Abstract
In 2002, the first III generation antipsychotic drug was registered—aripiprazole. Its partial dopaminergic agonism underlies its unique mechanism of action and the potentially beneficial influence on the positive, negative, or cognitive symptoms. Due to its relatively high intrinsic activity, the drug could often cause agitation, anxiety, or akathisia. For this reason, efforts were made to develop a drug which would retain the positive favorable actions of aripiprazole but present a more advantageous clinical profile. This turned out to be brexpiprazole, which was registered in 2015. Its pharmacodynamic and pharmacokinetic profile (similarly to the other most recent antipsychotics, i.e., lurasidone or cariprazine) shows promise of increasing the effectiveness of schizophrenia treatment in the dimensions in which the previous antipsychotics were not sufficiently effective, including negative, depressive, or cognitive symptoms. Like other new antipsychotics, it can also be useful in the treatment of mood disorders, for instance drug-resistant depression. Previous reviews focused on the use of brexpiprazole in specific diagnostic groups. The aim of this article is to provide the readers with an overview of data on the mechanism of action, clinical effectiveness in all studied diagnostic groups, as well as potential drug–food interactions, and the safety of brexpiprazole.
1. Pharmacological Profile of Brexipiprazole
1.1. Structure and Class
Brexpiprazole is a quinol derivative, its structure very much resembles that of aripiprazole (Figure 1) [1]. Compared to aripiprazole, brexpiprazole has an additional thiophene ring. This different structure contributes to dissimilar pharmacodynamic properties. As opposed to aripiprazole, brexpiprazole (1) has less intrinsic D2 activity which translates to lower risk of akathisia and EPS, (2) presents higher potency at 5HT1a which may result in better antidepressant, anxiolytic, and procognitive effectiveness, (3) shows higher potency at 5HT2a and therefore is less likely to cause akathisia and insomnia. According to the neuroscience-based Nomenclature 2 classification, it is a partial dopamine and serotonin agonist (D2, 5HT1a) as well as a serotonin antagonist (5HT2a) [2,3].
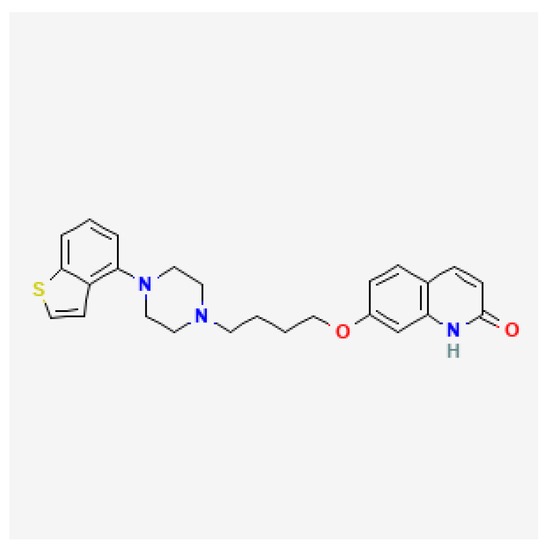
Figure 1.
Brexpiprazole chemical structure based on [1].
1.2. Pharmacodynamics
In in vitro studies, brexpiprazole acts as a partial agonist of 5HT1a, D2, and D3 receptors [4]. Its impact on 5HT1a receptors might translate into precognitive and mood-enhancing effects as well as a reduced risk of extrapyramidal symptoms (EPS). The partial agonism of D2 receptors accounts for the antipsychotic action of the drug, it also mandates that the risk of EPS and hyperprolactinemia (hPRL) is lower compared to antipsychotics which display the complete antagonism of D2. Regarding the D2 receptors, brexpiprazole demonstrates a level of intrinsic activity that is comparable to cariprazine or aripiprazole, meaning that it is for the most part antagonistic. Therefore, compared to aripiprazole, it might present a lower occurrence of adverse effects (AE) such as akathisia, nausea, or vomiting [5]. In the therapeutic dose range, brexpiprazole occupies 59–75% of dopaminergic receptors, approximately as much as the drugs from older generations of antipsychotics, which warrants good effectiveness on positive psychotic symptoms [6]. The partial agonism of D3 might result in increased effectiveness on negative symptoms of schizophrenia as well as recognitive and antidepressant potential [5]. The drug also acts as an antagonist on 5HT2a, 5HT2c, 5HT7, alfa-1, and H1 receptors. The antagonism on 5HT1a might cause sedative and anxiolytic activity, increased antipsychotic effect, and decreased risk of EPS and hPRL. The antagonism of 5HT7 translates to procognitive and antidepressant effect, especially in combination with the partial agonism of 5HT1a, and it might also have a positive impact on the negative schizophrenia symptoms. Although low, the antagonism of 5HT2c might produce antidepressant action but also increased appetite. The moderate antagonism of α1 might cause more sedation, higher risk of orthostatic hypotension, and impact the effectiveness of the drug in post-traumatic stress treatment, in particular nightmares (which was addressed in preliminary reports regarding this issue). The α2 antagonism might influence the antipsychotic action of the drug, however the H1 antagonism increases the risk of weight gain and sedation, and it also results in an anxiolytic and tranquilizing effect [7,8,9,10]. The in vitro studies in animal cell lines also indicated the inhibitory activity on monoamine, 5HT, NA, and DA transporters, which was comparable to that of serotonin and noradrenalin reuptake inhibitors (SNRI); however, studies on human cell lines reported only a small impact on these transporters. Additionally, these results were not replicated in other in vitro studies on animals [11]. The comparison of affinities of brexpiprazole and other partial agonists of D2 for specific receptors is presented in Table 1.

Table 1.
The comparison of affinities of brexpiprazole and other partial agonists of D2 for specific receptors. Additionally, clinical effects of agonism and antagonism of those receptors has been summarized based on [7,12,13]—modified.
1.3. Pharmacokinetics
After oral ingestion, brexpiprazole is easily absorbed, it is characterized by a high 95% bioavailability. Food has no significant effect on the pharmacokinetics of brexpiprazole, therefore it might be taken with or without a meal which is more convenient compared to lurasidone which has to be taken with a meal of at least 350 kcal or ziprasidone which should be taken with a meal of at least 500 kcal, and potentially increases the chance of good adherence. What is more, the time from the last meal or to the next meal has no influence on brexpiprazole absorption, which enhances the adherence compared to, i.e., quetiapine XR, which should be taken at least an hour before the next meal or sulpiride which should be taken either one hour before the meal or two hours after it [14]. The volume of distribution after intravenous administration is approximately 1.56 ± 0.42 L/kg, which suggests an extravascular distribution. The drug has significant lipophilic properties, which enables the blood–brain barrier transfer, but could also result in storage in fat tissue [15]. Maximum drug concentration is reached 4 h after oral ingestion, the biological half-life is 91 h [16]. Because the drug’s half-life is long, an omission of a dose has less of an impact on its effectiveness vs. other antipsychotics with shorter half-lives. A study comparing the risk of relapse after antipsychotic discontinuation indicated that 4 weeks after the brexpiprazole was stopped only 8% of patients reported the recurrence of disease. After this time, the drug–placebo separation was noted, which was a second-best result following the one for cariprazine relapse of 5% after 4 weeks, with drug–placebo separation after 6.6 weeks. For the other antipsychotics, the results ranged between 13 and 34% for the risk of relapse after 4 weeks and between 1.1 and 2.7 weeks for the drug–placebo separation [17]. Brexpiprazole is metabolized by cytochrome enzymes CYP2D6 and CYP3A4. Its metabolism produces an active metabolite DM-3411, which has a pharmacodynamic profile similar to the parent drug but is less potent and has a lower penetration to the central nervous system and therefore has no significant impact on the therapeutic effect. Both the parent drug and the active metabolite to a great extent (99%) bind to the serum proteins. Therefore, both brexpiprazole and DM-3411 could potentially compete with other drugs which bind to serum proteins which may potentially result in (1) increased levels of free form of the concomitant drug should it bind to serum proteins with less potency and higher risk of its adverse effects, (2) increased levels of free brexpiprazole and free DM-3411 should the concomitant drug bind to serum proteins with higher potency and higher effects as well as risk of adverse effects of brexpiprazole/DM-3411 despite the relatively low dosing. Nonetheless, as reported by the producer, the results of in vitro studied indicated that protein binding of brexpiprazole is not influenced by warfarin, digoxin, or diazepam [18]. After repeated doses, the steady state is reached in 10–12 days. Brexpiprazole is mainly eliminated with urine and feces, the unmetabolized drug accounts for 14% in the first and <1% in the second case [19]. The therapeutic concentration of brexpiprazole ranges between 40–140 ng/L and the alert level 280 ng/L. No standards advise routine plasma drug concentration monitoring; however, it could be clinically useful [20]. No significant differences were reported regarding the pharmacokinetics in patients 65 y or older. Subjects with moderate or severe liver or kidney damage are at risk of increased drug exposition, therefore the dose should be lower. Slow CYP2D6 metabolizers show about 30% lower drug clearance and are at risk of increased drug exposure, same as patients taking CYP2D6 inhibitors. In these circumstances, the Summary of Product Characteristics (SmPC) advises dose modification: a dose reduction by 50%. However, the physiological modeling of drug pharmacokinetics indicated that this might delay the occurrence of therapeutic effects and prolong the treatment. The authors of the abovementioned study suggest that the recommended dose should be administered two times a day (b.i.d) in the first week of treatment [21]. Subjects who simultaneously receive CYP3A4-inhibiting drugs are also at risk of higher brexpiprazole exposition.
Furthermore, drug and nutrient interactions need to be taken into account. Recently, a study on rats suggested that concurrent administration of brexpiprazole and grapefruit and pomegranate juices (CYP3A4 inhibitors) increased systemic exposure to brexpiprazole [22]. Several drugs (i.e., azoles, foods, and drinks) might inhibit CYP3A4, that is, red wine, beer, grapefruit, pomegranate, cranberry, lime, pomelo, onion and tomato juice, parsley, thyme, celeriac, legumes, garlic, licorice root, Seville orange products (juice, jam, marmalade), black/long pepper, and saffron. Some nutritive products may also inhibit CYP2D6, that is, Glycyrrhiza inflata liquorice root products (spice, confectionery, tea, sambuca), black/long/chili pepper, and saffron. Consequently, patients should be informed of the potential interactions with both drugs and foods and drinks. On the other hand, the risk of drug–drug or drug–nutrient interactions applies to the majority of antipsychotics (aside from amisuplride and sulpiride which are not metabolized by CYP 450 enzymes). Furthermore, the risk of drug–nutrient interactions is in most cases minimal and reaches the level of clinical significance only in patients who consume substantial amounts of the abovementioned products (i.e., due to consumption of the dietary supplements) [14]. Concurrent administration of CYP3A4 inductors could decrease the effectiveness of the drug. It should be noted that aside from drugs which are CYP3A4 inductors (i.e., carbamazepine, glucocorticosteroids), some foods (ginger) might also include CYP3A4 [14]. While in vitro studies indicate that brexpiprazole might act as a weak inhibitor of CYP2B6, 2D6, and 3A4, this effect seems to have no clinical impact on the risk of drug interactions, as no reports of studies or cases suggesting its significance are available [23]. Moreover, the product monograph states that oral brexpiprazole had no effect on the metabolism of single doses of dextromethorphan (a CYP2D6 substrate), lovastatin (a CYP3A4 substrate), or bupropion (a CYP2B6 substrate), and that brexpiprazole did not affect the absorption of single doses of drugs that are substrates of BCRP transporter (rosuvastatin) and PgP (P-glycoprotein) transporter (fexofenadine). The producer states that no dosage adjustment in needed in the case of CYP2D6, CYP3A4, CYP2B6, BCRP, and PgP substrates during concomitant administration with brexpiprazole [18]. The physical modeling of the drug’s pharmacokinetics suggests a potentially significant impact that obesity might have on the biological half-life of the drug and the time in which the steady state is reached, which in this study was extended by approximately 9 days, probably due to the high lipophilicity of brexpiprazole and an almost three times higher volume of distribution observed in patients with BMI > 35. Hence, the authors of the mentioned study suggest an algorithm alternative to SmPC in the first phase of the treatment—1 mg b.i.d. in days 1–4, 2 mg b.i.d. in days 5–7, and 4 mg once a day (q.d.) in the next days. For the obese patients who are also slow CYP2D6 metabolizers, they propose the following dosing regimen: 0.5 mg b.i.d. in days 1–4, 1 mg b.i.d. in days 5–7, 2 mg b.i.d. in days 8–18, and 2 mg q.d. in the next days [15].
2. Clinical Use
2.1. Schizophrenia—Acute Episode (up to 6 Weeks)
Short-term studies of brexpiprazole monotherapy indicate its effectiveness in reducing the positive, negative, and cognitive symptoms, severity of aggression, as well as the levels of improvement and functional remission. The number needed to treat (NNT) for treatment response in acute schizophrenia of brexpiprazole is 7, which is lower than observed in the case of cariprazine treatment (NNT 10) and higher than that reported for lurasidone treatment (NNT 4–7 depending on the dose of the drug) [24]. A meta-analysis of studies exploring the links between the effectiveness and drug dose showed that, regarding the negative symptoms, the dose of 2 mg/d resulted in the maximal effect and then a plateau was observed. Concerning the positive symptoms, the effective dose (ED95) was 4 mg/d, however the curve was upward, suggesting a potential increase in effectiveness in doses above 4 mg/d [25]. The studies on acute schizophrenia episode treatment are presented in Table 2.

Table 2.
The studies on acute schizophrenia episode treatment with the use of brexipiprazole.
2.2. Schizophrenia—Long-Term Treatment
Available randomized controlled trials (RCTs) and open-label studies indicate that the therapeutic effects observed in short-term studies are either sustained or increase with the treatment time. Additionally, brexpiprazole use in monotherapy provides a good chance of achieving improvement or functional remission. The worse the pretreatment functioning of the patient, the higher the probability of improvement; on the other hand, the better the initial level of functioning, the higher the likelihood of remission. Brexpiprazole treatment is also associated with a lower risk of relapse and a longer time to treatment discontinuation compared to other antipsychotics. The NNT for the relapse prevention of brexpiprazole is 4, which is similar to the ones reported for aripiprazole (NNT 5), cariprazine (NNT 5), or olanzapine (NNT 3) [39]. Studies on long-term schizophrenia treatment are displayed in Table 3.

Table 3.
Studies on long-term schizophrenia treatment with the use of brexipiprazole.
2.3. Treatment-Resistant Depression (TRD)
The effectiveness of brexpiprazole was extensively studied in augmenting the antidepressant treatment in TRD and its effects were noted in reducing the symptoms in all domains of depression. It improved the patients’ functioning and showed effects in patients with coexisting anxiety or anger. In previous studies it was also effective in the treatment of patients who had no treatment response to other therapies. NNT for treatment response in TRD of adjunctive brexpiprazole is 12, which is similar to that observed in the case of treatment augmented with quetiapine (NNT 12) and higher than that reported for adjunctive aripiprazole (NNT 9), risperidone (NNT 6), or the combination of olanzapine and fluoxetine (NNT 6) [45,46]. The therapeutic effects might be noted within a week of treatment. Studies assessing brexpiprazole as an adjunctive drug in TRD are presented in Table 4.

Table 4.
Studies assessing effects of the brexpiprazole in treatment-resistant depression.
2.4. Bipolar Disorder
Preliminary studies suggest the potential effectiveness of brexpiprazole as monotherapy or adjunctive drug in the treatment of bipolar depression. While pilot studies were conducted in small groups of patients, their results in TRD and the profile of action raise hope that further studies will confirm these preliminary data. On the other hand, no effectiveness of brexpiprazole monotherapy on manic episodes was shown. The studies of brexpiprazole effectiveness in bipolar disorders are summed up in Table 5.

Table 5.
The studies of brexpiprazole effectiveness in bipolar disorders.
3. Safety and Tolerance
The existing RCTs and open-label studies indicate the good safety and tolerance of brexpiprazole. The majority of AEs are mild or moderate, while severe AE are rare. The risk of discontinuation due to AEs is comparable to these observed for other new antipsychotics. The studies on safety are summed up in Table 6.

Table 6.
The studies on brexipiprazole treatment safety and tolerance.
3.1. Weight Increase and Metabolic Syndrome
Due to the relatively high affinity for the H1 receptor, brexpiprazole causes the weight increase more often than other III generation neuroleptics. In a meta-analysis of clinical studies it was noted that for the treatment with doses 2–4 mg/d, the mean weight gain was 0.95 kg and the number needed to harm (NNH) was 20 (for comparison, NNT for cariprazine was 50 and mean weight increase 0.55, for quetiapine NNH was 34 and mean weight gain 0.99 kg, for risperidone NNH was 12 and mean weight increase was 1.5 kg) [62]. Despite the significant risk of weight gain, no increase in the risk of metabolic syndrome was observed during brexpiprazole treatment. Moreover, in a study on 37 subjects, it was reported that a switch of antipsychotic (from risperidone, olanzapine, blonanserine, haloperidole, aripiprazole, paliperidone, and perospirone) to brexpiprazole was associated with the decrease in weight and improvement of metabolic parameters [63]. In a different study on 186 participants, who were switched from the previous neuroleptic to brexpiprazole, no changes in lipid profile and a slight increase in weight were noted in those initially treated with antipsychotics other than aripiprazole. The patients who were previously treated with aripiprazole had a mean increase in weight of 1.1 kg [64].
3.2. EPS
The most common EPS observed during brexpiprazole therapy is akathisia, which is significantly less common while using brexpiprazole vs. while using other new neuroleptics such as cariprazine, lurasidone, or aripiprazole. The low risk of EPS/akathisia due to brexpiprazole treatment could be explained by its relatively low (lower than this reported for aripiprazole) intrinsic D2 activity. Indeed, previous studies showed that regarding the risk of akathisia the NNH of brexpiprazole ranged from 15 (in depressed patients) to 112 (in patients with schizophrenia). The NNH for akathisia of aripiprazole ranged from 12 to 30 (in depressed patients) and 6 to 12 (in schizophrenia patients, depending on the dose of the drug). The NNH for EPS/akathisia of aripiprazole was 4 and NNH for akathisia in the case of cariprazine ranged from 7 (in patients with manic or mixed episodes) to 12 to 20 (in patients with schizophrenia) depending on the dose of the drug [45,46]. In the previously mentioned studies, a change from brexpiprazole to a different antipsychotic either did not produce an exacerbation of EPS [64] or resulted in an improvement of EPS [63].
3.3. hPRL and Sexual Functions
In the short-term studies, AEs linked to prolactin levels were noted in 1.8% of patients on brexpiprazole compared to 0.6% of those receiving placebo, while in the long-term studies AEs were noted in 1.7% of subjects treated with brexpiprazole. hPRL exceeding >3x the upper the limit of normal were noted in 1.5% of women and 1.6% of men using brexpiprazole and 3.6% of women and 3.4% of men receiving placebo. In the long-term observations, similar hPRL increases were noted in 5.3% of women and 2.0% of men [65]. Both in the short- and long-term studies an improvement of sexual functioning was noted in patients with TRD treated with brexpiprazole [66].
3.4. Other AE
Of studies on large patient groups, none reported a prolonged QTc interval or hematological AE, one case of seizure was noted. In a post hoc study on a group of 410 subjects, a link between prolonged QTc interval and brexpiprazole use was observed, however only five of all patients in this study received this drug [67]. No cases of significant hepatotoxic reactions were observed during the treatment.

Table 7.
The data on the adverse events of brexpiprazole vs. aripiprazole and cariprazine.
In Table 8 values of NNH for the most important AE of brexpiprazole, cariprazine, lurasidone, and aripiprazole are summed up [69,70].

Table 8.
Values of the number needed to harm for the most important adverse events of brexpiprazole, cariprazine, lurasidone, and aripiprazole in patients with schizophrenia according to [69,70].
3.5. Gaps in Knowledge Regarding Brexpiprazole
Little is known of the effectiveness of brexpiprazole in schizoaffective disorder. Only two of the conduced studies of brexpiprazole in schizophrenia included patients with schizoaffective disorder, however they constituted less than 10% of the subjects [63,71]. Considering the pharmacological profile of brexpiprazole and its effectiveness in schizophrenia, major depression, and probably also bipolar depression, the drug could potentially be effective in schizoaffective disorder. This issue needs to be addressed in future studies. As mentioned earlier, the studies assessing the effectiveness of brexpiprazole in bipolar depression are preliminary and further research on this issue is necessary.
4. Conclusions
Brexpiprazole is an antipsychotic showing effectiveness in both short- and long-term treatment of schizophrenia and TRD. It is also effective in bipolar depression as well as agitation and aggression related to the course of dementia. It has an advantageous pharmacokinetic profile which decreases the risk associated with a dose omission and a pharmacodynamic profile which ensures the low occurrence of AEs, good tolerance, and effectiveness in reducing the positive symptoms of psychosis as well as affective and cognitive symptoms.
Author Contributions
Conceptualization, M.S. and K.W.-B.; writing—original draft preparation, K.W.-B. and A.J.K.; writing—review and editing, M.S., A.A.C., K.W.-B. and A.J.K.; supervision, M.S. and A.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11978813, Brexpiprazole. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Brexpiprazole (accessed on 14 February 2023).
- Kikuchi, T.; Maeda, K.; Suzuki, M.; Hirose, T.; Futamura, T.; Mcquade, R.D. Discovery research and development history of the dopamine D 2 receptor partial agonists, aripiprazole and brexpiprazole. Neuropsychopharmacol. Rep. 2021, 41, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Aftab, A.; Gao, K. The preclinical discovery and development of brexpiprazole for the treatment of major depressive disorder. Expert Opin. Drug Discov. 2017, 12, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Sugino, H.; Akazawa, H.; Amada, N.; Shimada, J.; Futamura, T.; Yamashita, H.; Ito, N.; McQuade, R.D.; Mørk, A.; et al. Brexpiprazole I: In Vitro and In Vivo Characterization of a Novel Serotonin-Dopamine Activity Modulator. J. Pharmacol. Exp. Ther. 2014, 350, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Mechanism of action of cariprazine. CNS Spectrums 2016, 21, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.F.; Raoufinia, A.; Bricmont, P.; Brašić, J.R.; McQuade, R.D.; Forbes, R.A.; Kikuchi, T.; Kuwabara, H. An open-label, positron emission tomography study of the striatal D2/D3 receptor occupancy and pharmacokinetics of single-dose oral brexpiprazole in healthy participants. Eur. J. Clin. Pharmacol. 2021, 77, 717–725. [Google Scholar] [CrossRef]
- Siwek, M.; Wojtasik-Bakalarz, K. Leki Przeciwpsychotyczne; Rybakowski, J., Ed.; PWN: Warsaw, Poland, 2022; pp. 109–174. [Google Scholar]
- Siwek, M.; Wasik, A.; Krupa, A.; Gorostowicz, A. Nowe Leki Przeciwpsychotyczne; Medical Education: Warsaw, Poland, 2020. [Google Scholar]
- Siwek, M.; Krupa, A.J.; Wasik, A. Lurasidone–pharmacodynamic and pharmacokinetic properties, clinical potential and interaction risk. Pharmacother. Psychiatry Neurol. 2020, 34, 117–134. [Google Scholar] [CrossRef]
- Eaves, S.; Rey, J.A. Brexipiprazole (Rexulti): A new monotherapy for schizophrenia and adjunctive therapy for major depressive disorder. Pharm. Ther. 2016, 41, 418–422. [Google Scholar]
- Oosterhof, C.A.; El Mansari, M.; Blier, P. Acute Effects of Brexpiprazole on Serotonin, Dopamine, and Norepinephrine Systems: An In Vivo Electrophysiologic Characterization. J. Pharmacol. Exp. Ther. 2014, 351, 585–595. [Google Scholar] [CrossRef]
- Misiak, B.; Bieńkowski, P.; Samochowiec, J. Cariprazine—A novel antipsychotic drug and its place in the treatment of schizophrenia. Psychiatr. Polska 2018, 52, 971–981. [Google Scholar] [CrossRef]
- Miyamoto, S.; Miyake, N.; Jarskog, L.F.; Fleischhacker, W.W.; Lieberman, J.A. Pharmacological treatment of schizophrenia: A critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatry 2012, 17, 1206–1227. [Google Scholar] [CrossRef]
- Wasik, A.; Krupa, A.; Siwek, M. Interactions of antidepressants, mood-stabilisers and antipsychotics with food. Pharmacother. Psychiatry Neurol. 2019, 35, 51–74. [Google Scholar] [CrossRef]
- Bruno, C.D.; Elmokadem, A.; Housand, C.; Jordie, E.B.; Chow, C.R.; Laughren, T.P.; Greenblatt, D.J. Impact of Obesity on Brexpiprazole Pharmacokinetics: Proposal for Improved Initiation of Treatment. J. Clin. Pharmacol. 2022, 62, 55–65. [Google Scholar] [CrossRef]
- Frankel, J.S.; Schwartz, T.L. Brexpiprazole and cariprazine: Distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther. Adv. Psychopharmacol. 2016, 7, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Jain, R.; Meyer, J.; Periclou, A.; Carrothers, T.; Barabássy, Á.; Patel, M.; Earley, W. Relationship between the timing of relapse and plasma drug levels following discontinuation of cariprazine treatment in patients with schizophrenia: Indirect comparison with other second-generation antipsychotics after treatment discontinuation. Neuropsychiatr. Dis. Treat. 2019, 15, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Otsuka Pharmaceuticals Co., Ltd. REXULTI Product Monograph 2020. Available online: https://www.lundbeck.com/content/dam/lundbeck-com/americas/canada/products/files/rexulti_product_monograph_english.pdf (accessed on 18 February 2023).
- Mauri, M.C.; Paletta, S.; Di Pace, C.; Reggiori, A.; Cirnigliaro, G.; Valli, I.; Altamura, A.C. Clinical Pharmacokinetics of Atypical Antipsychotics: An Update. Clin. Pharmacokinet. 2018, 57, 1493–1528. [Google Scholar] [CrossRef] [PubMed]
- Schoretsanitis, G.; Kane, J.M.; Correll, C.U.; Marder, S.R.; Citrome, L.; Newcomer, J.W.; Robinson, D.G.; Goff, D.C.; Kelly, D.L.; Freudenreich, O.; et al. Blood levels to optimize antipsychotic treatment in clinical practice: A joint consensus statement of the American society of clinical psychopharmacology and the therapeutic drug monitoring task force of the arbeitsgemeinschaft für neuropsychopharmakologie und pharmakopsychiatrie. J. Clin. Psychiatry 2020, 81, 3649. [Google Scholar]
- Elmokadem, A.; Bruno, C.D.; Housand, C.; Jordie, E.B.; Chow, C.R.; Lesko, L.J.; Greenblatt, D.J. Brexpiprazole Pharmacokinetics in CYP2D6 Poor Metabolizers: Using Physiologically Based Pharmacokinetic Modeling to Optimize Time to Effective Concentrations. J. Clin. Pharmacol. 2022, 62, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, D.; Kate, A.S. Investigation of the impact of grapefruit juice, pomegranate juice and tomato juice on pharmacokinetics of brexpiprazole in rats using UHPLC–QTOF–MS. Biomed. Chromatogr. 2021, 35, e5201. [Google Scholar] [CrossRef]
- Sasabe, H.; Koga, T.; Furukawa, M.; Matsunaga, M.; Sasahara, K.; Hashizume, K.; Oozone, Y.; Amunom, I.; Torii, M.; Umehara, K.; et al. In vitro evaluations for pharmacokinetic drug-drug interactions of a novel serotonin-dopamine activity modulator, brexpiprazole. Xenobiotica 2021, 51, 522–535. [Google Scholar] [CrossRef]
- Citrome, L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: A systematic review of the efficacy and safety profile for this newly approved antipsychotic—What is the number needed to treat, number needed to harm and likelihood to be hel. Int. J. Clin. Pract. 2015, 69, 978–997. [Google Scholar] [CrossRef]
- Sabe, M.; Zhao, N.; Crippa, A.; Kaiser, S. Antipsychotics for negative and positive symptoms of schizophrenia: Dose-response meta-analysis of randomized controlled acute phase trials. Schizophrenia 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Skuban, A.; Hobart, M.; Ouyang, J.; Weiller, E.; Weiss, C.; Kane, J.M. Efficacy of brexpiprazole in patients with acute schizophrenia: Review of three randomized, double-blind, placebo-controlled studies. Schizophr. Res. 2016, 174, 82–92. [Google Scholar] [CrossRef]
- Kane, J.M.; Skuban, A.; Hobart, M.; Ouyang, J.; Weiller, E.; Weiss, C.; Correll, C.U. Overview of short- and long-term tolerability and safety of brexpiprazole in patients with schizophrenia. Schizophr. Res. 2016, 174, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Skuban, A.; Ouyang, J.; Hobart, M.; Pfister, S.; McQuade, R.D.; Nyilas, M.; Carson, W.H.; Sanchez, R.; Eriksson, H. Efficacy and Safety of Brexpiprazole for the Treatment of Acute Schizophrenia: A 6-Week Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Psychiatry 2015, 172, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Marder, S.R.; Eriksson, H.; Zhao, Y.; Hobart, M. Post-hoc analysis of a randomized, placebo-controlled, active-reference 6-week study of brexpiprazole in acute schizophrenia. Acta Neuropsychiatr. 2020, 32, 153–158. [Google Scholar] [CrossRef]
- Ishigooka, J.; Iwashita, S.; Tadori, Y. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia in Japan: A 6-week, randomized, double-blind, placebo-controlled study. Psychiatry Clin. Neurosci. 2018, 72, 692–700. [Google Scholar] [CrossRef]
- Citrome, L.; Ota, A.; Nagamizu, K.; Perry, P.; Weiller, E.; Baker, R.A. The effect of brexpiprazole (OPC-34712) and aripiprazole in adult patients with acute schizophrenia: Results from a randomized, exploratory study. Int. Clin. Psychopharmacol. 2016, 31, 192–201. [Google Scholar] [CrossRef] [PubMed]
- van Erp, T.G.; Baker, R.A.; Cox, K.; Okame, T.; Kojima, Y.; Eramo, A.; Potkin, S.G. Effect of brexpiprazole on control of impulsivity in schizophrenia: A randomized functional magnetic resonance imaging study. Psychiatry Res. Neuroimaging 2020, 301, 111085. [Google Scholar] [CrossRef]
- Correll, C.U.; He, Y.; Therrien, F.; MacKenzie, E.; Meehan, S.R.; Weiss, C.; Hefting, N.; Hobart, M. Effects of Brexpiprazole on Functioning in Patients with Schizophrenia: Post Hoc Analysis of Short- And Long-term Studies. J. Clin. Psychiatry 2022, 83, 39942. [Google Scholar] [CrossRef]
- Meade, N.; Shi, L.; Meehan, S.R.; Weiss, C.; Ismail, Z. Efficacy and safety of brexpiprazole in patients with schizophrenia presenting with severe symptoms: Post-hoc analysis of short- and long-term studies. J. Psychopharmacol. 2020, 34, 829–838. [Google Scholar] [CrossRef]
- Citrome, L.; Ouyang, J.; Shi, L.; Meehan, S.R.; Baker, R.A.; Weiss, C. Effect of Brexpiprazole on Agitation and Hostility in Patients with Schizophrenia: Post Hoc Analysis of Short- and Long-Term Studies. J. Clin. Psychopharmacol. 2019, 39, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Antoun Reyad, A.; Girgis, E.; Mishriky, R. Efficacy and safety of brexpiprazole in acute management of psychiatric disorders: A meta-analysis of randomized controlled trials. Int. Clin. Psychopharmacol. 2020, 35, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Nutt, D.J.; Davies, S.J.C. Visualizing classification of drugs used in psychotic disorders: A ‘subway map’ representing mechanisms, established classes and informal categories. J. Psychopharmacol. 2022, 36, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Ikuta, T.; Matsuda, Y.; Sakuma, K.; Iwata, N. Aripiprazole vs. brexpiprazole for acute schizophrenia: A systematic review and network meta-analysis. Psychopharmacology 2020, 237, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Citrome, L. Schizophrenia relapse, patient considerations, and potential role of lurasidone. Patient Prefer. Adherence 2016, 10, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, W.W.; Hobart, M.; Ouyang, J.; Forbes, A.; Pfister, S.; McQuade, R.D.; Carson, W.H.; Sanchez, R.; Nyilas, M.; Weiller, E. Efficacy and Safety of Brexpiprazole (OPC-34712) as Maintenance Treatment in Adults with Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. Int. J. Neuropsychopharmacol. 2016, 20, 11–21. [Google Scholar] [CrossRef]
- Ishigooka, J.; Iwashita, S.; Tadori, Y. Long-term safety and effectiveness of brexpiprazole in Japanese patients with schizophrenia: A 52-week, open-label study. Psychiatry Clin. Neurosci. 2018, 72, 445–453. [Google Scholar] [CrossRef]
- Inada, K.; Yamada, S.; Akiyoshi, H.; Kojima, Y.; Iwashita, S.; Ishigooka, J. Long-Term Efficacy and Safety of Brexpiprazole in Elderly Japanese Patients with Schizophrenia: A Subgroup Analysis of an Open-Label Study. Neuropsychiatr. Dis. Treat. 2020, 16, 2267–2275. [Google Scholar] [CrossRef]
- Forbes, A.; Hobart, M.; Ouyang, J.; Shi, L.; Pfister, S.; Hakala, M. A Long-Term, Open-Label Study to Evaluate the Safety and Tolerability of Brexpiprazole as Maintenance Treatment in Adults with Schizophrenia. Int. J. Neuropsychopharmacol. 2018, 21, 433–441. [Google Scholar] [CrossRef]
- Hishimoto, A.; Yasui-Furukori, N.; Sekine, D.; Matsukawa, M.; Yamada, S. Treatment Discontinuation Among Patients with Schizophrenia Treated with Brexpiprazole and Other Oral Atypical Antipsychotics in Japan: A Retrospective Observational Study. Adv. Ther. 2022, 39, 4299–4314. [Google Scholar] [CrossRef]
- Corponi, F.; Fabbri, C.; Bitter, I.; Montgomery, S.; Vieta, E.; Kasper, S.; Pallanti, S.; Serretti, A. Novel antipsychotics specificity profile: A clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. Eur. Neuropsychopharmacol. 2019, 29, 971–985. [Google Scholar] [CrossRef]
- Vázquez, G.H.; Bahji, A.; Undurraga, J.; Tondo, L.; Baldessarini, R.J. Efficacy and Tolerability of Combination Treatments for Major Depression: Antidepressants plus Second-Generation Antipsychotics vs. Esketamine vs. Lithium. J. Psychopharmacol. 2021, 35, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E.; Youakim, J.M.; Skuban, A.; Hobart, M.; Augustine, C.; Zhang, P.; McQuade, R.D.; Carson, W.H.; Nyilas, M.; Sanchez, R.; et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: A phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J. Clin. Psychiatry 2015, 76, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E.; Youakim, J.M.; Skuban, A.; Hobart, M.; Zhang, P.; McQuade, R.D.; Nyilas, M.; Carson, W.H.; Sanchez, R.; Eriksson, H. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: A phase 3, randomized, double-blind study. J. Clin. Psychiatry 2015, 76, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Hobart, M.; Skuban, A.; Zhang, P.; Augustine, C.; Brewer, C.; Hefting, N.; Sanchez, R.; McQuade, R.D. A Randomized, Placebo-Controlled Study of the Efficacy and Safety of Fixed-Dose Brexpiprazole 2 mg/d as Adjunctive Treatment of Adults with Major Depressive Disorder. J. Clin. Psychiatry 2018, 79, 5950. [Google Scholar] [CrossRef] [PubMed]
- Hobart, M.; Skuban, A.; Zhang, P.; Josiassen, M.K.; Hefting, N.; Augustine, C.; Brewer, C.; Sanchez, R.; McQuade, R.D. Efficacy and safety of flexibly dosed brexpiprazole for the adjunctive treatment of major depressive disorder: A randomized, active-referenced, placebo-controlled study. Curr. Med. Res. Opin. 2018, 34, 633–642. [Google Scholar] [CrossRef]
- Bauer, M.; Hefting, N.; Lindsten, A.; Josiassen, M.K.; Hobart, M. A randomised, placebo-controlled 24-week study evaluating adjunctive brexpiprazole in patients with major depressive disorder. Acta Neuropsychiatr. 2018, 31, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hobart, M.; Zhang, P.; Weiss, C.; Meehan, S.R.; Eriksson, H. Adjunctive Brexpiprazole and Functioning in Major Depressive Disorder: A Pooled Analysis of Six Randomized Studies Using the Sheehan Disability Scale. Int. J. Neuropsychopharmacol. 2018, 22, 173–179. [Google Scholar] [CrossRef]
- Katzman, M.A.; Therrien, F.; MacKenzie, E.M.; Wang, F.; de Jong-Laird, A.; Boucher, M. Efficacy of adjunctive brexpiprazole on symptom clusters of major depressive disorder: A post hoc analysis of four clinical studies. J. Affect. Disord. 2022, 316, 201–208. [Google Scholar] [CrossRef]
- Thase, M.E.; Weiller, E.; Zhang, P.; Weiss, C.; McIntyre, R.S. Adjunctive brexpiprazole in patients with major depressive disorder and anxiety symptoms: Post hoc analyses of three placebo-controlled studies. Neuropsychiatr. Dis. Treat. 2018, 15, 37–45. [Google Scholar] [CrossRef]
- Nelson, J.C.; Weiller, E.; Zhang, P.; Weiss, C.; Hobart, M. Efficacy of adjunctive brexpiprazole on the core symptoms of major depressive disorder: A post hoc analysis of two pooled clinical studies. J. Affect. Disord. 2018, 227, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Hobart, M.; Zhang, P.; Skuban, A.; Brewer, C.; Hefting, N.; Sanchez, R.; McQuade, R.D. A Long-Term, Open-Label Study to Evaluate the Safety and Tolerability of Brexpiprazole as Adjunctive Therapy in Adults with Major Depressive Disorder. J. Clin. Psychopharmacol. 2019, 39, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Mard, F.; Davidsen, C.K.; Baker, R.A. Adjunctive brexpiprazole in patients with major depressive disorder and irritability: An exploratory study. J. Clin. Psychiatry 2016, 77, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.L.; Ota, A.; Perry, P.; Tsuneyoshi, K.; Weiller, E.; Baker, R.A. Adjunctive brexpiprazole in patients with major depressive disorder and anxiety symptoms: An exploratory study. Brain Behav. 2016, 6, e00520. [Google Scholar] [CrossRef]
- Brown, E.S.; Khaleghi, N.; Van Enkevort, E.; Ivleva, E.; Nakamura, A.; Holmes, T.; Mason, B.L.; Escalante, C. A pilot study of brexpiprazole for bipolar depression. J. Affect. Disord. 2019, 249, 315–318. [Google Scholar] [CrossRef]
- Vieta, E.; Sachs, G.; Chang, D.; Hellsten, J.; Brewer, C.; Peters-Strickland, T.; Hefting, N. Two randomized, double-blind, placebo-controlled trials and one open-label, long-term trial of brexpiprazole for the acute treatment of bipolar mania. J. Psychopharmacol. 2021, 35, 971–982. [Google Scholar] [CrossRef]
- Lepola, U.; Hefting, N.; Zhang, D.; Hobart, M. Adjunctive brexpiprazole for elderly patients with major depressive disorder: An open-label, long-term safety and tolerability study. Int. J. Geriatr. Psychiatry 2018, 33, 1403–1410. [Google Scholar] [CrossRef]
- Barton, B.B.; Segger, F.; Fischer, K.; Obermeier, M.; Musil, R. Update on weight-gain caused by antipsychotics: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2020, 19, 295–314. [Google Scholar] [CrossRef]
- Ichinose, M.; Miura, I.; Horikoshi, S.; Yamamoto, S.; Kanno-Nozaki, K.; Watanabe, K.; Yabe, H. Effect of Switching to Brexpiprazole on Plasma Homovanillic Acid Levels and Antipsychotic-Related Side Effects in Patients with Schizophrenia or Schizoaffective Disorder. Neuropsychiatr. Dis. Treat. 2021, 17, 1047–1053. [Google Scholar] [CrossRef]
- Ishigooka, J.; Inada, K.; Niidome, K.; Aoki, K.; Kojima, Y.; Iwashita, S.; Yamada, S. Safety of switching to brexpiprazole in Japanese patients with schizophrenia: A post-hoc analysis of a long-term open-label study. Hum. Psychopharmacol. Clin. Exp. 2021, 36, e2777. [Google Scholar] [CrossRef]
- Ivkovic, J.; Lindsten, A.; George, V.; Eriksson, H.; Hobart, M. Effect of Brexpiprazole on Prolactin: An Analysis of Short-and Long-Term Studies in Schizophrenia. J. Clin. Psychopharmacol. 2019, 39, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.H.; Ivkovic, J.; Chen, D.; George, V.; Hobart, M. Effect of Brexpiprazole on Prolactin and Sexual Functioning: An Analysis of Short- And Long-Term Study Data in Major Depressive Disorder. J. Clin. Psychopharmacol. 2020, 40, 560–567. [Google Scholar] [CrossRef]
- Okayasu, H.; Shinozaki, T.; Takano, Y.; Sugawara, N.; Fujii, K.; Yasui-Furukori, N.; Ozeki, Y.; Shimoda, K. Effects of Antipsychotics on Arrhythmogenic Parameters in Schizophrenia Patients: Beyond Corrected QT Interval. Neuropsychiatr. Dis. Treat. 2021, 17, 239–249. [Google Scholar] [CrossRef]
- Wichniak, A.; Siwek, M.; Rymaszewska, J.; Janas-Kozik, M.; Wolańczyk, T.; Bieńkowski, P.; Dudek, D.; Heitzman, J.; Szulc, A.; Samochowiec, J. The position statement of the Working Group of the Polish Psychiatric Association on the use of D2/D3 dopamine receptor partial agonists in special populations. Psychiatr Pol. 2021, 55, 967–987. [Google Scholar] [CrossRef] [PubMed]
- Wichniak, A.; Samochowiec, J.; Szulc, A.; Dudek, D.; Heitzman, J.; Janas-Kozik, M.; Wolańczyk, T.; Rymaszewska, J.; Siwek, M.; Bieńkowski, P. The position statement of the Working Group of the Polish Psychiatric Association on the use of D2/D3 dopamine receptor partial agonists in the treatment of mental disorders. Psychiatr Pol. 2021, 55, 941–966. [Google Scholar] [CrossRef]
- Citrome, L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: Absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017, 37, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Shimizu, H.; Yamashita, R.; Washida, K.; Takeda, T.; Aoki, S. Association between previous high-dose antipsychotic therapy and brexpiprazole discontinuation after the initiation of brexpiprazole in patients with schizophrenia or schizoaffective disorder. Int. Clin. Psychopharmacol. 2020, 35, 98–104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).