Anhedonia in Relation to Reward and Effort Learning in Young People with Depression Symptoms
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.2.1. Questionnaires
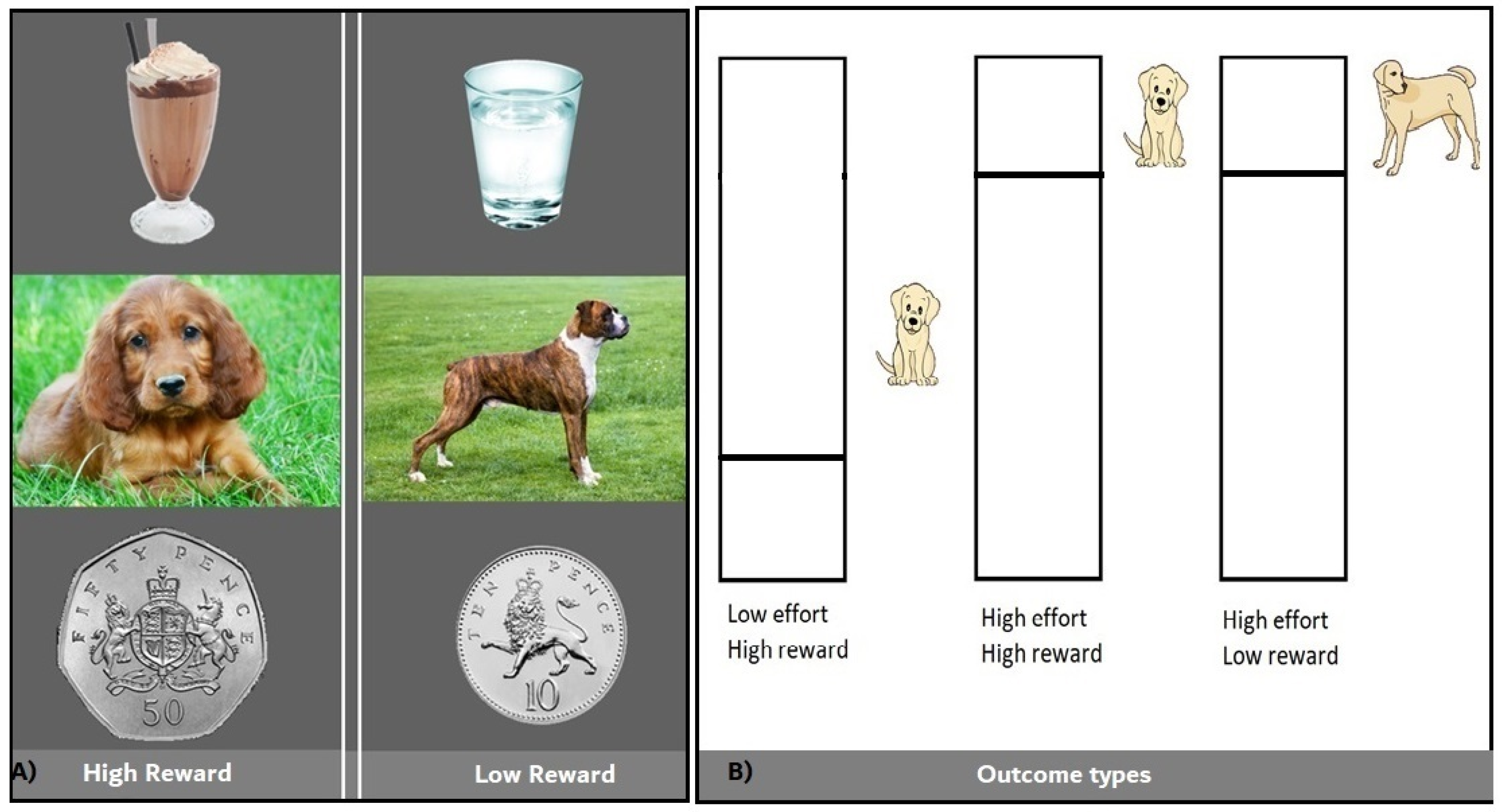
2.2.2. Learning Task
2.3. Analysis
3. Results
3.1. Demographics and Questionnaire Measures
3.2. Trait Anhedonia and Subjective Liking, Wanting and Willingness to Exert Effort
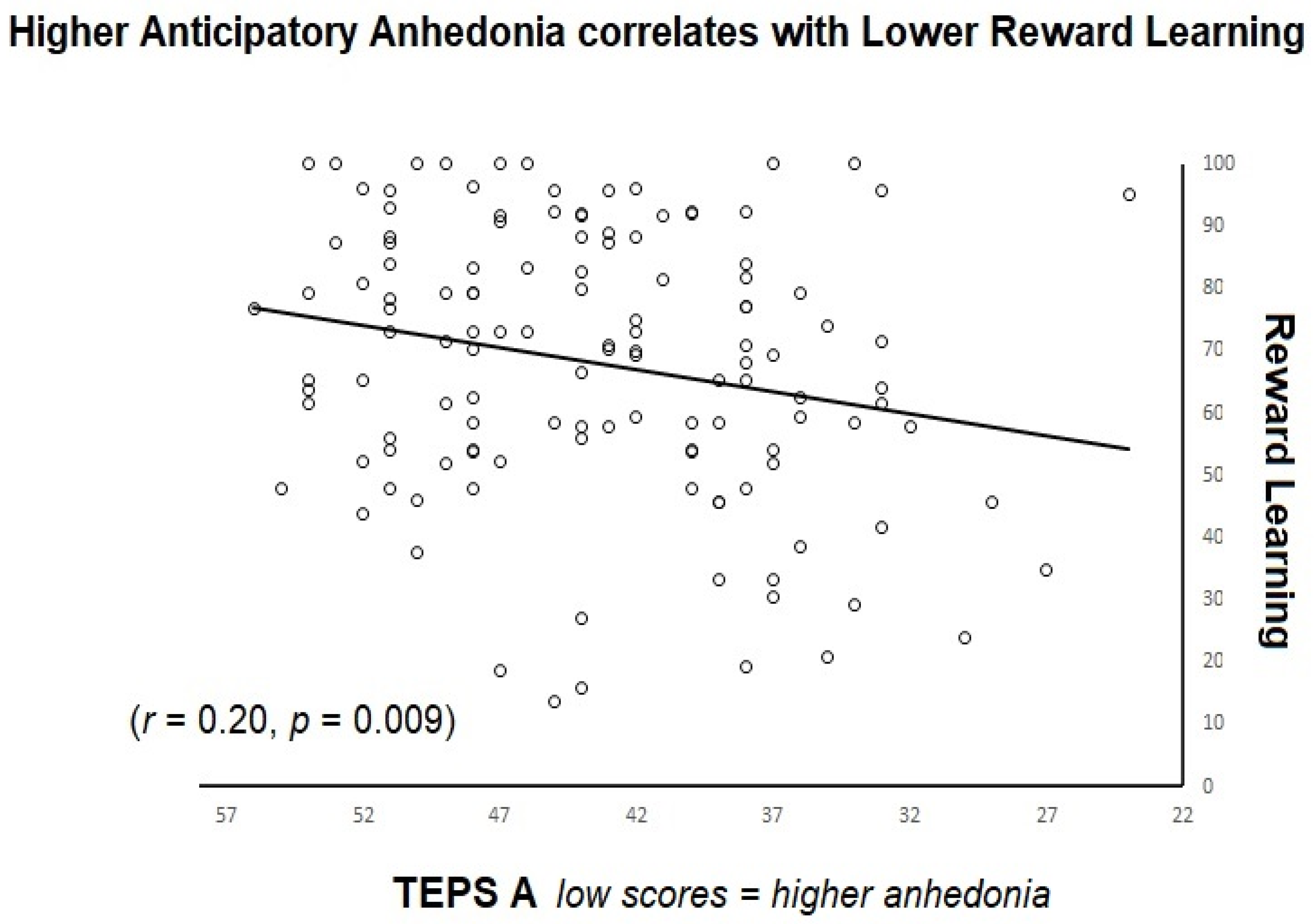
3.3. Trait Anhedonia and Task Measures
3.4. Differences between Trial Types and Reward Conditions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017.
- Argyropoulos, S.V.; Nutt, D.J. Anhedonia revisited: Is there a role for dopamine-targeting drugs for depression? J. Psychopharmacol. 2013, 27, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; McCabe, C. Can understanding reward help illuminate anhedonia? Curr. Behav. Neurosci. Rep. 2019, 6, 236–242. [Google Scholar] [CrossRef]
- Rizvi, S.J.; Pizzagalli, D.A.; Sproule, B.A.; Kennedy, S.H. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci. Biobehav. Rev. 2016, 65, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Treadway, M.T.; Zald, D.H. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011, 35, 537–555. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C. Linking anhedonia symptoms with behavioural and neural reward responses in adolescent depression. Curr. Opin. Behav. Sci. 2018, 22, 143–151. [Google Scholar] [CrossRef]
- Ely, B.A.; Nguyen, T.N.; Tobe, R.H.; Walker, A.M.; Gabbay, V. Multimodal Investigations of Reward Circuitry and Anhedonia in Adolescent Depression. Front. Psychiatry 2021, 12, 678709. [Google Scholar] [CrossRef]
- Forbes, E.E.; Dahl, R.E. Research Review: Altered reward function in adolescent depression: What, when and how? J. Child Psychol. Psychiatry 2012, 53, 3–15. [Google Scholar] [CrossRef]
- Blanco, N.J.; Otto, A.R.; Maddox, W.T.; Beevers, C.G.; Love, B.C. The influence of depression symptoms on exploratory decision-making. Cognition 2013, 129, 563–568. [Google Scholar] [CrossRef]
- Cooper, J.A.; Gorlick, M.A.; Denny, T.; Worthy, D.A.; Beevers, C.G.; Maddox, W.T. Training attention improves decision making in individuals with elevated self-reported depressive symptoms. Cogn. Affect. Behav. Neurosci. 2014, 14, 729–741. [Google Scholar] [CrossRef]
- Herzallah, M.M.; Moustafa, A.A.; Natsheh, J.Y.; Abdellatif, S.M.; Taha, M.B.; Tayem, Y.I.; Sehwail, M.A.; Amleh, I.; Petrides, G.; Myers, C.E. Learning from negative feedback in patients with major depressive disorder is attenuated by SSRI antidepressants. Front. Integr. Neurosci. 2013, 7, 67. [Google Scholar] [CrossRef]
- Kumar, P.; Goer, F.; Murray, L.; Dillon, D.G.; Beltzer, M.L.; Cohen, A.L.; Brooks, N.H.; Pizzagalli, D.A. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology 2018, 43, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Kunisato, Y.; Okamoto, Y.; Ueda, K.; Onoda, K.; Okada, G.; Yoshimura, S.; Suzuki, S.-i.; Samejima, K.; Yamawaki, S. Effects of depression on reward-based decision making and variability of action in probabilistic learning. J. Behav. Ther. Exp. Psychiatry 2012, 43, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Pechtel, P.; Dutra, S.J.; Goetz, E.L.; Pizzagalli, D.A. Blunted reward responsiveness in remitted depression. J. Psychiatr. Res. 2013, 47, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Robinson, O.J.; Cools, R.; Carlisi, C.O.; Sahakian, B.J.; Drevets, W.C. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am. J. Psychiatry 2012, 169, 152–159. [Google Scholar] [CrossRef]
- Kangas, B.D.; Der-Avakian, A.; Pizzagalli, D.A. Probabilistic reinforcement learning and anhedonia. In Anhedonia: Preclinical, Translational, and Clinical Integration; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Hankin, B.L.; Wetter, E.K.; Flory, K. Appetitive motivation and negative emotion reactivity among remitted depressed youth. J. Clin. Child Adolesc. Psychol. 2012, 41, 611–620. [Google Scholar] [CrossRef]
- Dickson, J.M.; Moberly, N.J.; O’Dea, C.; Field, M. Goal fluency, pessimism and disengagement in depression. PLoS ONE 2016, 11, e0166259. [Google Scholar] [CrossRef]
- Dickson, J.M.; MacLeod, A.K. Dysphoric adolescents’ causal explanations and expectancies for approach and avoidance goals. J. Adolesc. 2006, 29, 177–191. [Google Scholar] [CrossRef]
- Salmela-Aro, K.; Nurmi, J.-E. Depressive symptoms and personal project appraisals: A cross-lagged longitudinal study. Personal. Individ. Differ. 1996, 21, 373–381. [Google Scholar] [CrossRef]
- Silvia, P.J.; Eddington, K.M.; Harper, K.L.; Burgin, C.J.; Kwapil, T.R. Appetitive motivation in depressive anhedonia: Effects of piece-rate cash rewards on cardiac and behavioral outcomes. Motiv. Sci. 2020, 6, 259. [Google Scholar] [CrossRef]
- Hershenberg, R.; Satterthwaite, T.D.; Daldal, A.; Katchmar, N.; Moore, T.M.; Kable, J.W.; Wolf, D.H. Diminished effort on a progressive ratio task in both unipolar and bipolar depression. J. Affect. Disord. 2016, 196, 97–100. [Google Scholar] [CrossRef]
- Treadway, M.T.; Bossaller, N.A.; Shelton, R.C.; Zald, D.H. Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. J. Abnorm. Psychol. 2012, 121, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Huang, J.; Zhu, C.Y.; Wang, Y.F.; Cheung, E.F.C.; Chan, R.C.K.; Xie, G.R. Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 2014, 220, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Cléry-Melin, M.-L.; Schmidt, L.; Lafargue, G.; Baup, N.; Fossati, P.; Pessiglione, M. Why don’t you try harder? An investigation of effort production in major depression. PLoS ONE 2011, 6, e23178. [Google Scholar]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Hawton, K.; Saunders, K.E.; O’Connor, R.C. Self-harm and suicide in adolescents. Lancet 2012, 379, 2373–2382. [Google Scholar] [CrossRef]
- Clayborne, Z.M.; Varin, M.; Colman, I. Systematic review and meta-analysis: Adolescent depression and long-term psychosocial outcomes. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 72–79. [Google Scholar] [CrossRef]
- Dunn, V.; Goodyer, I.M. Longitudinal investigation into childhood-and adolescence-onset depression: Psychiatric outcome in early adulthood. Br. J. Psychiatry 2006, 188, 216–222. [Google Scholar] [CrossRef]
- Goodyer, I.M.; Reynolds, S.; Barrett, B.; Byford, S.; Dubicka, B.; Hill, J.; Holland, F.; Kelvin, R.; Midgley, N.; Roberts, C. Cognitive behavioural therapy and short-term psychoanalytical psychotherapy versus a brief psychosocial intervention in adolescents with unipolar major depressive disorder (IMPACT): A multicentre, pragmatic, observer-blind, randomised controlled superiority trial. Lancet Psychiatry 2017, 4, 109–119. [Google Scholar]
- McMakin, D.L.; Olino, T.M.; Porta, G.; Dietz, L.J.; Emslie, G.; Clarke, G.; Wagner, K.D.; Asarnow, J.R.; Ryan, N.D.; Birmaher, B.; et al. Anhedonia Predicts Poorer Recovery Among Youth With Selective Serotonin Reuptake Inhibitor Treatment-Resistant Depression. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 404–411. [Google Scholar] [CrossRef]
- Rzepa, E.; McCabe, C. Dimensional Anhedonia and the Adolescent brain: Reward and Aversion Anticipation, Effort and Consummation. BJPsych Open 2019, 5, e99. [Google Scholar] [CrossRef]
- Vinckier, F.; Jaffre, C.; Gauthier, C.; Smajda, S.; Abdel-Ahad, P.; Le Bouc, R.; Daunizeau, J.; Fefeu, M.; Borderies, N.; Plaze, M. Elevated effort cost identified by computational modeling as a distinctive feature explaining multiple behaviors in patients with depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Gard, D.E.; Kring, A.M.; Gard, M.G.; Horan, W.P.; Green, M.F. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophr. Res. 2007, 93, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Skvortsova, V.; Palminteri, S.; Pessiglione, M. Learning To Minimize Efforts versus Maximizing Rewards: Computational Principles and Neural Correlates. J. Neurosci. 2014, 34, 15621–15630. [Google Scholar] [CrossRef]
- Skvortsova, V.; Degos, B.; Welter, M.-L.; Vidailhet, M.; Pessiglione, M. A selective role for dopamine in learning to maximize reward but not to minimize effort: Evidence from patients with Parkinson’s disease. J. Neurosci. 2017, 37, 6087–6097. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Williams, J.B.; Gibbon, M.; First, M.B. Structured Clinical Interview for the DSM–IV (SCID–I/P); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J. Personal. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Clark, L.A.; Watson, D. Constructing validity: Basic issues in objective scale development. In Methodological Issues and Strategies in Clinical Research; American Psychological Association: Washington, DC, USA, 2016. [Google Scholar]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The eating attitudes test: Psychometric features and clinical correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef]
- Lehmann, V.; Huis, E.M.; Vingerhoets, A.J. The human and animal baby schema effect: Correlates of individual differences. Behav. Process. 2013, 94, 99–108. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple hypothesis testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Watson, R.; Harvey, K.; McCabe, C.; Reynolds, S. Understanding anhedonia: A qualitative study exploring loss of interest and pleasure in adolescent depression. Eur. Child Adolesc. Psychiatry 2020, 29, 489–499. [Google Scholar] [CrossRef]
- Renz, K.E.; Lincoln, T.M. The effect of salience of rewards on effort-based decision making in psychotic disorders. BMC Psychiatry 2022, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Palminteri, S.; Kilford, E.J.; Coricelli, G.; Blakemore, S.-J. The computational development of reinforcement learning during adolescence. PLoS Comput. Biol. 2016, 12, e1004953. [Google Scholar] [CrossRef]
- Thomsen, K.R. Measuring anhedonia: Impaired ability to pursue, experience, and learn about reward. Front. Psychol. 2015, 6, 1409. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, E.; Pizzagalli, D.A.; Demyttenaere, K.; Hompes, T.; Sienaert, P.; de Boer, P.; Schmidt, M.; Claes, S. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry 2013, 73, 639–645. [Google Scholar] [CrossRef] [PubMed]




| Mean | SD | |
|---|---|---|
| Age | 20.64 | 2.74 |
| BDI | 11.95 | 10.164 |
| EAT | 8.41 | 8.037 |
| TEPS_A | 43.50 | 6.759 |
| TEPS_C | 35.53 | 6.140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frey, A.-L.; Kaya, M.S.; Adeniyi, I.; McCabe, C. Anhedonia in Relation to Reward and Effort Learning in Young People with Depression Symptoms. Brain Sci. 2023, 13, 341. https://doi.org/10.3390/brainsci13020341
Frey A-L, Kaya MS, Adeniyi I, McCabe C. Anhedonia in Relation to Reward and Effort Learning in Young People with Depression Symptoms. Brain Sciences. 2023; 13(2):341. https://doi.org/10.3390/brainsci13020341
Chicago/Turabian StyleFrey, Anna-Lena, M. Siyabend Kaya, Irina Adeniyi, and Ciara McCabe. 2023. "Anhedonia in Relation to Reward and Effort Learning in Young People with Depression Symptoms" Brain Sciences 13, no. 2: 341. https://doi.org/10.3390/brainsci13020341
APA StyleFrey, A.-L., Kaya, M. S., Adeniyi, I., & McCabe, C. (2023). Anhedonia in Relation to Reward and Effort Learning in Young People with Depression Symptoms. Brain Sciences, 13(2), 341. https://doi.org/10.3390/brainsci13020341





