Social Brain Network of Children with Autism Spectrum Disorder: Characterization of Functional Connectivity and Potential Association with Stereotyped Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Psychological Assessment
2.3. MRI Acquisition
2.4. Data Preprocessing
2.5. Social-Related ROIs
2.6. Functional Connectivity Maps
2.7. Construction of the Brain Functional Network
2.8. Brain Network Topology Properties
2.9. Statistical Analysis
2.9.1. Demographic and Clinical Characteristics
2.9.2. Voxel-Wise Analysis
2.9.3. ROI-Wise Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Results of Voxel-Wise Analysis
3.2.1. Intergroup Analysis of FC Maps
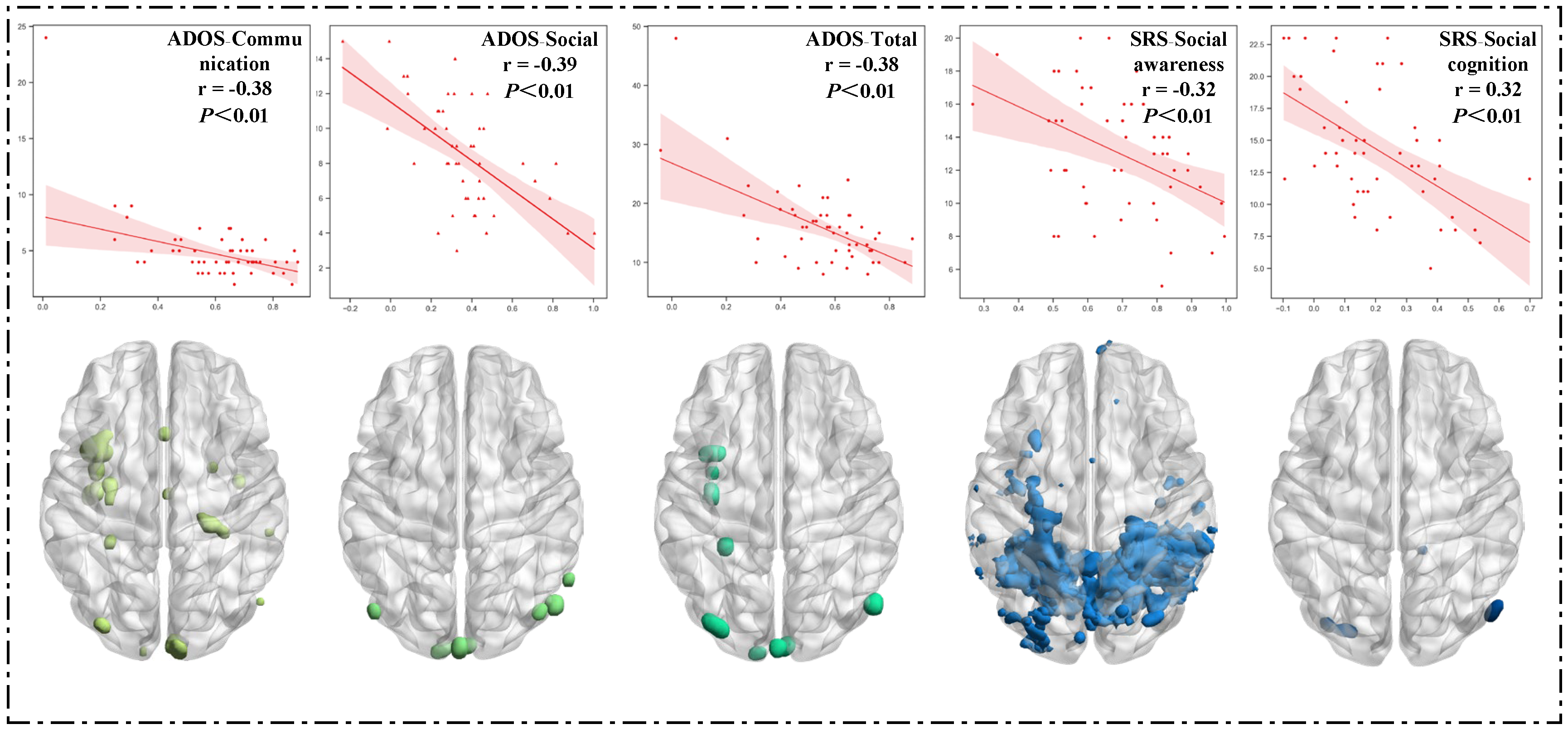
3.2.2. Correlation between Clinical Characteristics and FC Maps
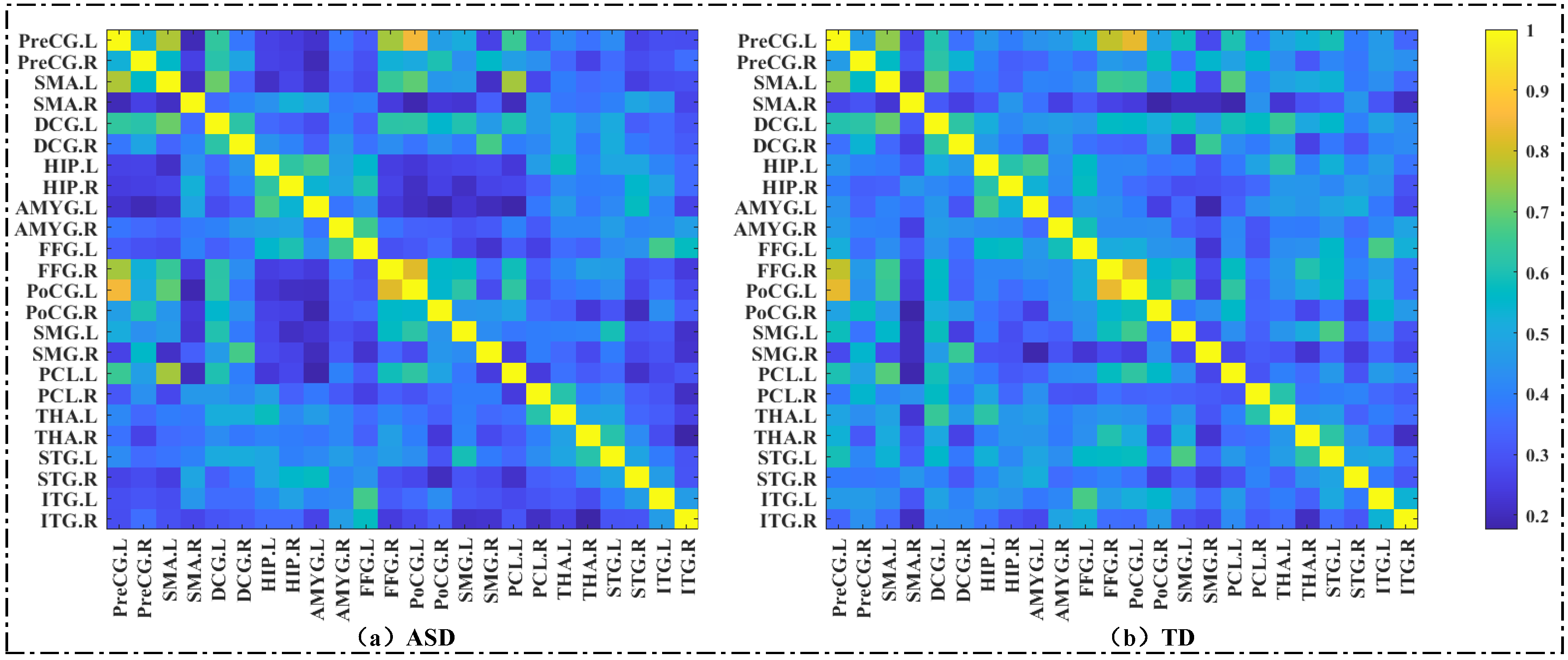
3.3. Results of ROI−Wise Analysis—Graph Metrics of Brain Functional Network
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fombonne, E.; MacFarlane, H.; Salem, A.C. Epidemiological surveys of ASD: Advances and remaining challenges. J. Autism Dev. Disord. 2021, 51, 4271–4290. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Strathearn, L.; Liu, B.; Bao, W. Prevalence of autism spectrum disorder amon2 US children and adolescents, 2014–2016. JAMA 2018, 319, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef]
- Kana, R.K.; Libero, L.E.; More, M.S. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Physics Life Rev. 2011, 8, 410–437. [Google Scholar] [CrossRef] [PubMed]
- Wadhera, T.; Kakkar, D. Multiplex temporal measures reflecting neural underpinnings of brain functional connectivity under cognitive load in Autism Spectrum Disorder. Neurol. Res. 2020, 42, 327–337. [Google Scholar] [CrossRef]
- Cao, W.; Zhu, H.; Li, Y.; Wang, Y.; Bai, W.; Lao, U.; Zhang, Y.; Ji, Y.; He, S.; Zou, X. The Development of Brain Network in Males with Autism Spectrum Disorders from Childhood to Adolescence: Evidence from fNIRS Study. Brain Sci. 2021, 11, 120. [Google Scholar] [CrossRef]
- Harlalka, V.; Bapi, R.S.; Vinod, P.K. A typical Flexibility in Dynamic Functional Connectivity Quantifies the Severity in Autism Spectrum Disorder. Front. Hum. Neurosci. 2019, 13, 6. [Google Scholar] [CrossRef]
- Maximo, J.O.; Cadena, E.J.; Kana, R.K. The implications of brain connectivity in the neuropsychology of autism. Neuropsychol. Rev. 2014, 24, 16–31. [Google Scholar] [CrossRef]
- Yao, Z.; Hu, B.; Xie, Y.; Zheng, F.; Liu, G.; Chen, X.; Zheng, W. Resting-State Time-Varying Analysis Reveals Aberrant Variations of Functional Connectivity in Autism. Front Hum. Neurosci. 2016, 10, 463. [Google Scholar] [CrossRef]
- Wadhera, T. Brain network topology unraveling epilepsy and ASD Association: Automated EEG-based diagnostic model. Expert Syst. Appl. 2021, 186, 115762. [Google Scholar] [CrossRef]
- Wu, X.; Lin, F.; Sun, W.; Zhang, T.; Sun, H.; Li, J. Relationship between Short-Range and Homotopic Long-Range Resting State Functional Connectivity in Temporal Lobes in Autism Spectrum Disorder. Brain Sci. 2021, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Sepulcre, J.; Liu, H.; Talukdar, T.; Martincorena, I.; Yeo, B.T.; Buckner, R.L. The organization of local and distant functional connectivity in the human brain. PLoS Comput. Biol. 2010, 6, e1000808. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.; Huangfu, H.; Liang, P.; Zhong, N. Analysis of dynamic functional connectivity of resting EEG signals. J. Phys. 2015, 64, 382–391. [Google Scholar]
- Harikumar, A.; Evans, D.W.; Dougherty, C.C.; Carpenter, K.L.; Michael, A.M. A Review of the Default Mode Network in Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. Brain Connect. 2021, 11, 253–263. [Google Scholar] [CrossRef]
- Leaf, J.B.; Cihon, J.H.; Ferguson, J.L.; Milne, C.M.; Leaf, R.; McEachin, J. Advances in Our Understanding of Behavioral Intervention: 1980 to 2020 for Individuals Diagnosed with Autism Spectrum Disorder. J. Autism Dev. Disord. 2021, 51, 4395–4410. [Google Scholar] [CrossRef]
- Afif, I.Y.; Farkhan, M.; Kurdi, O.; Maula, M.I.; Ammarullah, M.I.; Setiyana, B.; Jamari, J.; Winarni, T.I. Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study. Bioengineering 2022, 9, 48. [Google Scholar] [CrossRef]
- Esse Wilson, J.; Trumbo, M.C.; Wilson, J.K.; Tesche, C.D. Transcranial direct current stimulation (tDCS) over right temporoparietal junction (rTPJ) for social cognition and social skills in adults with autism spectrum disorder (ASD). J. Neural Transm. 2018, 125, 1857–1866. [Google Scholar] [CrossRef]
- Sellaro, R.; Nitsche, M.A.; Colzato, L.S. The stimulated social brain: Effects of transcranial direct current stimulation on social cognition. Ann. N Y Acad. Sci. 2016, 1369, 218–239. [Google Scholar] [CrossRef]
- Gómez, L.; Vidal, B.; Maragoto, C.; Morales, L.M.; Berrillo, S.; Vera Cuesta, H.; Baez, M.; Denis, M.; Marín, T.; Cabrera, Y.; et al. Non-invasive brain stimulation for children with autism spectrum disorders: A short-term outcome study. Behav. Sci. 2017, 7, 63–75. [Google Scholar] [CrossRef]
- Amatachaya, A.; Auvichayapat, N.; Patjanasoontorn, N.; Suphakunpinyo, C.; Ngernyam, N.; Aree-Uea, B.; Keeratitanont, K.; Auvichayapat, P. Effect of anodal transcranial direct current stimulation on autism: A randomized double-blind crossover trial. Behav. Neurol. 2014, 2014, 173073. [Google Scholar] [CrossRef]
- Morishima, Y.; Schunk, D.; Bruhin, A.; Christian Ruff, C.; Fehr, E. Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron 2012, 75, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Isik, L.; Koldewyn, K.; Beeler, D.; Kanwisher, N. Perceiving social interactions in the posterior superiortemporal sulcus. Proc. Natl. Acad. Sci. USA 2017, 114, E9145–E9152. [Google Scholar] [CrossRef] [PubMed]
- Báez-Mendoza, R.; Mastrobattista, E.P.; Wang, A.J.; Williams, Z.M. Social agent identity cells in the prefrontal cortex of interacting groups of primates. Science 2021, 374, eabb4149. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, P.L.; Wittmann, M.K. Ventral anterior cingulate cortex and social decision-making. Neurosci. Biobehav. Rev. 2018, 92, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.C.; Moura, P.J.; Cysneiros, R.M. Temporal lobe epilepsy and social behavior: An animal model for autism? Epilepsy Behav. 2008, 13, 43–46. [Google Scholar] [CrossRef]
- Choleris, E.; Devidze, N.; Kavaliers, M. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog. Brain Res. 2008, 170, 291–303. [Google Scholar]
- Lehr, A.B.; Kumar, A.; Tetzlaff, C. CA2 beyond social memory: Evidence for a fundamental role in hippocampal information processing. Neurosci. Biobehav. Rev. 2021, 126, 398–412. [Google Scholar] [CrossRef]
- Wilson, J.E.; Quinn, D.K.; Wilson, J.K.; Garcia, C.M.; Tesche, C.D. Transcranial Direct Current Stimulation to the Right Temporoparietal Junction for Social Functioning in Autism Spectrum Disorder: A Case Report. J. ECT 2018, 34, e10–e13. [Google Scholar] [CrossRef]
- Zhou, T.; Kang, J.; Li, Z.; Chen, H.; Li, X. Transcranial direct current stimulation modulates brain functional connectivity in autism. Neuroimage Clin. 2020, 28, 102500. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM -V); American Psychiatric Association: Washington, DC, USA, 2013; pp. 50–51. [Google Scholar]
- Zhang, H.C. Revision of the Chinese version of wechsler intelligence Scale for Children (WISC-IV). Psychol. Sci. 2009, 32, 155–157. [Google Scholar]
- Tang, Y.; Guo, Y.; Rice, C.E.; Wang, Y.; Cubells, J.F. Introduction of “autism diagnostic observation scale”, one of the gold criteria for the diagnosis of autism. Int. J. Psychiatry 2010, 37, 38–40. [Google Scholar]
- Gong, J.; Zou, S.; Liu, D. Reliability and validity analysis of social response scale. Chin. J. Med. Innov. 2019, 16, 19–23. [Google Scholar]
- Liu, J.; Xu, X. Application of social communication questionnaire in primary screening of autism spectrum disorder. Sci. China Life Sci. 2015, 8, 4. [Google Scholar]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Kelly Anne, B.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L. Functional network organization of the human brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef]
- Amodeo, D.A.; Lai, C.Y.; Hassan, O. Maternal immune activation impairs cognitive flexibility and alters transcription in frontal cortex. Neurobiol. Dis. 2019, 125, 211–218. [Google Scholar] [CrossRef]
- Solso, S.; Xu, R.; Proudfoot, J.; Courchesne, E. Diffusion Tensor Imaging Provides Evidence of Possible Axonal Overconnectivity in Frontal Lobes in Autism Spectrum Disorder Toddlers. Biol. Psychiatry 2016, 79, 676–684. [Google Scholar] [CrossRef]
- Carper, R.A.; Solders, S.; Treiber, J.M.; Fishman, I.; Müller, R.A. Corticospinal tract anatomy and functional connectivity of primary motor cortex in autism. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 859–867. [Google Scholar] [CrossRef]
- Lo, Y.C.; Chen, Y.J.; Hsu, Y.C. Altered frontal aslant tracts as a heritable neural basis of social communication deficits in autism spectrum disorder: A sibling study using tract-based automatic analysis. Autism Res. 2019, 12, 225–238. [Google Scholar] [CrossRef]
- Cascio, C.J.; Gu, C.; Schauder, K.B. Somatosensory Event-Related Potentials and Association with Tactile Behavioral Responsiveness Patterns in Children with ASD. Brain Topogr. 2015, 28, 895–903. [Google Scholar] [CrossRef]
- Cechmanek, B.; Johnston, H.; Vazhappilly, S. Somatosensory Regions Show Limited Functional Connectivity Differences in Youth with Autism Spectrum Disorder. Brain Connect. 2018, 8, 558–566. [Google Scholar] [CrossRef]
- Orefice, L.L.; Mosko, J.R.; Morency, D.T. Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models. Cell 2019, 178, 867–886.e24. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. A preliminary study of atypical cortical change ability of dynamic whole-brain functional connectivity in autism spectrum disorder. Int. J. Neurosci. 2022, 132, 213–225. [Google Scholar] [CrossRef]
- Wang, J.; Fu, K.; Chen, L. Increased Gray Matter Volume and Resting-State Functional Connectivity in Somatosensory Cortex and their Relationship with Autistic Symptoms in Young Boys with Autism Spectrum Disorder. Front. Physiol. 2017, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Y.; Wang, W. Sex Differences in Neural Responses to the Perception of Social Interactions. Front. Hum. Neurosci. 2020, 14, 565132. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Milardi, D.; Bertino, S. The Limbic and Sensorimotor Pathways of the Human Amygdala: A Structural Connectivity Study. Neuroscience 2018, 385, 166–180. [Google Scholar] [CrossRef]
- Fu, Z.; Tu, Y.; Di, X.; Du, Y.; Sui, J.; Biswal, B.B.; Zhang, Z.; de Lacy, N.; Calhoun, V.D. Transient increased thalamic-sensory connectivity and decreased whole-brain dynamism in autism. Neuroimage 2019, 190, 191–204. [Google Scholar] [CrossRef]
- Wilson, K.C.; Kornisch, M.; Ikuta, T. Disrupted functional connectivity of the primary auditory cortex in autism. Psychiatry Res. Neuroimaging 2022, 324, 111490. [Google Scholar] [CrossRef]
- Saugstad, L.F. Infantile autism: A chronic psychosis since infancy due to synaptic pruning of the supplementary motor area. Nutr. Health 2011, 20, 171–182. [Google Scholar] [CrossRef]
- Liang, D.; Xia, S.; Zhang, X. Analysis of Brain Functional Connectivity Neural Circuits in Children with Autism Based on Persistent Homology. Front. Hum. Neurosci. 2021, 15, 745671. [Google Scholar] [CrossRef]
- Knaus, T.A.; Burns, C.; Kamps, J. Atypical activation of action-semantic network in adolescents with autism spectrum disorder. Brain Cogn. 2017, 117, 57–64. [Google Scholar] [CrossRef]
- Hanaie, R.; Mohri, I.; Kagitani-Shimono, K.; Tachibana, M.; Matsuzaki, J.; Hirata, I.; Nagatani, F.; Watanabe, Y.; Katayama, T.; Taniike, M. Aberrant Cerebellar-Cerebral Functional Connectivity in Children and Adolescents with Autism Spectrum Disorder. Front. Hum. Neurosci. 2018, 12, 454. [Google Scholar] [CrossRef]
- Ramos, T.C.; Balardin, J.B.; Sato, J.R.; Fujita, A. Abnormal Cortico-Cerebellar Functional Connectivity in Autism Spectrum Disorder. Front. Syst. Neurosci. 2019, 12, 74. [Google Scholar] [CrossRef]
- Van Overwalle, F.; Manto, M.; Cattaneo, Z. Consensus Paper: Cerebellum and Social Cognition. Cerebellum 2020, 19, 833–868. [Google Scholar] [CrossRef]
- Chao, O.Y.; Zhang, H.; Pathak, S.S. Functional Convergence of Motor and Social Processes in Lobule IV/V of the Mouse Cerebellum. Cerebellum 2021, 20, 836–852. [Google Scholar] [CrossRef]
- D’Urso, G.; Toscano, E.; Sanges, V.; Sauvaget, A.; Sheffer, C.E.; Riccio, M.P.; Ferrucci, R.; Iasevoli, F.; Priori, A.; Bravaccio, C.; et al. Cerebellar Transcranial Direct Current Stimulation in Children with Autism Spectrum Disorder: A Pilot Study on Efficacy, Feasibility, Safety, and Unexpected Outcomes in Tic Disorder and Epilepsy. J. Clin. Med. 2021, 11, 143. [Google Scholar] [CrossRef]


| Subject | ASD Group (n = 51) | TD Group (n = 54) | Statistical | p |
|---|---|---|---|---|
| Age (years) | 8.16 ± 1.75 | 8.51 ± 1.82 | t = −0.999 | 0.320 a |
| Gender (male/female) | 45/6 | 45/9 | χ2 = 0.515 | 0.473 b |
| IQ | 101.16 ± 17.28 | 112.96 ± 16.54 | t = −3.576 | 0.001 a |
| ADOS-Communication | 5.02 ± 3.13 | - | - | - |
| ADOS-Social | 8.43 ± 3.08 | - | - | - |
| ADOS-Combined social–communication | 13.45 ± 5.32 | - | - | - |
| ADOS-Total | 16.12 ± 6.80 | - | - | - |
| SRS-Social awareness | 13.02 ± 3.65 | - | - | - |
| SRS-Social cognition | 14.49 ± 4.87 | - | - | - |
| SRS-Social communication | 21.84 ± 9.66 | - | - | - |
| SRS-Social motivation | 13.86 ± 4.83 | - | - | - |
| SRS-Autistic mannerisms | 13.10 ± 5.21 | - | - | - |
| SRS-Total | 76.31 ± 17.97 | - | - | - |
| SCQ-Total | 22.14 ± 6.14 | - | - | - |
| Attributes Groups | Clustering Coefficient | Betweenness Centrality | |||
|---|---|---|---|---|---|
| Brain Regions | ASD | TD | ASD | TD | |
| PreCG.L | 0.41 ± 0.25 | 0.61 ± 0.15 | 96.96 ± 155.99 | 9.35 ± 58.83 | |
| PreCG.R | 0.40 ± 0.25 | 0.58 ± 0.16 | 110.10 ± 171.46 | 13.67 ± 67.71 | |
| SMA.L | 0.40 ± 0.25 | 0.59 ± 0.15 | 102.78 ± 172.31 | 19.41 ± 87.20 | |
| SMA.R | 0.36 ± 0.20 | 0.48 ± 0.17 | 103.06 ± 148.17 | 78.80 ± 139.31 | |
| DCG.L | 0.45 ± 0.23 | 0.62 ± 0.14 | 52.04 ± 117.89 | 0.52 ± 2.99 | |
| DCG.R | 0.42 ± 0.21 | 0.55 ± 0.17 | 45.88 ± 116.65 | 50.22 ± 116.59 | |
| HIP.L | 0.39 ± 0.22 | 0.58 ± 0.15 | 103.12 ± 160.20 | 4.31 ± 18.94 | |
| HIP.R | 0.38 ± 0.23 | 0.56 ± 0.15 | 110.57 ± 164.67 | 16.19 ± 50.77 | |
| AMYG.L | 0.35 ± 0.22 | 0.56 ± 0.16 | 139.04 ± 166.83 | 23.78 ± 77.79 | |
| AMYG.R | 0.40 ± 0.23 | 0.56 ± 0.16 | 92.78 ± 150.58 | 29.59 ± 93.00 | |
| FFG.L | 0.39 ± 0.25 | 0.58 ± 0.15 | 127.08 ± 172.05 | 20.07 ± 75.18 | |
| FFG.R | 0.42 ± 0.24 | 0.60 ± 0.15 | 95.29 ± 142.22 | 9.81 ± 41.27 | |
| PoCG.L | 0.41 ± 0.25 | 0.60 ± 0.15 | 107.31 ± 146.61 | 16.37 ± 56.40 | |
| PoCG.R | 0.38 ± 0.25 | 0.56 ± 0.15 | 137.76 ± 164.96 | 21.11 ± 80.07 | |
| SMG.L | 0.39 ± 0.24 | 0.56 ± 0.16 | 98.55 ± 147.10 | 64.31 ± 134.05 | |
| SMG.R | 0.35 ± 0.23 | 0.50 ± 0.16 | 148.02 ± 181.61 | 74.17 ± 125.39 | |
| PCL.L | 0.38 ± 0.24 | 0.57 ± 0.15 | 123.67 ± 161.13 | 24.81 ± 70.56 | |
| PCL.R | 0.37 ± 0.21 | 0.55 ± 0.18 | 77.31 ± 134.41 | 56.87 ± 141.81 | |
| THA.L | 0.41 ± 0.22 | 0.57 ± 0.17 | 67.53 ± 146.93 | 45.46 ± 115.43 | |
| THA.R | 0.38 ± 0.22 | 0.56 ± 0.17 | 94.27 ± 155.80 | 53.09 ± 130.08 | |
| STG.L | 0.43 ± 0.22 | 0.60 ± 0.16 | 68.00 ± 129.87 | 13.46 ± 62.22 | |
| STG.R | 0.37 ± 0.22 | 0.54 ± 0.17 | 97.63 ± 137.11 | 33.33 ± 72.49 | |
| ITG.L | 0.38 ± 0.25 | 0.58 ± 0.15 | 113.90 ± 154.18 | 4.93 ± 27.41 | |
| ITG.R | 0.33 ± 0.25 | 0.53 ± 0.17 | 151.20 ± 171.27 | 52.33 ± 116.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, L.; Fang, H.; Wang, F.; Gao, T.; Zhu, Q.; Jiao, G.; Ke, X. Social Brain Network of Children with Autism Spectrum Disorder: Characterization of Functional Connectivity and Potential Association with Stereotyped Behavior. Brain Sci. 2023, 13, 280. https://doi.org/10.3390/brainsci13020280
Wang Y, Xu L, Fang H, Wang F, Gao T, Zhu Q, Jiao G, Ke X. Social Brain Network of Children with Autism Spectrum Disorder: Characterization of Functional Connectivity and Potential Association with Stereotyped Behavior. Brain Sciences. 2023; 13(2):280. https://doi.org/10.3390/brainsci13020280
Chicago/Turabian StyleWang, Yonglu, Lingxi Xu, Hui Fang, Fei Wang, Tianshu Gao, Qingyao Zhu, Gongkai Jiao, and Xiaoyan Ke. 2023. "Social Brain Network of Children with Autism Spectrum Disorder: Characterization of Functional Connectivity and Potential Association with Stereotyped Behavior" Brain Sciences 13, no. 2: 280. https://doi.org/10.3390/brainsci13020280
APA StyleWang, Y., Xu, L., Fang, H., Wang, F., Gao, T., Zhu, Q., Jiao, G., & Ke, X. (2023). Social Brain Network of Children with Autism Spectrum Disorder: Characterization of Functional Connectivity and Potential Association with Stereotyped Behavior. Brain Sciences, 13(2), 280. https://doi.org/10.3390/brainsci13020280






