Depression Is Associated with an Increased Risk of Subsequent Cancer Diagnosis: A Retrospective Cohort Study with 235,404 Patients
Abstract
Highlights
- Our findings indicate patients with depression have an increased risk of cancer, ranging from 10% to 39% increased risk depending on the type of cancer.
- Cancer risk was highest in patients with depression for lung, GI, breast, and urinary cancer.
- Our findings elucidate the rarely investigated direct impact of depression on cancer risk.
Abstract
1. Introduction
2. Method
2.1. Database
2.2. Study Outcomes and Variables
2.3. Study Population
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Cancer Diagnosis Risk
3.3. Incidence of Cancer Diagnosis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CG | Comparator group |
| COPD | Chronic obstructive pulmonary disease |
| DA | Disease Analyzer |
| GI | Gastro-intestinal |
| HPA | Hypothalamic–pituitary–adrenal |
| HR | Hazard ratio |
| IR | Incidence rate |
References
- Rehm, J.; Shield, K.D. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr. Psychiatry Rep. 2019, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, F.; Höfler, M.; Siegert, J.; Mack, S.; Gerschler, A.; Scholl, L.; Busch, M.A.; Hapke, U.; Maske, U.; Seiffert, I.; et al. Twelve-month prevalence, comorbidity and correlates of mental disorders in Germany: The Mental Health Module of the German Health Interview and Examination Survey for Adults (DEGS1-MH). Int. J. Methods Psychiatr. Res. 2014, 23, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Thornicroft, G.; Chatterji, S.; Evans-Lacko, S.; Gruber, M.; Sampson, N.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Andrade, L.; Borges, G.; et al. Undertreatment of people with major depressive disorder in 21 countries. Br. J. Psychiatry 2017, 210, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Nübel, J.; Jacobi, F.; Bätzing, J.; Holstiege, J. Mental and somatic comorbidity of depression: A comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC Psychiatry 2020, 20, 142. [Google Scholar] [CrossRef]
- Hartung, T.J.; Brähler, E.; Faller, H.; Härter, M.; Hinz, A.; Johansen, C.; Keller, M.; Koch, U.; Schulz, H.; Weis, J.; et al. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur. J. Cancer 2017, 72, 46–53. [Google Scholar] [CrossRef]
- Krebber, A.M.H.; Buffart, L.M.; Kleijn, G.; Riepma, I.C.; De Bree, R.; Leemans, C.R.; Becker, A.; Brug, J.; Van Straten, A.; Cuijpers, P.; et al. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psychooncology 2014, 23, 121–130. [Google Scholar] [CrossRef]
- Linden, W.; Vodermaier, A.; MacKenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef]
- Cuijpers, W.J.M.J.; Schoevers, R.A. Increased mortality in depressive disorders: A review. Curr. Psychiatry Rep. 2004, 6, 430–437. [Google Scholar] [CrossRef]
- Katon, W.; Lin, E.H.B.; Kroenke, K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen. Hosp. Psychiatry 2007, 29, 147–155. [Google Scholar] [CrossRef]
- DiMatteo, M.R.; Lepper, H.S.; Croghan, T.W. Depression Is a Risk Factor for Noncompliance with Medical Treatment: Meta-analysis of the Effects of Anxiety and Depression on Patient Adherence. Arch. Intern. Med. 2000, 160, 2101–2107. [Google Scholar] [CrossRef]
- Ko, A.; Kim, K.; Sik Son, J.; Park, H.Y.; Park, S.M. Association of pre-existing depression with all-cause, cancer-related, and noncancer-related mortality among 5-year cancer survivors: A population-based cohort study. Sci. Rep. 2019, 9, 18334. [Google Scholar] [CrossRef]
- Avila, C.; Holloway, A.C.; Hahn, M.K.; Morrison, K.M.; Restivo, M.; Anglin, R.; Taylor, V.H. An Overview of Links between Obesity and Mental Health. Curr. Obes. Rep. 2015, 4, 303–310. [Google Scholar] [CrossRef]
- Luger, T.M.; Suls, J.; Vander Weg, M.W. How robust is the association between smoking and depression in adults? A meta-analysis using linear mixed-effects models. Addict. Behav. 2014, 39, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Moussas, G.I.; Papadopoulou, A.G. Substance abuse and cancer. Psychiatriki 2017, 28, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef]
- Varela-Rey, M.; Woodhoo, A.; Martinez-Chantar, M.-L.; Mato, J.M.; Lu, S.C. Alcohol, DNA methylation, and cancer. Alcohol Res. Curr. Rev. 2013, 35, 25–35. [Google Scholar]
- Jacob, L.; Bleicher, L.; Kostev, K.; Kalder, M. Prevalence of depression, anxiety and their risk factors in German women with breast cancer in general and gynecological practices. J. Cancer Res. Clin. Oncol. 2016, 142, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Pinquart, M.; Duberstein, P.R. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.R. Depression in cancer patients: Pathogenesis, implications and treatment (review). Oncol. Lett. 2015, 9, 1509–1514. [Google Scholar] [CrossRef]
- Goodwin, J.S.; Zhang, D.D.; Ostir, G.V. Effect of Depression on Diagnosis, Treatment, and Survival of Older Women with Breast Cancer. J. Am. Geriatr. Soc. 2004, 52, 106–111. [Google Scholar] [CrossRef]
- Hjerl, K.; Andersen, E.W.; Keiding, N.; Mouridsen, H.T.; Mortensen, P.B.; Jørgensen, T. Depression as a prognostic factor for breast cancer mortality. Psychosomatics 2003, 44, 24–30. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Nietert, P.J.; Egede, L.E. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. Gen. Hosp. Psychiatry 2006, 28, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Li, J.Q.; Shi, J.F.; Que, J.Y.; Liu, J.J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.Q.; Qiao, Y.L.; et al. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 2020, 25, 1487–1499. [Google Scholar] [CrossRef]
- Rathmann, W.; Bongaerts, B.; Carius, H.J.; Kruppert, S.; Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Kalder, M.; Kostev, K. Incidence of depression and anxiety among women newly diagnosed with breast or genital organ cancer in Germany. Psychooncology 2017, 26, 1535–1540. [Google Scholar] [CrossRef]
- Robert Koch-Institut. Krebs in Deutschland für 2017/2018; Robert Koch-Institut: Berlin, Germany, 2021.
- Gathinji, M.; McGirt, M.J.; Attenello, F.J.; Chaichana, K.L.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quinones-Hinojosa, A. Association of preoperative depression and survival after resection of malignant brain astrocytoma. Surg. Neurol. 2009, 71, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA J. Am. Med. Assoc. 2011, 306, 737–745. [Google Scholar] [CrossRef]
- Gram, I.T.; Park, S.Y.; Wilkens, L.R.; Haiman, C.A.; Le Marchand, L. Smoking-Related Risks of Colorectal Cancer by Anatomical Subsite and Sex. Am. J. Epidemiol. 2020, 189, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Kispert, S.; McHowat, J. Recent insights into cigarette smoking as a lifestyle risk factor for breast cancer. Breast Cancer Targets Ther. 2017, 9, 127–132. [Google Scholar] [CrossRef]
- Nomura, A.M.Y.; Wilkens, L.R.; Henderson, B.E.; Epplein, M.; Kolonel, L.N. The association of cigarette smoking with gastric cancer: The multiethnic cohort study. Cancer Causes Control CCC 2012, 23, 51–58. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, H.; Zheng, S.; Ding, Z.; Chen, Z.; Jin, W.; Wang, L.; Wang, Z.; Fei, Y.; Zhang, S.; et al. Gender susceptibility for cigarette smoking-attributable lung cancer: A systematic review and meta-analysis. Lung Cancer 2014, 85, 351–360. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Franzen, P.L.; Buysse, D.J. Sleep disturbances and depression: Risk relationships for subsequent depression and therapeutic implications. Dialogues Clin. Neurosci. 2022, 10, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2018, 24, 18–33. [Google Scholar] [CrossRef]
- De Nunzio, C.; Andriole, G.L.; Thompson, I.M.; Freedland, S.J. Smoking and Prostate Cancer: A Systematic Review. Eur. Urol. Focus 2015, 1, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Patten, S.B.; Williams, J.V.A.; Lavorato, D.H.; Wang, J.L.; Jetté, N.; Sajobi, T.T.; Fiest, K.M.; Bulloch, A.G.M. Patterns of association of chronic medical conditions and major depression. Epidemiol. Psychiatr. Sci. 2018, 27, 42–50. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Musselman, D.; Nemeroff, C. The biology of depression in cancer and the relationship between depression and cancer progression. Int. Rev. Psychiatry 2014, 26, 16–30. [Google Scholar] [CrossRef]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry 2013, 70, 31–41. [Google Scholar] [CrossRef]
- Mogavero, M.P.; DelRosso, L.M.; Fanfulla, F.; Bruni, O.; Ferri, R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med. Rev. 2021, 56, 101409. [Google Scholar] [CrossRef]
- DiMatteo, M.R.; Haskard-Zolnierek, K.B. Impact of Depression on Treatment Adherence and Survival from Cancer. In Depression and Cancer; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 101–124. [Google Scholar] [CrossRef]
- Gleason, O.C.; Pierce, A.M.; Walker, A.E.; Warnock, J.K. The two-way relationship between medical illness and late-life depression. Psychiatr. Clin. N. Am. 2013, 36, 533–544. [Google Scholar] [CrossRef]
- Zimmaro, L.A.; Sephton, S.E.; Siwik, C.J.; Phillips, K.M.; Rebholz, W.N.; Kraemer, H.C.; Giese-Davis, J.; Wilson, L.; Bumpous, J.M.; Cash, E.D. Depressive symptoms predict head and neck cancer survival: Examining plausible behavioral and biological pathways. Cancer 2018, 124, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Giese-Davis, J.; Collie, K.; Rancourt, K.M.; Neri, E.; Kraemer, H.C.; Spiegel, D. Decrease in Depression Symptoms Is Associated with Longer Survival in Patients with Metastatic Breast Cancer: A Secondary Analysis. J. Clin. Oncol. 2010, 29, 413–420. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Risk Factors: Age—NCI. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/age (accessed on 16 July 2022).
- Fiske, A.; Gatz, M.; Pedersen, N.L. Depressive Symptoms and Aging: The Effects of Illness and Non-Health-Related Events. J. Gerontol. Ser. B 2003, 58, P320–P328. [Google Scholar] [CrossRef] [PubMed]
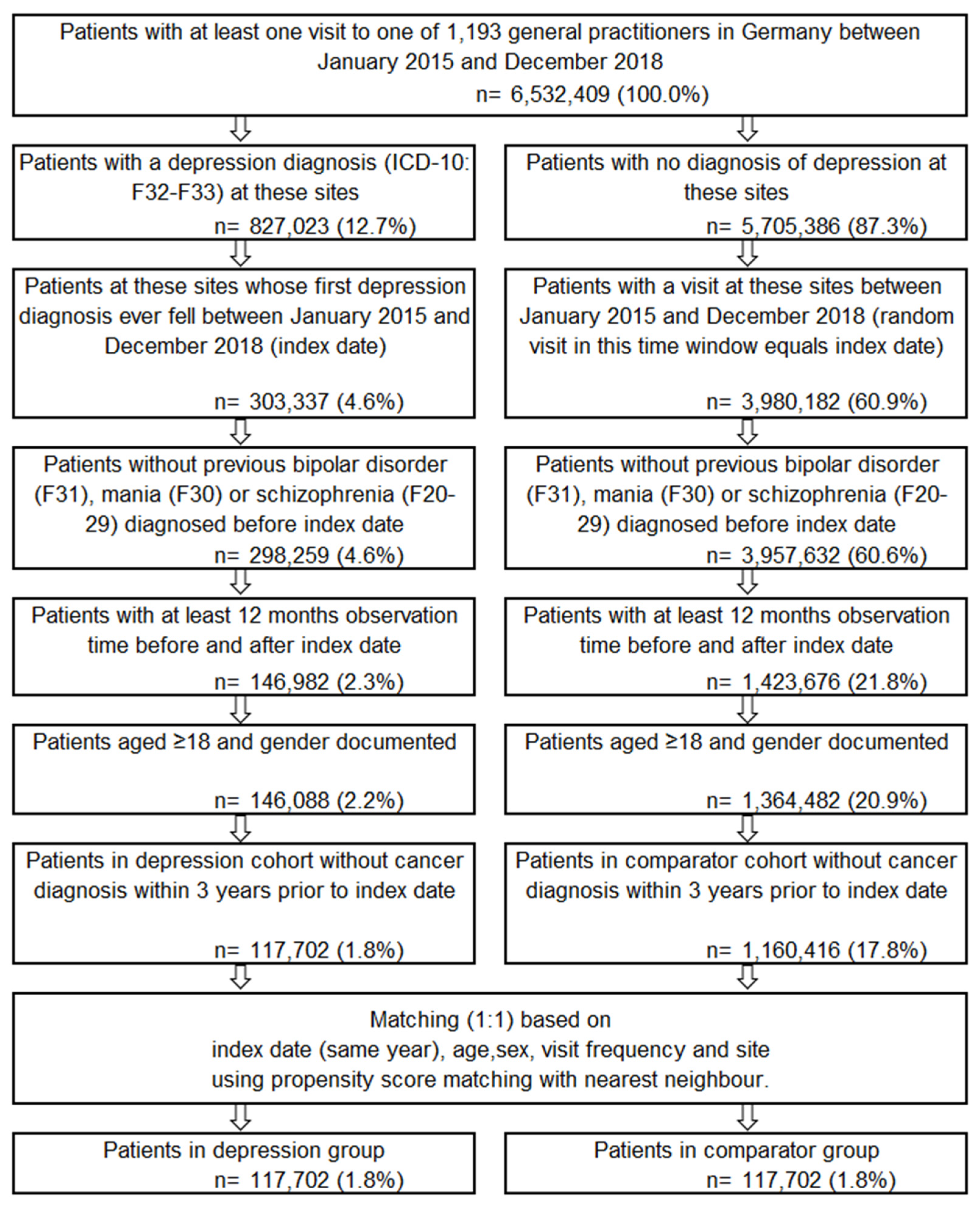
| Matched Groups | Group Difference (p-Values) + | ||||
|---|---|---|---|---|---|
| Patients with Depression | Patients without Depression | ||||
| N Patients | 117,702 | 117,702 | - | ||
| Diagnosis year | |||||
| 2015 | 26,740 | 22.7% | 29,496 | 25.1% | - |
| 2016 | 31,544 | 26.8% | 27,825 | 23.6% | - |
| 2017 | 29,848 | 25.4% | 28,922 | 24.6% | - |
| 2018 | 29,570 | 25.1% | 31,459 | 26.7% | - |
| Sex | |||||
| Female | 71,073 | 60.4% | 71,138 | 60.4% | 0.78 a |
| Male | 46,629 | 39.6% | 46,564 | 39.6% | |
| Age | |||||
| Mean (SD) | 55.0 | 18.4 | 55.0 | 19.9 | 0.8 b |
| 18–30 | 12,945 | 11.0% | 17,096 | 14.5% | - |
| >30–40 | 16,133 | 13.7% | 15,710 | 13.3% | - |
| >40–50 | 17,956 | 15.3% | 15,186 | 12.9% | - |
| >50–60 | 26,018 | 22.1% | 21,109 | 17.9% | - |
| >60–70 | 20,523 | 17.4% | 19,188 | 16.3% | - |
| >70–80 | 10,826 | 9.2% | 15,050 | 12.8% | - |
| >80–90 | 10,589 | 9.0% | 11,603 | 9.9% | - |
| >90 | 2712 | 2.3% | 2760 | 2.3% | - |
| Insurance type | |||||
| Statutory | 112,369 | 95.5% | 107,273 | 91.1% | <0.0001 a |
| Private | 5333 | 4.5% | 10,429 | 8.9% | |
| Index diagnosis severity | |||||
| Mild | 12,183 | 10.4% | 0 | 0.0% | - |
| Moderate | 27,165 | 23.1% | 0 | 0.0% | - |
| Severe | 6615 | 5.6% | 0 | 0.0% | - |
| Other and undefined | 71,739 | 60.9% | 0 | 0.0% | - |
| None, missing | 0 | 0.0% | 117,702 | 100.0% | - |
| Visit frequency 1 | |||||
| Mean (SD) | 2.5 | 1.1 | 2.5 | 1.1 | 0.8 c |
| Comorbidities 2 | |||||
| Hypertension (I10) | 1,440,870 | 15.4% | 1,689,483 | 21.0% | - |
| Diabetes (E10–14) | 462,626 | 4.9% | 514,175 | 6.4% | - |
| Lipid metabolism disorder (E78) | 304,267 | 3.2% | 342,310 | 4.3% | - |
| Ischemic heart diseases (I20–I25) | 236,564 | 2.5% | 243,337 | 3.0% | - |
| Osteoarthritis (M15–M19) | 164,548 | 1.8% | 138,282 | 1.7% | - |
| Atrial fibrillation (I48) | 96,077 | 1.0% | 102,862 | 1.3% | - |
| Obesity (E65–E68) | 51,108 | 0.5% | 38,802 | 0.5% | - |
| Heart failure (I50) | 79,010 | 0.8% | 75,803 | 0.9% | - |
| Osteoporosis (M80–M81) | 58,795 | 0.6% | 56,608 | 0.7% | - |
| Dementia (F01, F03, G30) | 34,190 | 0.4% | 19,896 | 0.2% | - |
| Cerebrovascular disease (I60–63, G45) | 29,052 | 0.3% | 23,300 | 0.3% | - |
| COPD (j44) | 123,727 | 1.3% | 102,722 | 1.3% | - |
| Other, non-chronic | 5,226,238 | 55.8% | 4,411,909 | 54.9% | - |
| Other psychiatric disorders (F) | 970,870 | 10.4% | 207,620 | 2.6% | - |
| Neuroses, somatoform disorders (F45–48) | 161,893 | 1.7% | 57,996 | 0.7% | - |
| Anxiety disorders (F40–43) | 206,737 | 2.2% | 62,595 | 0.8% | - |
| Substance use (F10–F19) | 76,484 | 0.8% | 42,907 | 0.5% | - |
| Behavioral disorder with physical disfunction (F50–F59) | 29,756 | 0.3% | 17,419 | 0.2% | - |
| Other not specified (F99) | 7981 | 0.1% | 3126 | 0.0% | - |
| Baseline observation time (years) 3 | |||||
| Mean (SD) | 7.6 | 5.8 | 7.3 | 5.5 | <0.0001 a |
| Follow-up time (years) 3 | |||||
| Mean (SD) | 3.9 | 1.4 | 3.8 | 1.4 | <0.0001 a |
| Matching | |||||
| Mean caliper | 0.8 | - | |||
| Number of Events | Comparative Risk | |||||
|---|---|---|---|---|---|---|
| Patients with Depression | Patients without Depression | Patients with Depression vs. Patients without Depression (Ref) | ||||
| Group | N Events (% 1) | N Events (% 1) | HR | 96% CI (Lower–Upper) | p-Value | |
| Any cancer diagnosis 2 | 4.9% | 4.1% | 1.18 | 1.14 | 1.23 | <0.0001 |
| By age group (in years) | ||||||
| 18–30 | 0.5% | 0.4% | 1.36 | 0.97 | 1.90 | 0.0725 |
| >30–40 | 1.0% | 0.7% | 1.40 | 1.10 | 1.78 | 0.0062 |
| >40–50 | 2.2% | 1.4% | 1.49 | 1.26 | 1.76 | <0.0001 |
| >50–60 | 3.8% | 2.9% | 1.30 | 1.17 | 1.43 | <0.0001 |
| >60–70 | 6.8% | 5.3% | 1.26 | 1.16 | 1.36 | <0.0001 |
| >70–80 | 10.4% | 8.6% | 1.22 | 1.13 | 1.32 | <0.0001 |
| >80–90 | 12.6% | 10.7% | 1.18 | 1.10 | 1.28 | <0.0001 |
| >90 | 11.6% | 9.3% | 1.26 | 1.07 | 1.48 | 0.0068 |
| By sex | ||||||
| Female | 4.9% | 3.8% | 1.27 | 1.21 | 1.33 | <0.0001 |
| Male | 4.9% | 4.5% | 1.08 | 1.02 | 1.15 | 0.0112 |
| Incidence Rate Cancer Diagnosis 1 | Rate Ratio 2 | |||||
|---|---|---|---|---|---|---|
| Patients with Depression | Patients without Depression | Patients with Depression vs. Patients without Depression (Ref) | ||||
| IR/1000 Person-Years | Est. | 95% CL (Lower-Upper) | p-Value | |||
| Any cancer diagnosis 3 | 15.23 | 12.62 | 1.22 | 1.18 | 1.27 | <0.0001 |
| By age group | ||||||
| 18–30 | 1.58 | 1.17 | 1.38 | 0.98 | 1.95 | 0.0639 |
| >30–40 | 2.92 | 2.04 | 1.41 | 1.11 | 1.8 | 0.0057 |
| >40–50 | 6.34 | 4.29 | 1.52 | 1.29 | 1.79 | <0.0001 |
| >50–60 | 11.03 | 8.43 | 1.35 | 1.22 | 1.49 | <0.0001 |
| >60–70 | 20.40 | 15.42 | 1.32 | 1.22 | 1.43 | <0.0001 |
| >70–80 | 31.65 | 25.39 | 1.24 | 1.15 | 1.33 | <0.0001 |
| >80–90 | 39.42 | 33.31 | 1.18 | 1.09 | 1.27 | <0.0001 |
| >90 | 41.23 | 32.53 | 1.27 | 1.08 | 1.49 | 0.0037 |
| By sex | ||||||
| Female | 15.10 | 11.50 | 1.32 | 1.26 | 1.39 | <0.0001 |
| Male | 15.45 | 14.30 | 1.09 | 1.03 | 1.16 | 0.0027 |
| By depression severity | ||||||
| Mild | 14.99 | - | - | - | - | - |
| Moderate | 14.24 | - | - | - | - | - |
| Severe | 16.77 | - | - | - | - | - |
| Other, undefined | 15.51 | - | - | - | - | - |
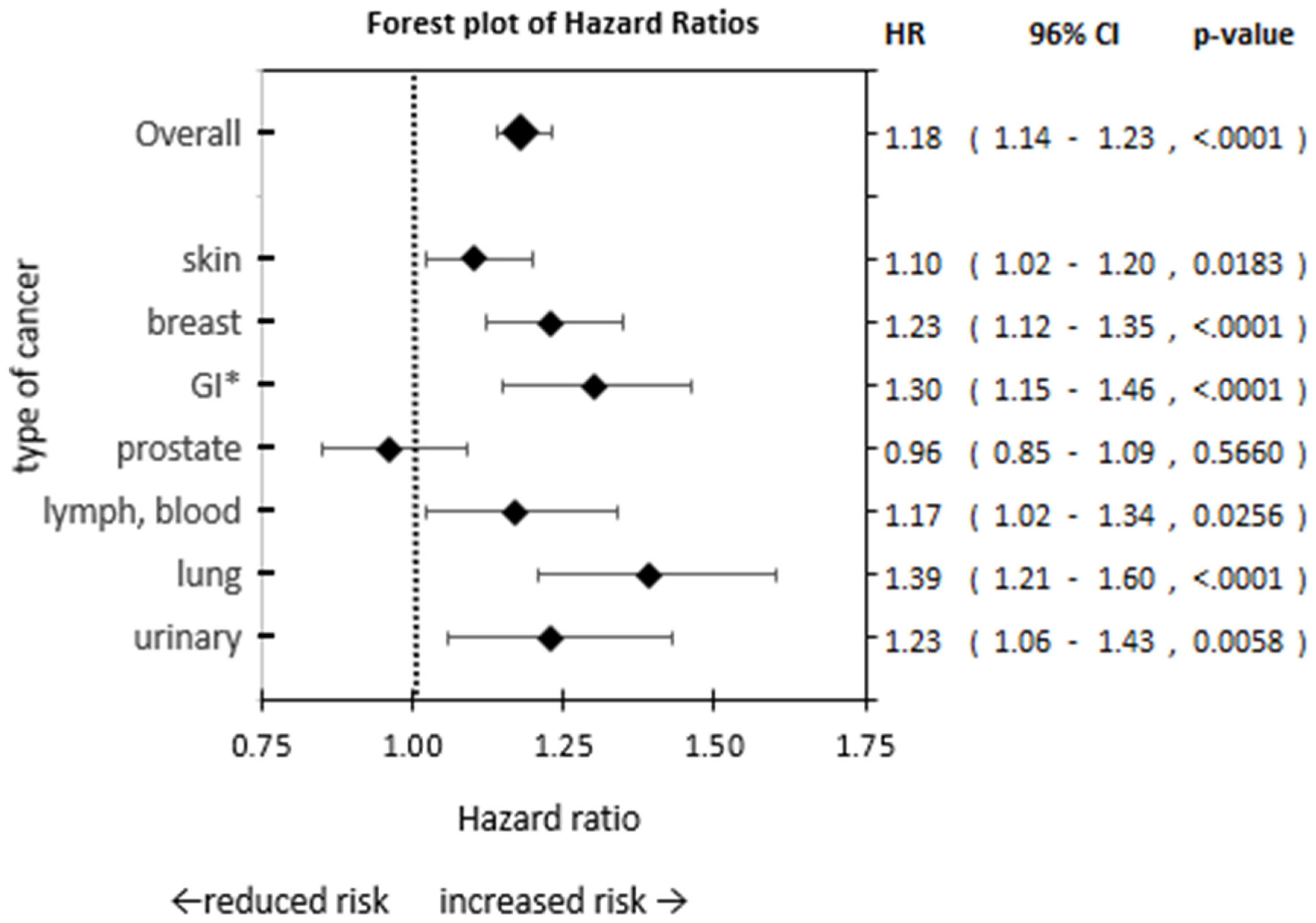
| By type of cancer | ||||||
| Skin | 2.62 | 2.37 | 1.12 | 1.03 | 1.22 | 0.0074 |
| Breast | 2.07 | 1.68 | 1.25 | 1.13 | 1.37 | <0.0001 |
| Gastro-intestinal | 1.44 | 1.11 | 1.31 | 1.17 | 1.47 | <0.0001 |
| Prostate | 1.05 | 1.09 | 0.98 | 0.86 | 1.11 | 0.7397 |
| Lymph, blood | 0.99 | 0.84 | 1.19 | 1.03 | 1.36 | 0.0144 |
| Lung 4 | 1.03 | 0.74 | 1.23 | 1.21 | 1.24 | <0.0001 |
| Urinary | 0.86 | 0.70 | 1.25 | 1.08 | 1.45 | 0.0031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mössinger, H.; Kostev, K. Depression Is Associated with an Increased Risk of Subsequent Cancer Diagnosis: A Retrospective Cohort Study with 235,404 Patients. Brain Sci. 2023, 13, 302. https://doi.org/10.3390/brainsci13020302
Mössinger H, Kostev K. Depression Is Associated with an Increased Risk of Subsequent Cancer Diagnosis: A Retrospective Cohort Study with 235,404 Patients. Brain Sciences. 2023; 13(2):302. https://doi.org/10.3390/brainsci13020302
Chicago/Turabian StyleMössinger, Hannah, and Karel Kostev. 2023. "Depression Is Associated with an Increased Risk of Subsequent Cancer Diagnosis: A Retrospective Cohort Study with 235,404 Patients" Brain Sciences 13, no. 2: 302. https://doi.org/10.3390/brainsci13020302
APA StyleMössinger, H., & Kostev, K. (2023). Depression Is Associated with an Increased Risk of Subsequent Cancer Diagnosis: A Retrospective Cohort Study with 235,404 Patients. Brain Sciences, 13(2), 302. https://doi.org/10.3390/brainsci13020302







