Development and Validation of a Prediction Model for Anxiety Improvement after Deep Brain Stimulation for Parkinson Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Outcome Assessment
2.3. Statistical Analysis
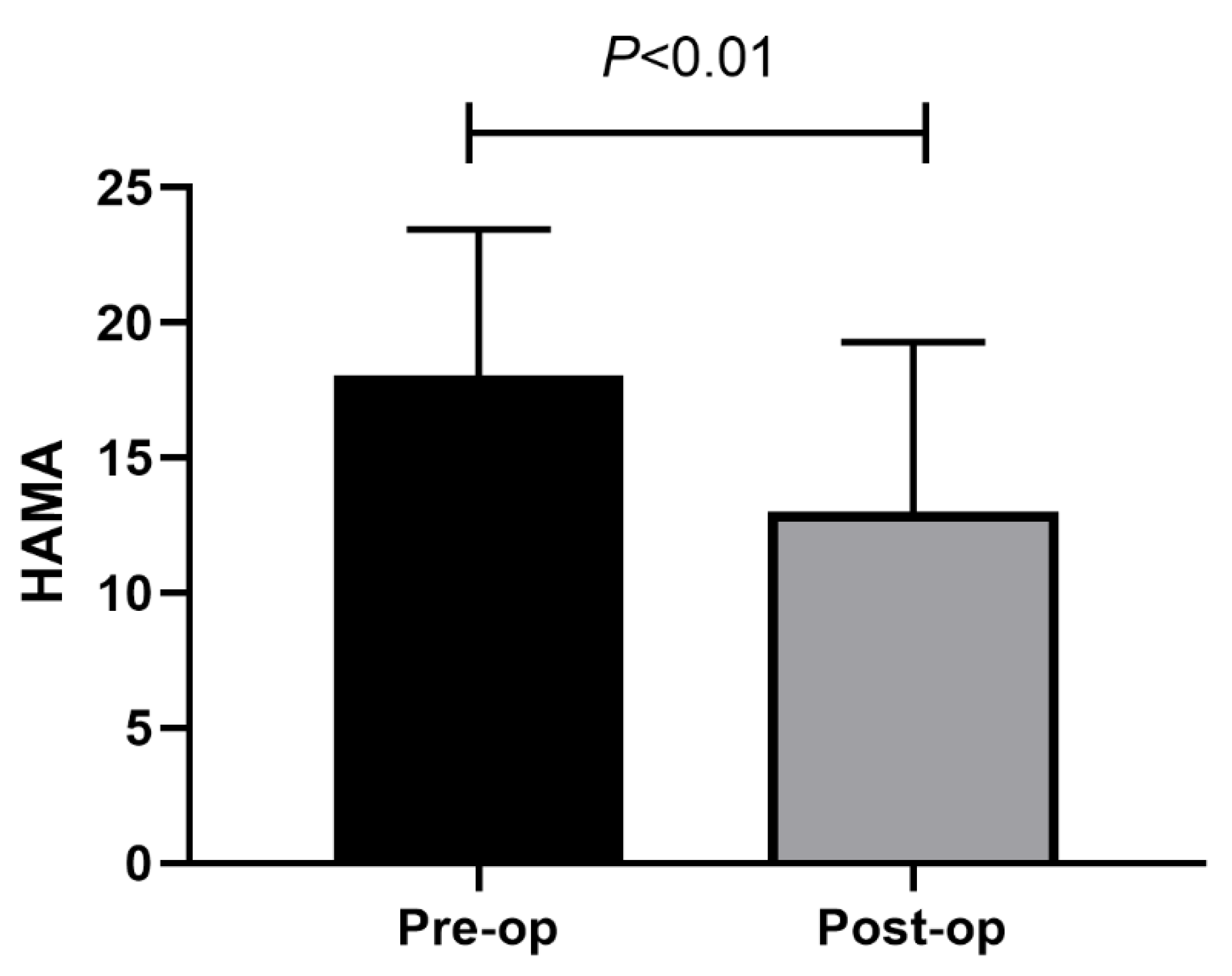
3. Results
3.1. Patients
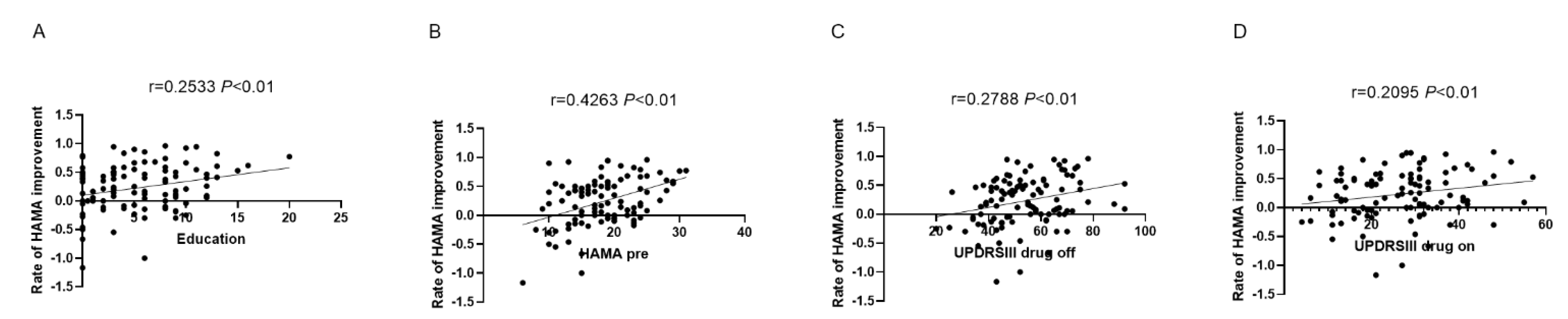
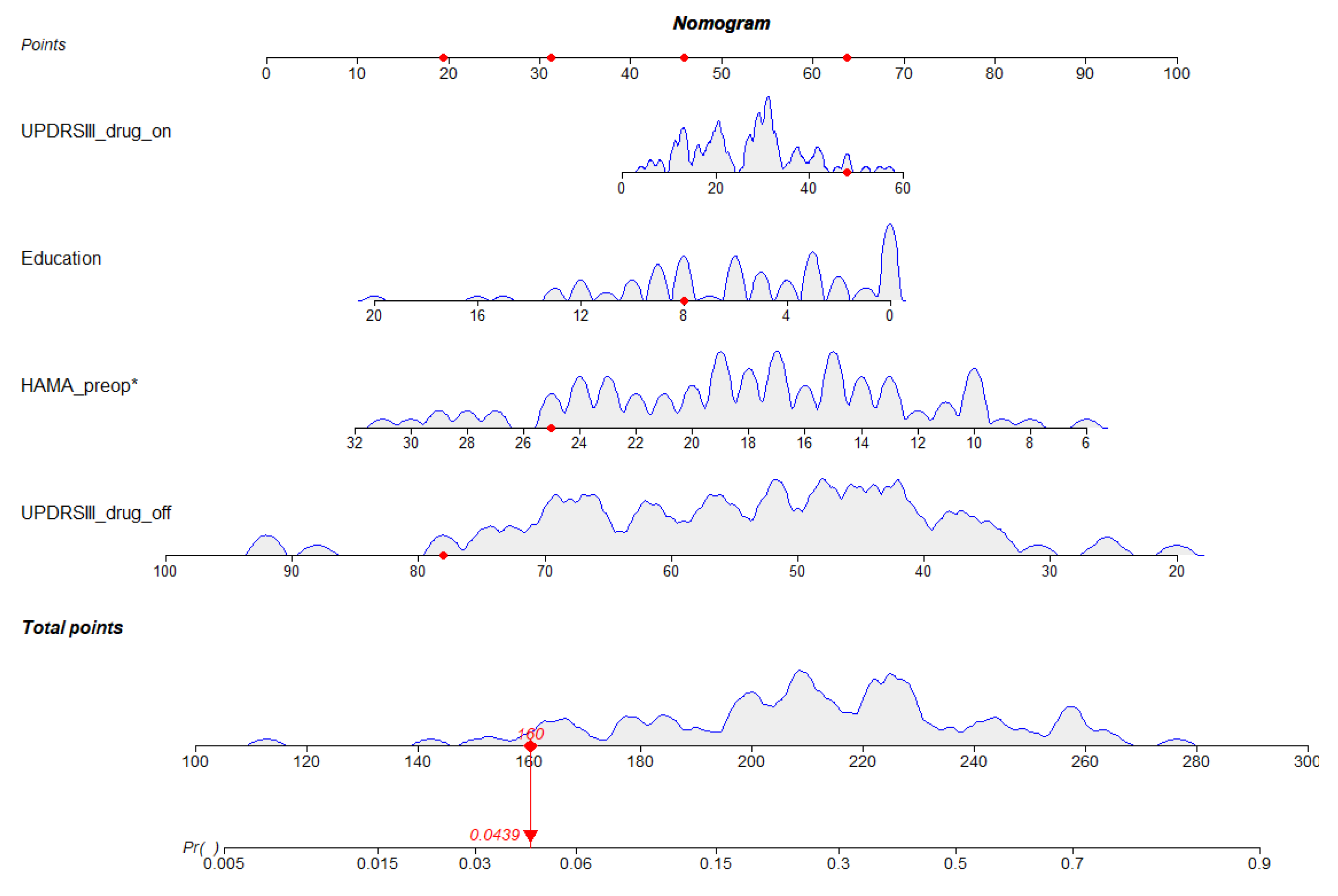
3.2. Development of the Nomogram
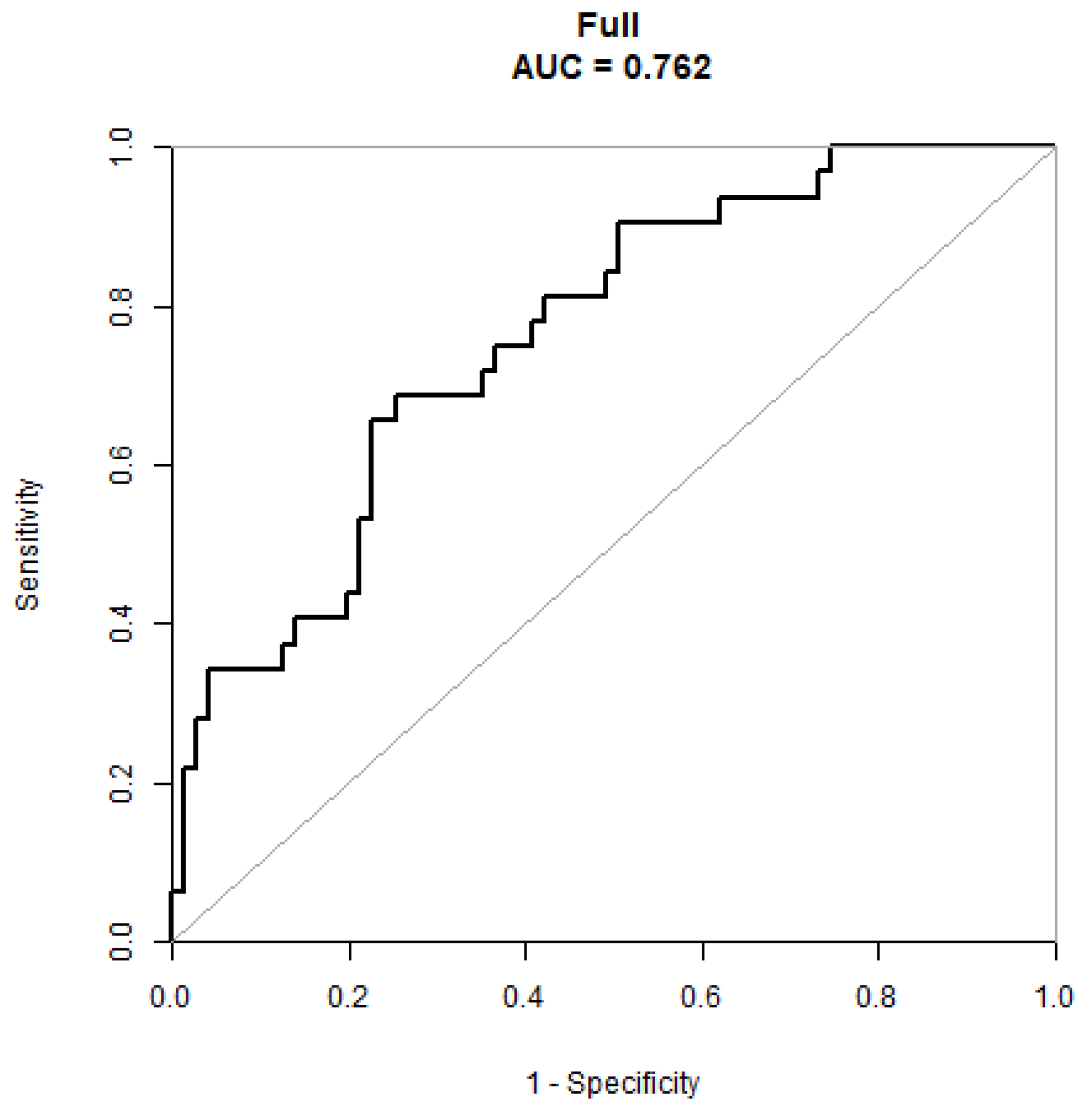
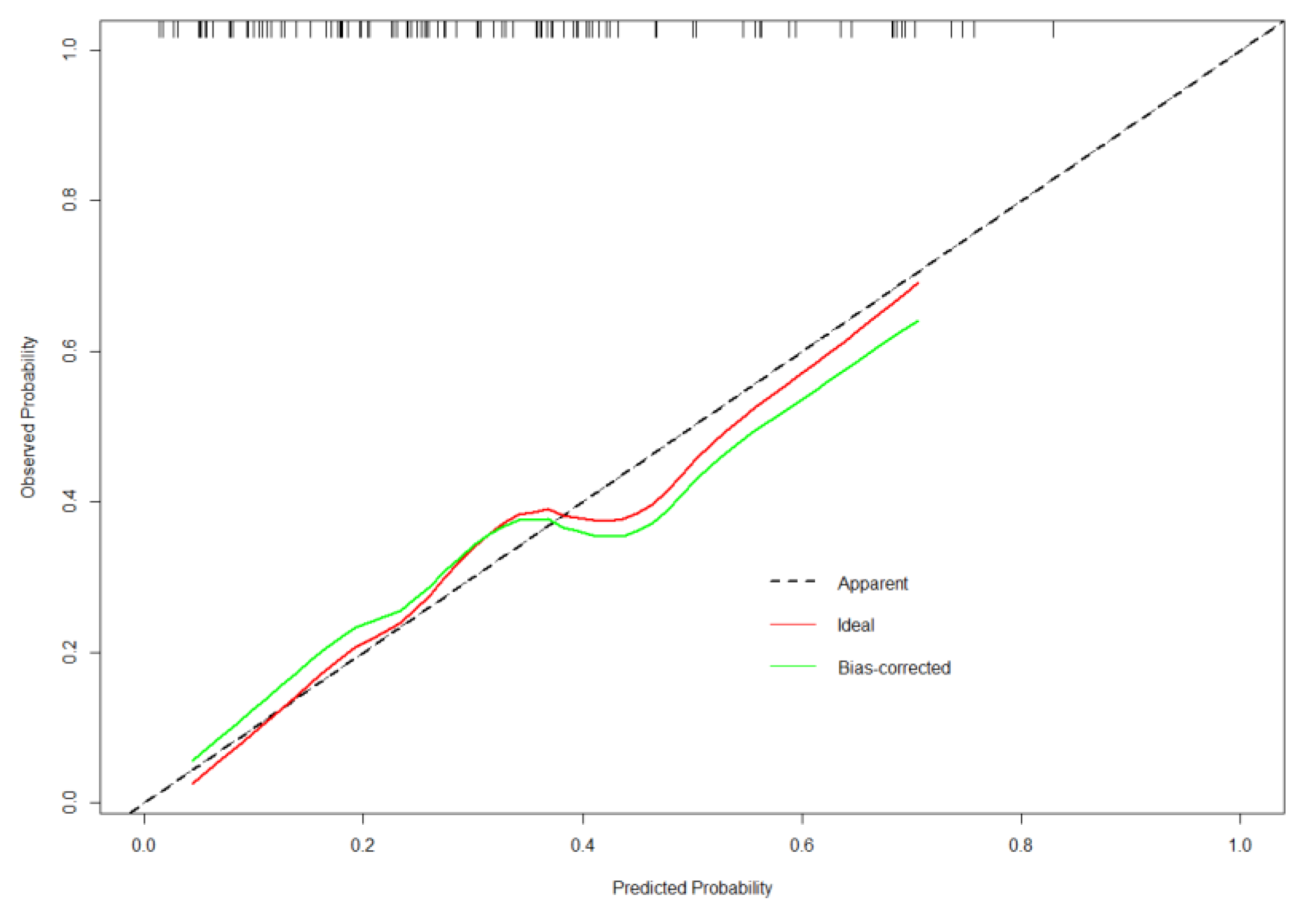
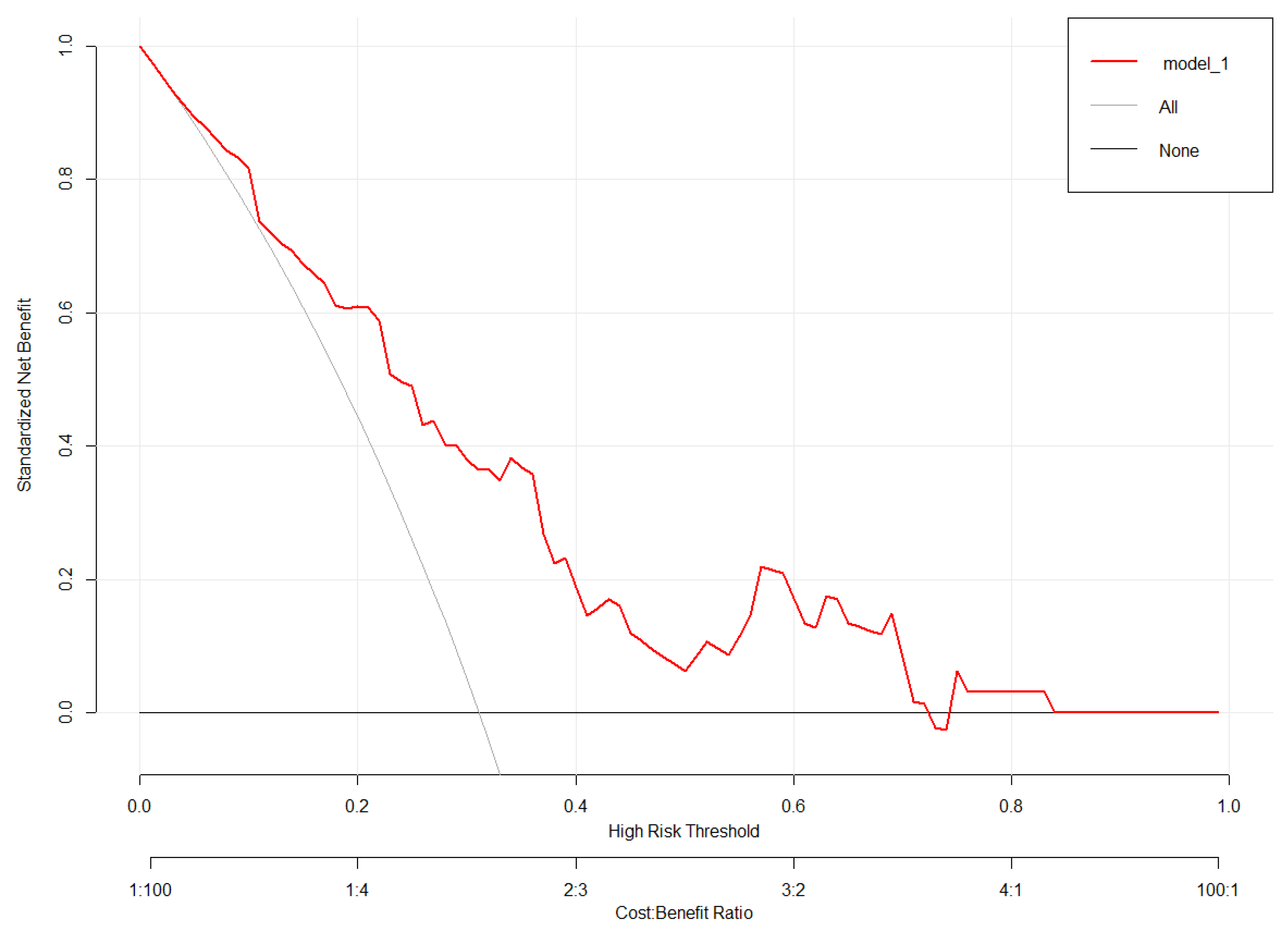
3.3. Validation of the Nomogram
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Ni, C.; Mei, J.; Xiong, C.; Chen, P.; Jiang, M.; Niu, C. Nomogram for Predicting Depression Improvement after Deep Brain Stimulation for Parkinson’s Disease. Brain Sci. 2022, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Ni, C.; Zhang, W.; Mei, J.; Xiong, C.; Chen, P.; Jiang, M.; Niu, C. Nomogram to Predict Cognitive State Improvement after Deep Brain Stimulation for Parkinson’s Disease. Brain Sci. 2022, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Agarwal, P. Depression and Anxiety in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 93–104. [Google Scholar] [CrossRef]
- Wen, M.C.; Chan, L.L.; Tan, L.C.; Tan, E.K. Depression, anxiety, and apathy in Parkinson’s disease: Insights from neuroimaging studies. Eur. J. Neurol. 2016, 23, 1001–1019. [Google Scholar] [CrossRef]
- Mei, J.; Chang, B.; Xiong, C.; Jiang, M.; Niu, C. A New Application of Functional Zonal Image Reconstruction in Programming for Parkinson’s Disease Treated Using Subthalamic Nucleus-Deep Brain Stimulation. Front. Neurol. 2022, 13, 916658. [Google Scholar] [CrossRef]
- Beudel, M.; Brown, P. Adaptive deep brain stimulation in Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 22 (Suppl. 1), S123–S126. [Google Scholar] [CrossRef]
- Cartmill, T.; Skvarc, D.; Bittar, R.; McGillivray, J.; Berk, M.; Byrne, L.K. Deep Brain Stimulation of the Subthalamic Nucleus in Parkinson’s Disease: A Meta-Analysis of Mood Effects. Neuropsychol. Rev. 2021, 31, 385–401. [Google Scholar] [CrossRef]
- Chang, C.; Li, N.; Wu, Y.; Geng, N.; Ge, S.; Wang, J.; Wang, X.; Wang, X. Associations between bilateral subthalamic nucleus deep brain stimulation (STN-DBS) and anxiety in Parkinson’s disease patients: A controlled study. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Lintel, H.; Corpuz, T.; Paracha, S.U.; Grossberg, G.T. Mood Disorders and Anxiety in Parkinson’s Disease: Current Concepts. J. Geriatr. Psychiatry Neurol. 2021, 34, 280–288. [Google Scholar] [CrossRef]
- Khatri, D.K.; Choudhary, M.; Sood, A.; Singh, S.B. Anxiety: An ignored aspect of Parkinson’s disease lacking attention. Biomed. Pharmacother. 2020, 131, 110776. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, A.F.; Dujardin, K.; Marsh, L.; Martinez-Martin, P.; Richard, I.H.; Starkstein, S.E. Symptomatology and markers of anxiety disorders in Parkinson’s disease: A cross-sectional study. Mov. Disord. 2011, 26, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.M.; Williams, J.R.; Anderson, K.E.; Chase, G.; Goldstein, S.A.; Grill, S.; Hirsch, E.S.; Lehmann, S.; Little, J.T.; Margolis, R.L.; et al. Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson’s disease. Mov. Disord. 2009, 24, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, E.C.; Freeman, A.; Vogel, R.L. Differential DSM-III psychiatric disorder prevalence profiles in dystonia and Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 29–36. [Google Scholar] [CrossRef]
- Umemura, A.; Oyama, G.; Shimo, Y.; Hattori, N. Deep brain stimulation for Parkinson’s disease. Nihon Rinsho. Jpn. J. Clin. Med. 2017, 75, 83–88. [Google Scholar] [CrossRef]
- Limousin, P.; Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 2019, 15, 234–242. [Google Scholar] [CrossRef]
- Witt, K.; Daniels, C.; Volkmann, J. Factors associated with neuropsychiatric side effects after STN-DBS in Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18 (Suppl. 1), S168–S170. [Google Scholar] [CrossRef]
- Accolla, E.A.; Pollo, C. Mood Effects After Deep Brain Stimulation for Parkinson’s Disease: An Update. Front. Neurol. 2019, 10, 617. [Google Scholar] [CrossRef]
- Carey, G.; Görmezoğlu, M.; de Jong, J.J.A.; Hofman, P.A.M.; Backes, W.H.; Dujardin, K.; Leentjens, A.F.G. Neuroimaging of Anxiety in Parkinson’s Disease: A Systematic Review. Mov. Disord. 2021, 36, 327–339. [Google Scholar] [CrossRef]
- Perepezko, K.; Naaz, F.; Wagandt, C.; Dissanayaka, N.N.; Mari, Z.; Nanavati, J.; Bakker, A.; Pontone, G.M. Anxiety in Parkinson’s Disease: A Systematic Review of Neuroimaging Studies. J. Neuropsychiatry Clin. Neurosci. 2021, 33, 280–294. [Google Scholar] [CrossRef]
- Dissanayaka, N.N.; White, E.; O’Sullivan, J.D.; Marsh, R.; Pachana, N.A.; Byrne, G.J. The clinical spectrum of anxiety in Parkinson’s disease. Mov. Disord. 2014, 29, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Bucur, M.; Papagno, C. Deep Brain Stimulation in Parkinson Disease: A Meta-analysis of the Long-term Neuropsychological Outcomes. Neuropsychol. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Leigh, E.; Chiu, K.; Clark, D.M. Is concentration an indirect link between social anxiety and educational achievement in adolescents? PLoS ONE 2021, 16, e0249952. [Google Scholar] [CrossRef] [PubMed]
| Improvement Group | Non-Improvement Group | p-Value | |
|---|---|---|---|
| No. | 71 | 32 | |
| Age (years) | 58.58 ± 8.24 | 59.97 ± 7.57 | 0.418 |
| Education (years) | 6.18 ± 4.56 | 4.11 ± 3.62 | 0.025 |
| LED | 658.10 ± 409.99 | 664.06 ± 236.40 | 0.939 |
| Drug improvement rate | 0.51 ± 0.15 | 0.54 ± 0.13 | 0.304 |
| UPDRSIII drug off | 56.86 ± 13.72 | 47.66 ± 13.16 | 0.002 |
| UPDRSIII drug on | 28.28 ± 11.57 | 23.00 ± 10.51 | 0.030 |
| NMSS Preop | 90.32 ± 28.71 | 81.34 ± 32.31 | 0.161 |
| PDQ39 Preop | 75.42 ± 16.52 | 68.41 ± 14.74 | 0.042 |
| MOCA Preop | 17.92 ± 5.89 | 20.34 ± 6.49 | 0.064 |
| MMSE Preop | 24.89 ± 3.54 | 25.22 ± 4.20 | 0.679 |
| HAMD Preop | 16.58 ± 7.06 | 13.84 ± 5.31 | 0.054 |
| HAMA Preop | 19.20 ± 5.27 | 15.53 ± 4.81 | 0.001 |
| Duration (years) | 8.90 ± 3.88 | 8.25 ± 3.63 | 0.423 |
| Gender | 0.533 | ||
| male | 42 (59.15%) | 21 (65.62%) | |
| female | 29 (40.85%) | 11 (34.38%) | |
| H-Y | 0.537 | ||
| 2 | 2 (2.82%) | 0 (0.00%) | |
| 2.5 | 9 (12.68%) | 7 (21.88%) | |
| 3 | 34 (47.89%) | 16 (50.00%) | |
| 4 | 20 (28.17%) | 8 (25.00%) | |
| 5 | 6 (8.45%) | 1 (3.12%) |
| Statistics | OR (95% CI) p-Value | |
|---|---|---|
| Age (years) | 59.01 ± 8.03 | 1.02 (0.97, 1.08) 0.4146 |
| Education (years) | 5.54 ± 4.38 | 0.89 (0.80, 0.99) 0.0287 |
| LED | 659.95 ± 363.80 | 1.00 (1.00, 1.00) 0.9383 |
| Drug improvement rate | 0.52 ± 0.14 | 4.78 (0.25, 92.97) 0.3019 |
| UPDRSIII drug off | 54.00 ± 14.15 | 0.95 (0.91, 0.98) 0.0034 |
| UPDRSIII drug on | 26.64 ± 11.47 | 0.96 (0.92, 1.00) 0.0332 |
| NMSS Preop | 87.53 ± 30.01 | 0.99 (0.98, 1.00) 0.1623 |
| PDQ39 Preop | 73.24 ± 16.25 | 0.97 (0.95, 1.00) 0.0469 |
| MOCA Preop | 18.67 ± 6.15 | 1.07 (1.00, 1.15) 0.0661 |
| MMSE Preop | 24.99 ± 3.74 | 1.02 (0.91, 1.15) 0.6758 |
| HAMD Preop | 15.73 ± 6.66 | 0.93 (0.87, 1.00) 0.0565 |
| HAMA Preop | 18.06 ± 5.38 | 0.86 (0.79, 0.95) 0.0022 |
| Duration (years) | 8.70 ± 3.80 | 0.95 (0.85, 1.07) 0.4201 |
| Gender | ||
| male | 63 (61.17%) | 1.0 |
| female | 40 (38.83%) | 0.76 (0.32, 1.81) 0.5335 |
| H-Y | ||
| 2 | 2 (1.94%) | 1.0 |
| 2.5 | 16 (15.53%) | 4,478,298.90 (0.00, Inf) 0.9881 |
| 3 | 50 (48.54%) | 2,709,559.00 (0.00, Inf) 0.9885 |
| 4 | 28 (27.18%) | 2,303,125.15 (0.00, Inf) 0.9886 |
| 5 | 7 (6.80%) | 959,635.48 (0.00, Inf) 0.9893 |
| Non-Adjusted | Model I | Model II | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Education (years) | 0.89 (0.80, 0.99) | 0.0287 | 0.88 (0.79, 0.99) | 0.0266 | 0.88 (0.79, 0.99) | 0.0259 |
| UPDRSIII drug off | 0.95 (0.91, 0.98) | 0.0034 | 0.95 (0.91, 0.98) | 0.0026 | 0.95 (0.91, 0.98) | 0.0028 |
| UPDRSIII drug on | 0.93 (0.87, 0.99) | 0.0332 | 0.95 (0.91, 0.99) | 0.0203 | 0.94 (0.86, 1.02) | 0.1110 |
| PDQ-39 Preop | 0.97 (0.95, 1.00) | 0.0469 | 0.97 (0.94, 1.00) | 0.0282 | 0.97 (0.94, 1.01) | 0.1379 |
| HAMA Preop | 0.86 (0.79, 0.95) | 0.0022 | 0.86 (0.78, 0.94) | 0.0017 | 0.86 (0.77, 0.96) | 0.0061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, B.; Mei, J.; Ni, C.; Xiong, C.; Chen, P.; Jiang, M.; Niu, C. Development and Validation of a Prediction Model for Anxiety Improvement after Deep Brain Stimulation for Parkinson Disease. Brain Sci. 2023, 13, 219. https://doi.org/10.3390/brainsci13020219
Chang B, Mei J, Ni C, Xiong C, Chen P, Jiang M, Niu C. Development and Validation of a Prediction Model for Anxiety Improvement after Deep Brain Stimulation for Parkinson Disease. Brain Sciences. 2023; 13(2):219. https://doi.org/10.3390/brainsci13020219
Chicago/Turabian StyleChang, Bowen, Jiaming Mei, Chen Ni, Chi Xiong, Peng Chen, Manli Jiang, and Chaoshi Niu. 2023. "Development and Validation of a Prediction Model for Anxiety Improvement after Deep Brain Stimulation for Parkinson Disease" Brain Sciences 13, no. 2: 219. https://doi.org/10.3390/brainsci13020219
APA StyleChang, B., Mei, J., Ni, C., Xiong, C., Chen, P., Jiang, M., & Niu, C. (2023). Development and Validation of a Prediction Model for Anxiety Improvement after Deep Brain Stimulation for Parkinson Disease. Brain Sciences, 13(2), 219. https://doi.org/10.3390/brainsci13020219







