial Pressure Signals During Infusion at the Subarachnoid Spinal Space of Pigs. In Acta Neurochirurgica Supplement; Springer: Cham, Switzerland, 2018; Volume 126, pp. 75–77. [Google Scholar] [CrossRef]
- Andrade, R.d.A.P.; Oshiro, H.E.; Miyazaki, C.K.; Hayashi, C.Y.; Morais, M.A.; Brunelli, R.; Carmo, J.P. A nanometer resolution wearable wireless medical device for non invasive intracranial pressure monitoring. IEEE Sens. J. 2021, 21, 22270–22284. [Google Scholar] [CrossRef]
- Vasconcelos, T.F.; Menegueti, M.G.; Corsi, C.A.C.; Michelon-Barbosa, J.; Sato, L.; Basile-Filho, A.; Becari, C.; Dantas, R.A.S.; Auxiliadora-Martins, M. Assessment of physicians’ knowledge about brain death and organ donation and associated factors. Medicine 2022, 101, e30793. [Google Scholar] [CrossRef]
- Lange, M.C.; Zetola, V.H.; Miranda-Alves, M.; Moro, C.H.; Silvado, C.E.; Rodrigues, D.L.; Gregorio, E.G.; Silva, G.S.; Oliveira-Filho, J.; Perdatella, M.T.; et al. Brazilian guidelines for the application of transcranial ultrasound as a diagnostic test for the confirmation of brain death. Arq. Neuro-Psiquiatr. 2012, 70, 373–380. [Google Scholar] [CrossRef]
- Hassett, C.E.; Uysal, S.P.; Butler, R.; Moore, N.Z.; Cardim, D.; Gomes, J.A. Assessment of Cerebral Autoregulation Using Invasive and Noninvasive Methods of Intracranial Pressure Monitoring. Neurocrit. Care 2022, 26, 562–569. [Google Scholar] [CrossRef]
- Cardoso, E.R.; Rowan, J.O.; Galbraith, S. Analysis of the cerebrospinal fluid pulse wave in intracranial pressure. J. Neurosurg. 1983, 59, 817–821. [Google Scholar] [CrossRef]
- Brasil, S.; Solla, D.J.F.; Nogueira, R.d.C.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.S. Intracranial Compliance Assessed by Intracranial Pressure Pulse Waveform. Brain Sci. 2021, 11, 971. [Google Scholar] [CrossRef]
- Scherzer, E. Intracranial pressure and brain death. Wien. Med. Wochenschr. 1990, 140, 562–564. [Google Scholar]
- Agapejev, S.; Da Silva, P.P.; Zanini, M.A.; Piza, E.T. Intracranial pressure monitoring as a complementary tests for diagnosing brain death. Preliminary observation through the report of 2 cases. Arq. Neuro-Psiquiatr. 1997, 55, 310–314. [Google Scholar] [CrossRef]
- Salih, F.; Holtkamp, M.; Brandt, S.A.; Hoffmann, O.; Masuhr, F.; Schreiber, S.; Weissinger, F.; Vajkoczy, P.; Wolf, S. Intracranial pressure and cerebral perfusion pressure in patients developing brain death. J. Crit. Care 2016, 34, 908–914. [Google Scholar] [CrossRef]
- Roth, C.; Ferbert, A.; Matthaei, J.; Kaestner, S.; Engel, H.; Gehling, M. Progress of intracranial pressure and cerebral perfusion pressure in patients during the development of brain death. J. Neurol. Sci. 2019, 398, 171–175. [Google Scholar] [CrossRef]
- Dominguez-Roldan, J.M.; Barrera-Chacon, J.M.; Martin-Bermudez, R.; Murillo-Cabezas, F.; Garcia-Alfaro, C.; Rincon-Ferrari, M.D. Changes in the intracranial pulse pressure waveform associated with brain death. Transplant. Proc. 1999, 31, 2597–2598. [Google Scholar] [CrossRef]
- Nucci, C.G.; De Bonis, P.; Mangiola, A.; Santini, P.; Sciandrone, M.; Risi, A.; Anile, C. Intracranial pressure wave morphological classification: Automated analysis and clinical validation. Acta Neurochir. 2016, 158, 581–588. [Google Scholar] [CrossRef]
- Rozsa, L.; Szabo, S.; Gombi, R.; Miko, L.; Balazs, E. Intracranial pressure increase and changes in cerebrovascular circulation, associated with brain death, studied by transcranial Doppler sonography. Orv. Hetil. 1991, 132, 2785–2788. [Google Scholar]
- Karaali, K.; Cevikol, C.; Senol, U.; Arici, G.; Kabaalioglu, A.; Ramazanoglu, A.; Bircan, O. Orbital Doppler sonography findings in cases of brain death. Am. J. Neuroradiol. 2000, 21, 945–947. [Google Scholar]
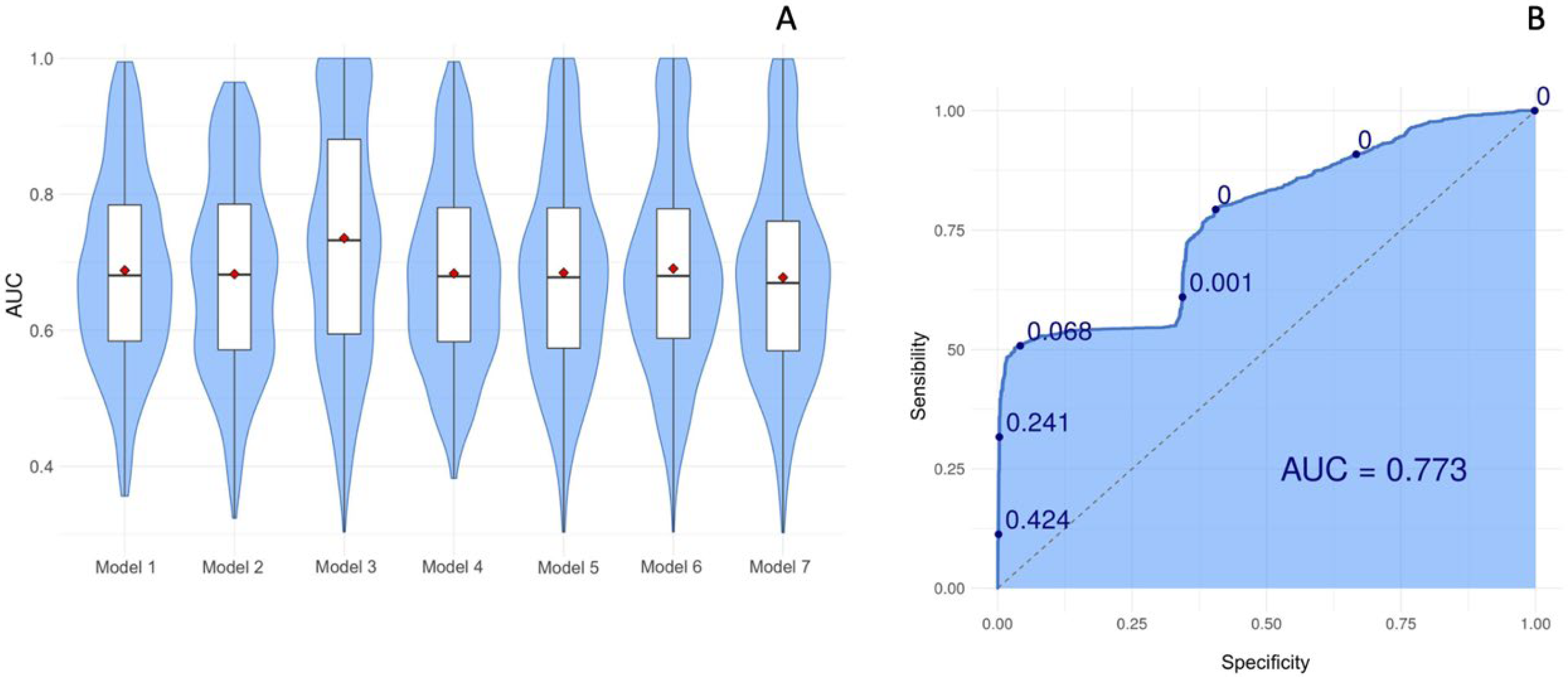
| Model 1 | Group~P2/P1 ratio |
| Model 2 | group~ttp |
| Model 3 | group~pulse_amp |
| Model 4 | group~P2/P1 ratio + ttp |
| Model 5 | group~ttp + pulse_amp |
| Model 6 | group~P2/P1 ratio + pulse_amp |
| Model 7 | Group~P2/P1 ratio + ttp + pulse_amp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ideta, M.M.L.; Oliveira, L.M.; Gonçalves, D.B.; Paschoalino, M.C.; Carvalho, N.A.; Della Coletta, M.V.; Paiva, W.; Brasil, S.; Amorim, R.L.O. Qualitative Evaluation of Intracranial Pressure Slopes in Patients Undergoing Brain Death Protocol. Brain Sci. 2023, 13, 401. https://doi.org/10.3390/brainsci13030401
Ideta MML, Oliveira LM, Gonçalves DB, Paschoalino MC, Carvalho NA, Della Coletta MV, Paiva W, Brasil S, Amorim RLO. Qualitative Evaluation of Intracranial Pressure Slopes in Patients Undergoing Brain Death Protocol. Brain Sciences. 2023; 13(3):401. https://doi.org/10.3390/brainsci13030401
Chicago/Turabian StyleIdeta, Mylena Miki Lopes, Louise Makarem Oliveira, Daniel Buzaglo Gonçalves, Mylla Christie Paschoalino, Nise Alessandra Carvalho, Marcus Vinicius Della Coletta, Wellingson Paiva, Sérgio Brasil, and Robson Luís Oliveira Amorim. 2023. "Qualitative Evaluation of Intracranial Pressure Slopes in Patients Undergoing Brain Death Protocol" Brain Sciences 13, no. 3: 401. https://doi.org/10.3390/brainsci13030401
APA StyleIdeta, M. M. L., Oliveira, L. M., Gonçalves, D. B., Paschoalino, M. C., Carvalho, N. A., Della Coletta, M. V., Paiva, W., Brasil, S., & Amorim, R. L. O. (2023). Qualitative Evaluation of Intracranial Pressure Slopes in Patients Undergoing Brain Death Protocol. Brain Sciences, 13(3), 401. https://doi.org/10.3390/brainsci13030401








