Abstract
Glioblastoma multiforme (GBM) represents the most common and aggressive central nervous system tumor associated with a poor prognosis. The aim of this study was to depict the role of intraoperative imaging techniques in GBM surgery and how they can ensure the maximal extent of resection (EOR) while preserving the functional outcome. The authors conducted a systematic review following PRISMA guidelines on the PubMed/Medline and Scopus databases. A total of 1747 articles were identified for screening. Studies focusing on GBM-affected patients, and evaluations of EOR and functional outcomes with the aid of advanced image-guided techniques were included. The resulting studies were assessed for methodological quality using the Risk of Bias in Systematic Review tool. Open Science Framework registration DOI 10.17605/OSF.IO/3FDP9. Eighteen studies were eligible for this systematic review. Among the selected studies, eight analyzed Sodium Fluorescein, three analyzed 5-aminolevulinic acid, two evaluated IoMRI imaging, two evaluated IoUS, and three evaluated multiple intraoperative imaging techniques. A total of 1312 patients were assessed. Gross Total Resection was achieved in the 78.6% of the cases. Follow-up time ranged from 1 to 52 months. All studies assessed the functional outcome based on the Karnofsky Performance Status scale, while one used the Neurologic Assessment in Neuro-Oncology score. In 77.7% of the cases, the functional outcome improved or was stable over the pre-operative assessment. Combining multiple intraoperative imaging techniques could provide better results in GBM surgery than a single technique. However, despite good surgical outcomes, patients often present a neurocognitive decline leading to a marked deterioration of the quality of life. Advanced intraoperative image-guided techniques can allow a better understanding of the anatomo-functional relationships between the tumor and the surrounding brain, thus maximizing the EOR while preserving functional outcomes.
1. Introduction
Glioblastoma multiforme (GBM) is the most aggressive and frequent primary malignant tumor of the central nervous system in adults [1]. The mean survival rate is 12–15 months following the gold-standard treatment, including maximal safe resection, radiation therapy, and adjuvant chemotherapy [2]. To date, a few therapeutical approaches have been shown to increase the overall survival (OS) of affected patients, although their efficacy still needs to be supported by large clinical trials [3,4,5].
The OS is mainly associated with the extent of resection (EOR). Lacroix et al. and subsequent studies demonstrated that an EOR > 98% of the tumor mass accounted for a better prognosis [6,7,8]. In this context, surgical planning becomes crucial to extend the OS of affected patients [9,10]. Moreover, due to the growing evidence regarding the biologic behavior of glioma neoplasms, the surgical approach aims to extend the resection beyond the contrast-enhanced tumor borders [11]. Therefore, careful evaluation of the tumor characteristics and its relationships with the adjacent parenchyma and white matter bundles is mandatory and currently possible through advanced imaging techniques. These technological advancements have been shown to assist the neurosurgeon to better understand the brain’s anatomo-functional organization, both maximizing the EOR and minimizing postoperative neurological morbidity [12,13].
The connection between EOR and quality of life (QoL) is the cornerstone of the “onco-functional balance”, based on glioma’s surgical resection tailored on the preservation of cortico-subcortical functions instead of the classical oncological boundaries [14,15]. Such a strategy relies on a detailed pre- and postoperative neuropsychological evaluation, along with a thorough knowledge of the functional anatomy of brain networks.
Recently, it has been shown that connectomic data may play a critical role, providing highly detailed neuroanatomical and neurofunctional maps of the human brain. This strategy could allow evaluating patients’ QoL through the study of a group of functions called “higher-order cognitive functions”, often underestimated during intraoperative monitoring [16,17]. This paradigm shift leads to a network-based approach to glioma surgery, transforming the traditional lesion-oriented approach of neuro-oncologic surgery into functionally tailored resection [18,19]. This shift is certainly made possible by the exceptional technological progress in the field of neuroimaging. The implementation of intraoperative imaging techniques and the development of new strategies aimed at accurate anatomo-functional, metabolic, genomic, and transcriptomic definition is revolutionizing the surgical management of GBM, increasingly emphasizing the importance of patients’ residual QoL.
This systematic review details the role of advanced image-guided techniques in GBM surgery, focusing on their impact on the EOR while preserving the postoperative patients’ QoL. The authors reviewed the feasibility and effectiveness of the main intraoperative imaging techniques such as Intraoperative MRI (IoMRI), Intraoperative Ultrasound (IoUS), sodium fluorescein (SF), 5-aminolevulinic acid (5-ALA), and neuronavigation with/out DTI-fiber tractography, alone or in association. According to the recently released WHO classification, the glioma nomenclature has been modified. Considering the lack of studies analyzing the relationship between intraoperative technique and clinical outcome in GBM surgery according to the most recent classification, it was necessary to maintain the previous WHO classification for our analysis.
2. Materials and Methods
2.1. Search of the Literature
Preferred Reporting Items for Systematic reviews and Meta-analyses guidelines (PRISMA) were followed to conduct this systematic review [20] (Figure 1). We performed a broad systematic literature search in Pubmed/Medline and Scopus for all studies investigating the application of intraoperative imaging techniques in GBM surgery to achieve the best onco-functional balance. We searched for studies published from 2017 up to the 9th of November 2022, using the following MeSH: “Intraoperative MRI (IoMRI)”, “Intraoperative Ultrasound (IoUS), “Sodium Fluorescin”, “5-ALA”, “intraoperative Neuronavigation”, “intraoperative tractography”, “High-grade glioma (HGG)”, “Glioblastoma (GBM)”, “Quality of Life (QoL)”, “Karnofsky Performance Status (KPS)”, and “extent of resection (EOR)”, combined using Boolean operators “AND” and “OR”. Furthermore, we manually screened reference lists of the most relevant systematic reviews and meta-analyses related to this study. Duplicate articles were eliminated using Microsoft Excel 16.37. Since the search combing GBM with the other MeSHs and free text terms revealed limited results, we enlarged the research including in our query “high grade glioma” (HGG). The protocol of this review has been prospectively registered in Open Science Framework and it is available online at https://doi.org/10.17605/OSF.IO/3FDP9 (accessed on 16 December 2022).
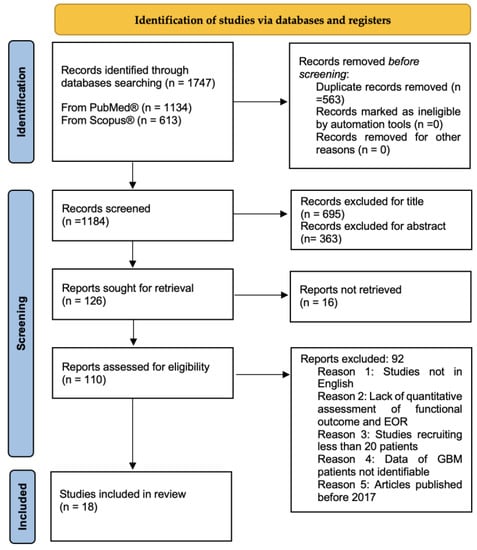
Figure 1.
PRISMA flow chart of selection process.
2.2. Study Selection and Risk of Bias Assessment
The research strategy initially relied on the title and abstract analysis. The article’s full text was retrieved for further investigation if the title and abstract met the inclusion criteria. Two authors (F.B. and P.M.S.) independently assessed eligibility, and differences were resolved with the help of a third author (L.B). We decided to include in the review studies published from 2017, to screen the large amount of data from the literature and, consequently, analyze the most recent evidence. The risk of bias was evaluated using the Risk of Bias in Systematic Reviews (ROBIS) assessment tool [21]. The ROBIS outlines four domains of biases divided into study eligibility criteria assessment, identification and study selection process, data collection and study appraisal evaluation, and synthesis and findings assessment. The data collection process was conducted without using any automated tools. No ethical approval was required for this study.
2.3. Eligibility Criteria
The articles were selected according to the following inclusion criteria:
- Full articles in English;
- Clinical studies;
- Studies including patients affected by GBM and/or HGG (studies focusing on HGG were considered only if characteristics of GBM patients were identifiable);
- Age > 18 years old;
- Studies assessing functional outcomes (expressed using standardized scales, i.e., KPS or NANO score);
- Studies assessing EOR.
Exclusion criteria:
- Meta-analysis, reviews, case reports, editorials, technical notes;
- Lack of quantitative assessment of functional outcome and extent of resection;
- Recruited less than 20 patients;
- Article published before 2017.
2.4. Data Extraction
Two authors (F.B. and P.M.S.) collected data on study characteristics (authors, publication year, study design, and country), patients’ characteristics (age and sex), type of intraoperative imaging modality used (IoMRI, IoUS, 5-ALA, SF, Neuronavigation and Tractography, Indocyanine green), preoperative mean tumor volume (cm3), number of tumors located into eloquent area (%), surgical and functional outcomes (EOR and KPS), and follow-up duration.
3. Results
3.1. Study Selection
The search performed yielded 1747 articles. After removing duplicates, the studies screened were 1184. Based on the title and abstract, 1058 articles were excluded and another 16 were not retrieved. A further 92 were not considered due to incompatibility with our eligibility criteria. Full texts of the remaining 18 studies were selected for the analysis. Among the selected studies, 2 were prospective studies, 1 was a case series, 1 Randomized Control Trial (RCT), 1 multicenter cross-sectional study, and 13 retrospective studies. Most studies evaluated multiple intraoperative imaging techniques to achieve maximal EOR while preserving the patient’s neurologic function and QoL. Demographic and study design data are summarized in Table 1.

Table 1.
Demographic and study design data included in review.
3.2. Study Characteristics
Three studies analyzed the intraoperative use of 5-ALA, alone or in association with other techniques, highlighting its ability to achieve GTR, and thus allowing an increase in OS and progression-free survival (PFS). Picart et al. [37] investigated the impact of 5-ALA florescence-guided surgery associated with IONM (5-ALA group), compared to surgery on white light (control group), in 51 patients affected by GBM located in eloquent areas. Three-month postoperative motor and language deficits rates were similar between groups (5-ALA group: 12.5%, 12.5%; control group: 29.6%, 14.8%) (p = 0.180; p = 0.990). There were no significant differences in EOR and OS between groups. Interestingly, the 12-month progression-free survival was significantly higher in the 5-ALA group (60%) than in the control group (21%; p = 0.006).
Bettag and coauthors [38], in a retrospective series of 20 patients with GBM treated endoscopically, showed that in all the patients, endoscopic fluorescence-guided tumor resection was beyond the contrast-enhanced tumor borders, with a mean postoperative KPS of 89.3. Finally, a multicenter cross-sectional study [30] compared two different groups of GBM patients based on independent variables such as EOR and + surgery (model 1), as well as intraoperative imaging (model 2), and on dependent variables from the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30/BN20). They found a positive correlation between EOR/awake surgery and QoL. On the other hand, in model 2, the impact of intraoperative imaging on QoL has been evaluated, demonstrating that the highest mean scores for functioning and the lowest for symptoms were found in cases where IoMRI was used alone or in combination with 5-ALA.
Eight studies focused on intraoperative use of SF in association with awake surgery [23,31,32,33,34,35,36,39]. Neira’s group [23] showed that with light fluorescence it could be possible to achieve GTR in 84% of patients. In particular, authors divided their cohorts into two groups: “amenable to GTR” and “not amenable to GTR”, based on tumor location. The rate of GTR was higher for patients with a tumor “amenable to GTR” (93.1% of patients). Moreover, they observed a mild reduction in postoperative KPS score (83.3 ± 12.0 preoperative vs. 78.4 ± 13.4 postoperative), especially in the recurrent GBM cohort. Chen et al. [33] showed that DTI with SF improved EOR and postoperative KPS for GBM located in the eloquent area. In their cohort, GTR was achieved in 41 patients (83.7%) while a subtotal resection (STR) was obtained in 8 patients (16.3%). KPS improved in 36 out of 49 patients. Francaviglia and collaborators [35] investigating SF obtained a GTR in 53.2% of the cases and STR in 29.8%. KPS improved in 31.9% of the cases. Ming Lu et al. [36], in their retrospective study, showed a maximal safe extent of resection (>25% FLAIRectomy) by using awake surgery and SF compared to awake surgery alone. Patients who received combined SF and awake craniotomy (AC) resulted in a greater median FLAIR resection percentage (31.90%) as compared with patients treated with AC alone (16.04%). Moreover, although no statistical differences were found in postoperative KPS score between the groups with FLAIR resection above and below 25% of thresholds, the extent of FLAIR GBM abnormality resection was directly related to OS and PFS in a univariate and multivariate analysis. By the way, KPS, PFS, and OS data had to be related to the percentage of tumor located in the eloquent area (58.7%).
Three investigations evaluated the role of IoMRI alone or in association with other intraoperative techniques in extending tumor resection [24,29,30]. Marongiu et al. [24] showed that in the group operated on with the aid of IoMRI, a GTR was achieved in 88.5% of the cases, with an improvement of KPS, at a discharge of 20.5%. In their study, Bassaganyas-Vancell et al. [29] included 118 affected patients according to 5-ALA surgical guidance (5A-group), iMRI (iMRI-group), or both (5A-iMRI-group). An EOR > 90% was more frequent in the 5-ALA group (75%), followed by the IoMRI group (73.7%), and finally the 5A-iMRI-group (71.8%). However, these differences were not statistically significant (p value = 0.94), due to the lower rate of tumor located in eloquent areas in the 5-ALA group (26.7%). Further, the occurrence of new neurologic deficits was 21.7%, 20.5%, and 15.8% in 5-ALA, 5-ALA/iMRI, and IoMRI, respectively (p value = 0.86).
Two studies focused on the use of IoUS [25,27]. Moiraghi et al. [25], using neuronavigated IoUS (N-IoUS), obtained a gross total resection (GTR) in 61.2% of the patients (vs. 44.8% in Neuronavigated (NN) group). At discharge, the difference between pre- and postoperative KPS was significantly higher for the N-ioUS (p < 0.01) compared to the other group.
Different studies [28,33] evaluated the usefulness of intraoperative tractography to maximize EOR while preserving the white matter tracts nearby the tumor. In this regard, Barbagallo et al. [28] reported about recurrent GBM treated with a multimodal approach, including DTI. It has been demonstrated that, with a multimodal approach (neuronavigation with MRI, iCT, 11C-methionine–positron emission tomography (11C-MET-PET), 5-ALA fluorescence, IONM, and N-IoUS), it was possible to reach 100% of GTR with unchanged or improved median KPS over the follow-up period. Chen D. et al. [33] demonstrated that combining preoperative DTI with intraoperative use of SF led to greater EOR for tumor located in the eloquent area: 83.7% of GTR vs. 45.7% in the control group. The authors also assessed the prognosis assessment by evaluating changes in muscle strength and KPS score in the first month after surgery. As a result, they showed that patients treated with preoperative DTI had an improvement in KPS (in 73.5% of cases vs. 47.8% in control group) and less postoperative reduction in muscle strength (20.4% vs. 47.8%) [33]. (Table 2). Three of 18 studies [24,33,38] also enrolled patients with recurrent HGGs in their series, even though the clinical outcome (i.e., KPS) and EOR was not compared between newly diagnosed GBMs and recurrent ones. One of them [33] included only recurrent GBM in its cohort.

Table 2.
Intraoperative technique, surgical, and functional outcomes included in the study.
3.3. Risk of Bias Assessment
The overall methodological quality assessment of this systematic review revealed a moderate-to-high risk of bias (Supplementary Materials). Overall, the quality of the studies retrieved (13 retrospective studies, 2 perspective and 1 RCT) suggests a severe risk of bias in participant selection. Additionally, the high heterogeneity in the patients’ samples, the type of intraoperative imaging techniques, and the lack of reporting of confounders add further concerns. Considering these limitations, the quality of evidence for the included studies was downgraded to low.
3.4. Study Synthesis
A total of 1312 patients were evaluated. The mean patient age was 56.5 ± 7.07 years, and a mean M/F ratio was 1.36. On average, GTR was achieved in 78.6% of the evaluated population. An EOR ≥ 90% was obtained in all the analyzed patients. All studies evaluated functional outcomes based on the KPS, while one study used the Neurologic Assessment in Neuro-Oncology (NANO) score. In 77.7% of the studies included (14/18) a variable degree of KPS improvement or its stability, compared with the preoperative period, was recorded. Notably, in 22.2% of the cases (4/18), the functional outcome worsened. The follow-up time was 8.4 ± 15.53 (ranging from 1 to 52 months). Giving the extensive range of follow-up time, it could not be possible to standardize KPS at the same follow-up.
4. Discussion
To date, in patients with GBM a maximal surgical resection has been shown to be related with an improvement in OS and PFS. In addition, the EOR beyond the FLAIR’s limits has been demonstrated to be associated with a higher percentage of survivors [40,41]. Despite the best surgical outcome, patients often present a postoperative neurocognitive decline, affecting the QoL.
Thus, it seems clear that there is a need to carefully evaluate which treatment strategy represents the optimal solution, taking into consideration both the OS and subsequent QoL. In this context, this study aims to show a comprehensive review of advanced image-guided intraoperative techniques in GBM surgery.
4.1. Fluorescence Techniques
Fluorescence techniques have been used in neurosurgery to obtain a more targeted and safer tumor resection [42]. Four types of intraoperative fluorescence can be distinguished as follows: tissue fluorescence based on passive permeability (i.e., ICG or SF); tissue fluorescence induced by specific metabolic characteristics (5-ALA); auto fluorescence; and, finally, fluorescence derived by fluorescent probes (i.e., near-infrared dyes) [43]. The most promising dyes, frequently used in GBM surgery, are 5-ALA and SF.
4.1.1. Aminolaevulinic Acid
The synthetic amino acid 5-ALA is a compound that, once metabolized, determines the formation of an intermediate fluorescent metabolite known as protoporphyrin IX (PpIX). This molecule accumulates in neoplastic cells in high concentrations [44]. Its value in maximizing EOR in glioma surgery has been highlighted in several studies [45,46,47]. Picart’s group showed that 5-ALA-aided surgery in GBM located in eloquent areas was associated with a significantly higher PFS (p value = 0.02), with no modification on the OS or worsening neurological and functional outcomes [37].
Several studies have shown that the use of 5-ALA in GBM surgery leads to an increased rate of GTR along with a good outcome in terms of PFS [48,49,50,51] and OS [52,53,54]. The fluorescence of 5-ALA can usefully differentiate boundaries between lesion core (ALA+), healthy parenchyma (ALA-), and areas of neoplastic infiltration (ALA-PALE), optimizing resection’s limits considering the intratumoral heterogeneity that characterizes GBM [55].
The compound 5-ALA has also been effectively used in recurrent GBM resection [56]. However, the risk of false-positive fluorescence for reactive non-tumor tissue is more remarkable in relapse forms, likely due to altered BBB following adjuvant therapies [57,58]. To be noted, not all types of GBMs uptake 5-ALA [59,60,61]. Suzuki and collaborators [62] found in U251 cell lines an over-expression of the gene encoding Cadherin-13 that would have a negative regulator role in 5-ALA metabolism, making some molecular subtypes of GBM “fluorescence negative” [63].
Interestingly, according to Bonnin et al. [64], different fluorescence intensity could be due to the histological tumor subtype. The Neural GBM subtype has a pattern of reduced fluorescence or even a non-fluorescence signal depending on the genotype analyzed. In this scenario, the association of multiple tracers may offer a viable solution [65]. Della Puppa et al. [53] combined 5-ALA with SF, highlighting the possibility of exploiting two different tracers to maximize EOR, thus improving oncology outcome [66,67].
Finally, Coburger et al. [68] compared the efficacy of IoMRI alone (group 1) versus IoMRI plus 5-ALA fluorescence (group 2) in GBM surgery, showing a significantly higher rate of maximal safe resection in group 2.
4.1.2. Sodium Fluorescein
SF is a fluorescent compound that exploits the altered permeability of the staining brain areas with abnormal cellularity and vascularization. SF is generally thought to bind to blood proteins. Protein-bound SF should be excluded from normal tissue by the BBB because of its dimension, while extravasating in regions where the tumor has compromised this barrier, providing tumor-to-normal contrast [69]. Since its function is based on the altered BBB integrity, SF could provide real-time images of the tumor, especially of its margins. As a matter of fact, a more significant metabolic activity of GBM cells has been seen in such areas [70]. However, it has been reported that BBB’s integrity could be preserved in some GBM, leading to the occurrence of false negatives [71]. Finally, some studies on the biokinetics of SF have shown that this tracer can also accumulate in non-tumor areas, especially where there has been surgical tissue manipulation [42,43].
Microsurgical resection of GBM using SF fluorescence has been associated with an increased GTR rate and OS [72,73,74]. For instance, Chen D et al. [33], in their retrospective cohort study, have evaluated the feasibility and clinical value of magnetic resonance diffusion tensor imaging (MR-DTI) associated with fluorescein in the resection of GBM, demonstrating that the EOR and postoperative KPS were significantly higher in the observation group than in the control group (83.7% vs. 45.7%, respectively; p < 0.001 and 73.5% vs. 47.8%, respectively; p < 0.029). Similar results were obtained by Raffa et al. [32]. Their study supports the role of the combination of SF-guided resection and Transcranial Magnetic Stimulation (TMS) for the surgical resection of the tumor involving the motor pathway. They compared patients managed by a multimodal approach versus controls, obtaining a higher GTR rate (73.17% vs. 51.22%; p = 0.04) and a reduction in cases with new permanent motor deficits (9.75% vs. 29.27%; p = 0.04) or worse KPS (12.19% vs. 31.71%; p = 0.03).
A safe maximal resection of GBM extended over the contrast-enhanced margins by using SF fluorescence is reported in a study on 32 patients [23]. A GTR was observed in 84% of patients with an average resected volume of 95%. Further studies confirmed the role of SF, alone or in association with different techniques, in gaining the maximum EOR and the best functional outcomes [32,75,76,77,78,79]. Schebesch et al. have recently analyzed 347 patients of a prospective HGG registry, showing significantly more complete resections were achieved in the SF group than in the white-light group (p < 0.003) [80].
Finally, it has been shown that an EOR of more than 25% of the FLAIR zone (the so-called FLAIRectomy) for those GBM extending into eloquent areas was safe and associated with a good outcome when the resection was integrated with SF-fluorescence use [36].
4.2. Neuronavigation and Tractography
Neuronavigation (NN) is a tool that has gained popularity over the years, giving the opportunity to visualize the surgical scenario in a 3-D model. Years from its first introduction, NN has evolved and integrated with new neuroimaging technologies and new improved algorithms, becoming a useful technique for planning the surgical approach and increasing the accuracy for tumor resection [81]. Some limitations, however, exist and should be taken into consideration. First, intraoperative changes in the surgical field may occur, such as brain shift and brain distortion during surgical maneuvers, limiting the use of NN as a real-time control for the radical resection. Additionally, cyst decompression or deliquoration may provide confounding information in navigational data [82]. This drawback can be overcome through the integration with real-time intraoperative techniques, such as the IoUS or IoMRI. NN has offered outstanding results when associated with brain-mapping techniques such as awake mapping and electrocorticography in the resection of lesions located in the eloquent motor and language areas [83].
Kubben et al. [84] compared the use of NN alone or in association with IoMRI in GBM surgery. The parameters evaluated were the EOR, clinical performance, and the patient’s OS. Interestingly, they found no significant difference between the two imaging techniques. To date, it is also possible to load DTI sequences in the neuronavigator, thus reconstructing tractographic maps that deterministically or probabilistically show three dimensionally the location of eloquent beams.
This tool offers an “in vivo” tracking of the white matter (WM) fiber bundles, providing useful qualitative and quantitative information for the surgical planning on the tracts inside and/or around an intracranial tumor [16,85,86].
The accurate localization of eloquent areas and white matter tracts, such as the corticospinal tract (CST), arcuate fasciculus, and corpus callosum may avoid or reduce the appearance of postoperative neurological deficits [87]. For instance, GBM resection performed in the fronto-parieto-temporal regions of the dominant hemisphere requires the identification of specific functional tracts related to eloquent areas at both cortical and subcortical levels, such as the inferior fronto-occipital fasciculus (IFOF), the inferior longitudinal fasciculus (ILF), the uncinate fasciculus (UF), and the pyramidal tract (PT). Tractography can show the spatial relationship between the tumor and the white matter bundles [88] and estimate the degree of radicality to be reached by evaluating the displacement or the infiltration of the WM fascicles. Their involvement is a strong predictor of the surgical outcome, since the chance of achieving a complete resection is higher when bundles are intact [85].
Interestingly, in a randomized controlled trial of 238 patients (affected by both LGG and HGG comparing NN with or without DTI of the PT), Wu et al. [89] demonstrated that EOR was higher and postoperative neurologic deficits were less frequent with the addition of DTI to the navigational dataset (p value < 0.001), especially in HGG group. Furthermore, in patients with motor-eloquent GBM, a novel tractography algorithm called Multi-Level Fiber Tracking (MLFT) has been introduced. This technical refinement adds branches to the pathways previously reconstructed nearby the tumor, improving the reconstruction of the CST compared to conventionally used DTI-based tractography, thus increasing accuracy and safety during surgery [90].
A tractography-related issue is the edema involving the WM around the tumor, which may reduce sensitivity of the technique. In these situations, High-Definition Fiber Tractography (HDFT) may clearly reveal the complex fiber bundles in the perilesional edematous area around the glioma in a three-dimensional way [16]. Further information can be retrieved when tractography is associated with functional Magnetic Resonance Imaging (fMRI) or a 3D ultrasound [87,91,92,93,94].
4.3. Intraoperative Ultrasound
IoUS is a safe, non-invasive, intraoperative real-time technique. It is fast, harmless, and cost-effective. B-mode US can provide anatomical information about tumor location [95]; Contrast-Enhanced UltraSound (CEUS) offers practical information about the tumor and its relationships with normal brain parenchyma, having the potential to identify residual tumor volume during surgery. Moreover, CEUS includes valuable information about tumor biological characteristics through direct visualization of its pattern of vascularization [96,97,98,99,100].
It has been suggested that the use of ultrasound in GBM surgery can facilitate complete tumor resection more than standard surgery, maximizing neurological outcome, functional performance, and health-related QoL. Wang et al. [101] have investigated the role of IoUS in improving the survival time of patients with either low- or high-grade gliomas and found that survival rates at 1 and 2 years were significantly increased compared to those of control patients (survival rates at 6 months, 1 year, and 2 years were 83.3% and 93.4%, 43.3% in the observational group, and 59.2%, and 13.3%, and 32.8% in the control group, respectively). As mentioned above, IoUS allows the continuous monitoring of the tumor remnants and the changes after surgical maneuvers, such as brain shift and brain deformation, thus reducing the Residual Tumor Volume (RTV) and increasing the EOR [25,95,102,103,104,105]. In these regards, Mahboob and colleagues [106] found in their meta-analysis that using by IoUS, GTR was obtained in 77% of patients affected by glioma. Incekara et al. [27] observed that complete tumor resection was increased when IoUS was used. Cases in which complete resection was thought to be achieved during the operation corresponded with radiological complete resection in only 11.8% in the standard surgery group and in 46.7% in the IoUS group. Moreover, median operative time with IoUS was not different from standard surgery, thus promoting the use of IoUS as an advantageous intraoperative tool for GBM resection without prolonging operation time. On the other hand, ioUS is an operator-dependent technique and, thus, an effective RTV detection can be achieved by experienced operators. Lastly, IoUS can be associated with other intraoperative techniques, such as NN, 5-ALA, and IoMRI to maximize the extent of contrast-enhancing GBM resection [27,107]. However, further investigations are needed to better understand which association results in the best surgical and functional outcomes.
4.4. Intraoperative MRI
IoMRI represents a valuable tool in neurosurgeons’ armamentarium, alone or in association with other techniques [108,109]. While a variety of intraoperative imaging modalities exists, IoMRI provides the highest quality evaluation of surgical resection and assessment of the dynamic changes that occur during surgery [110,111]. Additionally, in providing near real-time information about the dynamic changes occurring during surgery, IoMRI reduces the impact of brain shift phenomenon and improves the accuracy and definition of tumor remnant. In this regard, Hatiboglu et colleagues [112] found that in glioma surgery, even when the resection was considered complete, IoMRI demonstrated an unexpected residual tumor, resulting in additional resection in 47% of cases. Furthermore, IoMRI has been shown to provide useful information to reduce postoperative neurological deficit. In a clinical study, IoMRI improved the EOR by 17.8% and increases the GTR rate by 8.9% up to 73.2%, without additional neurological deficit [113]. Golub et al. [114] have investigated various intraoperative imaging techniques, showing that IMRI and 5-ALA are individually superior to conventional NN for achieving GTR of HGG (p < 0.001). Marongiu et al. [24] have analyzed the impact of IoMRI on EOR and KPS, comparing two groups of HGG patients. They demonstrated that the overall GTR for IoMRI group was 88.5%, whereas the non-IoMRI group was 44%. KPS score in IoMRI group was unchanged in 65.4%, improved in 20.5%, and worsened in 14.1% of cases, while in the other group the KPS score was unchanged in 69.5%, improved in 13.8%, and worsened in 16.7%. In another study, Nickel et al. [30] focused on GTR and health-related Quality of Life (HR-QoL), showing a statistically higher GTR when using IoMRI. In addition, they observed a higher score of HR-QoL associated with the use of IoMRI, although the results were not statistically significant. In contrast, it should be considered that ioMRI is a high-cost and time-consuming technique, for reasons that limit the diffusion of this technique.
Though several studies have demonstrated the ability of IoMRI to maximize EOR, to date its impact on the OS has not been fully clarified [115,116,117,118]. Main concerns related to the use of IoMRI are the lack of evidence for a clear benefit and the cost-effectiveness balance.
4.5. Future Directions–Digital Biopsy
Due to the continuous evolution in science and technology, new diagnostic and therapeutic tools and strategies have emerged, especially in the neuro-oncological field. Among these promising tools, Raman spectroscopy and confocal laser endomicroscopy have been shown to have a significant impact on glioblastoma surgery.
Raman spectroscopy is a biophotonic tool that can differentiate between different tissue types. It is non-destructive and no sample preparation is required [119,120]. Livermore JL et al. [121] has demonstrated that Raman spectroscopy has excellent sensitivity, specificity, and accuracy in predicting tumor versus normal brain. Moreover, in the glioblastoma cases in which 5-ALA-induced fluorescence was used, the performance of Raman spectroscopy was significantly better than the predictive value of 5-ALA-induced fluorescence (p = 0.0009). Certainly, Raman spectroscopy is an extremely innovative option, both for diagnostic and prognostic purposes, as several studies have already shown [122,123,124,125,126], and could become a crucial component in the neurosurgical armamentarium for identifying residual tumors and improving the surgical management of brain tumors [127].
Confocal laser endomicroscopy (CLE) represents a promising technology that provides the real-time histological visualization of living tissue [128,129]. In this context, CLE is an encouraging tool to obtain near real-time intraoperative histological data in neurosurgery. Furthermore, its utility in identifying brain tumor microvasculature, tumor margins, and tumor residual is now well accepted [130]. Höhne J and colleagues [131] evaluated the benefit of CLE in the operative workflow in a series of 12 patients. They found CLE beneficial in terms of high-quality visualization of fine structures and for displaying hidden anatomical details, pointing out its potential to change intracranial tumor surgery. Such evidence also confirms the results obtained by other groups [132,133,134]. However, there are still limitations to be addressed (i.e., the lack of standardized protocols for some of its uses), and further large clinical trials are needed [135].
5. Conclusions
In GBM surgery, tumor location strongly influences a radical resection, especially when the tumor involves eloquent areas. Moreover, the ability to infiltrate and spread through WM fiber bundles makes GBM a “whole-brain” pathology. Based on these principles, it is challenging to obtain a complete tumor resection while preserving patient QoL. In the recent years, many intraoperative imaging techniques have blossomed and enriched the surgical armamentarium. Each of them has shown to be of help during the surgical resection, although their limitations must be taken into consideration. The simultaneous use of different techniques identifies the paradigm of the multimodal approach, which, in combining the use of different intraoperative tools, holds promise in reaching a maximal safe resection while preserving neurological functions. Data from this systematic review suggest that multimodal imaging approaches are associated with better surgical and functional outcomes. However, tailored studies focusing on neuropsychological assessment and higher-order cognitive functions are mandatory to better evaluate the link between EOR and QoL. Although the concept of onco-functional balance seems to be well established in low-grade glioma, strong data on GBM and other HGG are still missing. It is well known that the survival of patients affected by HGG is strongly related to surgery, chemo, and radiation therapy and in this scenario the OS is strictly related with EOR. However, an aggressive resection can lead to a dramatic decline in the patient’s cognitive function and QoL, preventing the chance for subsequent adjuvant therapies.
This review has some limitations. First, all studies included in our review do not consider the new WHO classification on gliomas, which incorporates new molecular features. This fact may have led to considering different tumor entities as a single category, predisposing to risks of bias in the assessment of functional outcome and prognosis. Second, the studies included in this systematic review show a great heterogeneity in terms of sample size and imaging techniques used, leading to misinterpretation in the results. Furthermore, most of the included studies are retrospective, implying drawbacks for patient selection and study design. Finally, the evaluation of QoL is related to a simple assessment of the degree of autonomy in the performance of activity of daily life (ADL and BADL), measured by the KPS score. The use of new standardized scales that allow for a comprehensive neuropsychological assessment, including the evaluation of so-called higher cognitive functions (such as the different aspects of memory, comprehension, planning), is essential.
In conclusion, data from this systematic review suggest that in GBM surgery, multimodal imaging approaches are associated with better surgical and functional outcomes. However, the absence of well-tailored studies addressing the onco-functional balance and higher cognitive functions assessment in GBM patients strongly support future well-tailored studies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/brainsci13020216/s1. PRISMA Checklist, ROBIS tool.
Author Contributions
Conceptualization, F.T., L.B. and G.G.; methodology, F.B. and M.P.S.; software, F.B.; validation, F.T. and L.B.; formal analysis, F.B. and M.P.S.; investigation, M.P., U.E.B., S.M. (Salvatore Marrone) and S.M. (Sofia Musso); data curation, L.B. and F.T.; writing—original draft preparation, S.M. (Salvatore Marrone), U.E.B., S.M. (Sofia Musso) and M.P.; writing—review and editing, F.T., L.B. and G.G.; visualization, F.T., L.B. and G.G.; supervision, F.T. and G.G.; project administration, G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. The authors declare no conflict of interest.
References
- Salcman, M. Glioblastoma Multiforme. Am. J. Med. Sci. 1980, 279, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zigiotto, L.; Annicchiarico, L.; Corsini, F.; Vitali, L.; Falchi, R.; Dalpiaz, C.; Rozzanigo, U.; Barbareschi, M.; Avesani, P.; Papagno, C.; et al. Effects of Supra-Total Resection in Neurocognitive and Oncological Outcome of High-Grade Gliomas Comparing Asleep and Awake Surgery. J. Neuro-Oncol. 2020, 148, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, F.; Aguennouz, M.; la Torre, D.; Sfacteria, A.; Grasso, G. Role of Erythropoietin in Cerebral Glioma: An Innovative Target in Neuro-Oncology. World Neurosurg. 2019, 131, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Midiri, M.; Catalano, C.; Gagliardo, C. Transcranial Magnetic Resonance-Guided Focused Ultrasound Surgery for Brain Tumor Ablation: Are We Ready for This Challenging Treatment? World Neurosurg. 2018, 119, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Torregrossa, F. When Neuroprotection Becomes a Potential Ally of High-Grade Glioma. World Neurosurg. 2019, 125, 529–530. [Google Scholar] [CrossRef]
- Kotrotsou, A.; Elakkad, A.; Sun, J.; Thomas, G.A.; Yang, D.; Abrol, S.; Wei, W.; Weinberg, J.S.; Bakhtiari, A.S.; Kircher, M.F.; et al. Multi-Center Study Finds Postoperative Residual Non-Enhancing Component of Glioblastoma as a New Determinant of Patient Outcome. J. Neuro-Oncol. 2018, 139, 125–133. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma a Systematic Review and Meta-Analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A Multivariate Analysis of 416 Patients with Glioblastoma Multiforme: Prognosis, Extent of Resection, and Survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Grasso, G. Extent of Resection and Survival in Glioblastoma Multiforme. JAMA Oncol. 2016, 2, 1508–1509. [Google Scholar] [CrossRef]
- Tomlinson, S.B.; Hendricks, B.K.; Torregrossa, F.; Grasso, G.; Cohen-Gadol, A.A. Innovations in the Art of Microneurosurgery for Reaching Deep-Seated Cerebral Lesions. World Neurosurg. 2019, 131, 321–327. [Google Scholar] [CrossRef]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The Influence of Maximum Safe Resection of Glioblastoma on Survival in 1229 Patients: Can We Do Better than Gross-Total Resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Torregrossa, F.; Leone, L. Maximizing the Extent of Resection in High-Grade Glioma. World Neurosurg. 2019, 123, 256–258. [Google Scholar] [CrossRef]
- Di Ieva, A.; Magnussen, J.S.; McIntosh, J.; Mulcahy, M.J.; Pardey, M.; Choi, C. Magnetic Resonance Spectroscopic Assessment of Isocitrate Dehydrogenase Status in Gliomas: The New Frontiers of Spectrobiopsy in Neurodiagnostics. World Neurosurg. 2020, 133, e421–e427. [Google Scholar] [CrossRef]
- Duffau, H.; Mandonnet, E. The “Onco-Functional Balance” in Surgery for Diffuse Low-Grade Glioma: Integrating the Extent of Resection with Quality of Life. Acta Neurochir. 2013, 155, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Nibali, M.C.; Torregrossa, F.; Bello, L.; Grasso, G. Innovation in Neurosurgery: The Concept of Cognitive Mapping. World Neurosurg. 2019, 131, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Abhinav, K.; Yeh, F.-C.; Mansouri, A.; Zadeh, G.; Fernandez-Miranda, J.C. High-Definition Fiber Tractography for the Evaluation of Perilesional White Matter Tracts in High-Grade Glioma Surgery. Neuro. Oncol. 2015, 17, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Dadario, N.B.; Brahimaj, B.; Yeung, J.; Sughrue, M.E. Reducing the Cognitive Footprint of Brain Tumor Surgery. Front. Neurol. 2021, 12, 711646. [Google Scholar] [CrossRef]
- Samuel, N.; Vetkas, A.; Pancholi, A.; Sarica, C.; Loh, A.; Germann, J.; Harmsen, I.E.; Tasserie, J.; Milano, V.; Yamamoto, K.; et al. A Network-Based Approach to Glioma Surgery: Insights from Functional Neurosurgery. Cancers 2021, 13, 6127. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G. Innovation in Neurosurgery: Integration Between Cutting-Edge Devices and “Old-Fashioned” Surgical Technique. World Neurosurg. 2019, 131, 311–312. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A New Tool to Assess Risk of Bias in Systematic Reviews Was Developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Kelm, A.; Ille, S.; Schröder, A.; Zimmer, C.; Ringel, F.; Meyer, B.; Krieg, S.M. Setup presentation and clinical outcome analysis of treating highly language-eloquent gliomas via preoperative navigated transcranial magnetic stimulation and tractography. Neurosurg Focus. 2018, 44, E2. [Google Scholar] [CrossRef]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive Resection at the Infiltrative Margins of Glioblastoma Facilitated by Intraoperative Fluorescein Guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, A.; D’Andrea, G.; Raco, A. 1.5-T Field Intraoperative Magnetic Resonance Imaging Improves Extent of Resection and Survival in Glioblastoma Removal. World Neurosurg. 2017, 98, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Moiraghi, A.; Prada, F.; Delaidelli, A.; Guatta, R.; May, A.; Bartoli, A.; Saini, M.; Perin, A.; Wälchli, T.; Momjian, S.; et al. Navigated Intraoperative 2-Dimensional Ultrasound in High-Grade Glioma Surgery: Impact on Extent of Resection and Patient Outcome. Oper. Neurosurg. 2020, 18, 363–373. [Google Scholar] [CrossRef]
- Barbagallo, G.M.V.; Altieri, R.; Garozzo, M.; Maione, M.; Di Gregorio, S.; Visocchi, M.; Peschillo, S.; Dolce, P.; Certo, F. High Grade Glioma Treatment in Elderly People: Is It Different Than in Younger Patients? Analysis of Surgical Management Guided by an Intraoperative Multimodal Approach and Its Impact on Clinical Outcome. Front Oncol. 2021, 24, 631255. [Google Scholar] [CrossRef]
- Incekara, F.; Smits, M.; Dirven, L.; Bos, E.M.; Balvers, R.K.; Haitsma, I.K.; Schouten, J.W.; Vincent, A.J.P.E. Intraoperative B-Mode Ultrasound Guided Surgery and the Extent of Glioblastoma Resection: A Randomized Controlled Trial. Front. Oncol. 2021, 11, 649797. [Google Scholar] [CrossRef]
- Vincenzo Barbagallo, G.M.; Certo, F.; di Gregorio, S.; Maione, M.; Garozzo, M.; Peschillo, S.; Altieri, R. Recurrent High-Grade Glioma Surgery: A Multimodal Intraoperative Protocol to Safely Increase Extent of Tumor Resection and Analysis of Its Impact on Patient Outcome. Neurosurg. Focus 2021, 50, E20. [Google Scholar] [CrossRef]
- Bassaganyas-Vancells, C.; Roldán, P.; González, J.J.; Ferrés, A.; García, S.; Culebras, D.; Hoyos, J.; Reyes, L.; Torales, J.; Enseñat, J. Combined Use of 5-Aminolevulinic Acid and Intraoperative Low-Field Magnetic Resonance Imaging in High-Grade Glioma Surgery. World Neurosurg. 2019, 130, e206–e212. [Google Scholar] [CrossRef]
- Nickel, K.; Renovanz, M.; König, J.; Stöckelmaier, L.; Hickmann, A.K.; Nadji-Ohl, M.; Engelke, J.; Weimann, E.; Freudenstein, D.; Ganslandt, O.; et al. The Patients’ View: Impact of the Extent of Resection, Intraoperative Imaging, and Awake Surgery on Health-Related Quality of Life in High-Grade Glioma Patients—Results of a Multicenter Cross-Sectional Study. Neurosurg. Rev. 2018, 41, 207–219. [Google Scholar] [CrossRef]
- Luzzi, S.; Lucifero, A.G.; Martinelli, A.; del Maestro, M.; Savioli, G.; Simoncelli, A.; Lafe, E.; Preda, L.; Galzio, R. Supratentorial High-Grade Gliomas: Maximal Safe Anatomical Resection Guided by Augmented Reality High-Definition Fiber Tractography and Fluorescein. Neurosurg. Focus 2021, 51, E5. [Google Scholar] [CrossRef]
- Raffa, G.; Scibilia, A.; Conti, A.; Cardali, S.M.; Rizzo, V.; Terranova, C.; Quattropani, M.C.; Marzano, G.; Ricciardo, G.; Vinci, S.L.; et al. Multimodal Surgical Treatment of High-Grade Gliomas in the Motor Area: The Impact of the Combination of Navigated Transcranial Magnetic Stimulation and Fluorescein-Guided Resection. World Neurosurg. 2019, 128, e378–e390. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, X.; Zhu, X.; Wu, L.; Ma, S.; Yan, J.; Yan, D. Diffusion Tensor Imaging with Fluorescein Sodium Staining in the Resection of High-Grade Gliomas in Functional Brain Areas. World Neurosurg. 2019, 124, e595–e603. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chen, B.; Yao, X.; Yang, Y. Outcome Comparisons of High-Grade Glioma Resection with or without Fluorescein. Curr. Probl. Cancer 2019, 43, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Francaviglia, N.; Iacopino, D.G.; Costantino, G.; Villa, A.; Impallaria, P.; Meli, F.; Maugeri, R. Fluorescein for Resection of High-Grade Gliomas: A Safety Study Control in a Single Center and Review of the Literature. Surg. Neurol. Int. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Fu, Z.; He, X.; Lu, J.; Deng, X.; Lin, D.; Gu, Y.; Fan, Y.; Lai, M.; Li, J.; et al. T2 Fluid-Attenuated Inversion Recovery Resection for Glioblastoma Involving Eloquent Brain Areas Facilitated Through Awake Craniotomy and Clinical Outcome. World Neurosurg. 2020, 135, e738–e747. [Google Scholar] [CrossRef] [PubMed]
- Picart, T.; Armoiry, X.; Berthiller, J.; Dumot, C.; Pelissou-guyotat, I.; Signorelli, F. Is Fluorescence-Guided Surgery with 5-Ala in Eloquent Areas for Malignant Gliomas a Reasonable and Useful Technique? Neurochirurgie 2017, 63, 189–196. [Google Scholar] [CrossRef]
- Bettag, C.; Hussein, A.; Behme, D.; Maragkou, T.; Rohde, V.; Mielke, D. Endoscopic Fluorescence-Guided Resection Increases Radicality in Glioblastoma Surgery. Oper. Neurosurg. 2020, 18, 41–46. [Google Scholar] [CrossRef]
- Catapano, G.; Sgulò, F.G.; Seneca, V.; Lepore, G.; Columbano, L.; di Nuzzo, G. Fluorescein-Guided Surgery for High-Grade Glioma Resection: An Intraoperative “Contrast-Enhancer”. World Neurosurg. 2017, 104, 239–247. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Brunasso, L.; Costanzo, R.; Paolini, F.; Umana, G.E.; Scalia, G.; Gagliardo, C.; Gerardi, R.M.; Basile, L.; Graziano, F.; et al. Brain Mapping-Aided Supratotal Resection (Sptr) of Brain Tumors: The Role of Brain Connectivity. Front. Oncol. 2021, 11, 645854. [Google Scholar] [CrossRef]
- Jackson, C.; Choi, J.; Khalafallah, A.M.; Price, C.; Bettegowda, C.; Lim, M.; Gallia, G.; Weingart, J.; Brem, H.; Mukherjee, D. A Systematic Review and Meta-Analysis of Supratotal versus Gross Total Resection for Glioblastoma. J. Neurooncol. 2020, 148, 419–431. [Google Scholar] [CrossRef]
- Folaron, M.; Strawbridge, R.; Samkoe, K.S.; Filan, C.; Roberts, D.W.; Davis, S.C. Elucidating the Kinetics of Sodium Fluorescein for Fluorescence-Guided Surgery of Glioma. J. Neurosurg. 2019, 131, 724–734. [Google Scholar] [CrossRef]
- Stummer, W.; Suero Molina, E. Fluorescence Imaging/Agents in Tumor Resection. Neurosurg. Clin. N. Am. 2017, 28, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA Approval for Glioma Surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef] [PubMed]
- della Pepa, G.M.; Ius, T.; la Rocca, G.; Gaudino, S.; Isola, M.; Pignotti, F.; Rapisarda, A.; Mazzucchi, E.; Giordano, C.; Dragonetti, V.; et al. 5-Aminolevulinic Acid and Contrast-Enhanced Ultrasound: The Combination of the Two Techniques to Optimize the Extent of Resection in Glioblastoma Surgery. Neurosurgery 2020, 86, E529–E540. [Google Scholar] [CrossRef] [PubMed]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA Fluorescence-Guided Resection of High-Grade Glioma Leads to Greater Extent of Resection with Better Outcomes: A Systematic Review. J. Neurooncol. 2022, 156, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Dadario, N.B.; Khatri, D.; Reichman, N.; Nwagwu, C.D.; D’Amico, R.S. 5-Aminolevulinic Acid–Shedding Light on Where to Focus. World Neurosurg. 2021, 150, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Eljamel, S. 5-ALA Fluorescence Image Guided Resection of Glioblastoma Multiforme: A Meta-Analysis of the Literature. Int. J. Mol. Sci. 2015, 16, 10443–10456. [Google Scholar] [CrossRef]
- Ba, N.L. Fluorescence—Guided Surgery for High-Grade Gliomas. J. Surg. Oncol. 2018, 118, 356–361. [Google Scholar] [CrossRef]
- Kiesel, B.; Wadiura, L.I.; Mischkulnig, M.; Makolli, J.; Sperl, V.; Borkovec, M.; Freund, J.; Lang, A.; Millesi, M.; Berghoff, A.S.; et al. Efficacy, Outcome and Safety of Elderly Patients with Glioblastoma in the 5-ALA Era: Single Center Experience of More Than 10 Years. Cancers 2021, 13, 6119. [Google Scholar] [CrossRef]
- Baig Mirza, A.; Christodoulides, I.; Lavrador, J.P.; Giamouriadis, A.; Vastani, A.; Boardman, T.; Ahmed, R.; Norman, I.; Murphy, C.; Devi, S.; et al. Aminolevulinic Acid-Guided Resection Improves the Overall Survival of Patients with Glioblastoma-a Comparative Cohort Study of 343 Patients. Neuro-Oncol. Adv. 2021, 3, vdab047. [Google Scholar] [CrossRef]
- Schupper, A.J.; Yong, R.L.; Hadjipanayis, C.G. The Neurosurgeon’ s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection. J. Clin. Med. 2021, 10, 236. [Google Scholar] [CrossRef]
- Cordova, J.S.; Gurbani, S.S.; Holder, C.A.; Olson, J.J.; Schreibmann, E.; Shi, R.; Guo, Y.; Shu, H.G.; Shim, H.; Hadjipanayis, C.G. Semi-Automated Volumetric and Morphological Assessment of Glioblastoma Resection with Fluorescence-Guided Surgery. Mol. Imaging Biol. 2016, 18, 454–462. [Google Scholar] [CrossRef]
- Bettag, C.; Schregel, K.; Langer, P.; Thomas, C.; Behme, D.; Stadelmann, C.; Rohde, V.; Mielke, D. Endoscope-Assisted Fluorescence-Guided Resection Allowing Supratotal Removal in Glioblastoma Surgery. Neurosurg. Focus 2021, 50, E3. [Google Scholar] [CrossRef] [PubMed]
- Pathways, T.; Manini, I.; Caponnetto, F.; Dalla, E.; Ius, T.; Maria, G.; Pepa, D.; Pegolo, E.; Bartolini, A.; la Rocca, G.; et al. Cancers Heterogeneity Matters: Different Regions of Glioblastoma Are Characterized by Distinctive. Cancers 2020, 12, 2960. [Google Scholar]
- Wachter, D.; Kallenberg, K.; Wrede, A. Fluorescence-Guided Operation in Recurrent Glioblastoma Multiforme Treated with Bevacizumab?—Fluorescence of the Noncontrast Enhancing Tumor Tissue? J. Neurol. Surg. Part A Central Eur. Neurosurg. 2012, 73, 401–406. [Google Scholar]
- Kamp, M.A.; Felsberg, J.; Sadat, H.; Kuzibaev, J.; Steiger, H.J.; Rapp, M.; Reifenberger, G.; Dibué, M.; Sabel, M. 5-ALA-Induced Fluorescence Behavior of Reactive Tissue Changes Following Glioblastoma Treatment with Radiation and Chemotherapy. Acta Neurochir. 2015, 157, 207–214. [Google Scholar] [CrossRef]
- Chohan, M.O.; Berger, M.S. 5-Aminolevulinic Acid Fluorescence Guided Surgery for Recurrent High-Grade Gliomas. J. Neurooncol. 2019, 141, 517–522. [Google Scholar] [CrossRef]
- Kiesel, B.; Mischkulnig, M.; Woehrer, A.; Martinez-Moreno, M.; Millesi, M.; Mallouhi, A.; Czech, T.; Preusser, M.; Hainfellner, J.A.; Wolfsberger, S.; et al. Systematic Histopathological Analysis of Different 5-Aminolevulinic Acid–Induced Fluorescence Levels in Newly Diagnosed Glioblastomas. J. Neurosurg. 2018, 129, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.A.; Krause Molle, Z.; Munoz-Bendix, C.; Rapp, M.; Sabel, M.; Steiger, H.J.; Cornelius, J.F. Various Shades of Red—A Systematic Analysis of Qualitative Estimation of ALA-Derived Fluorescence in Neurosurgery. Neurosurg. Rev. 2018, 41, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Mischkulnig, M.; Kiesel, B.; Borkovec, M.; Wadiura, L.I.; Benner, D.; Hosmann, A.; Hervey-Jumper, S.; Knosp, E.; Roessler, K.; Berger, M.S.; et al. High Interobserver Agreement in the Subjective Classification of 5-Aminolevulinic Acid Fluorescence Levels in Newly Diagnosed Glioblastomas. Lasers Surg. Med. 2020, 52, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Wada, S.; Eguchi, H.; Adachi, J.I.; Mishima, K.; Matsutani, M.; Nishikawa, R.; Nishiyama, M. Cadherin 13 Overexpression as an Important Factor Related to the Absence of Tumor Fluorescence in 5-Aminolevulinic Acid-Guided Resection of Glioma. J. Neurosurg. 2013, 119, 1331–1339. [Google Scholar] [CrossRef]
- Sánchez-Ortega, J.F.; Aguas-Valiente, J.; Sota-Ochoa, P.; Calatayud-Pérez, J.; Sánchez-Ortega, J.F. Glioblastoma with Primitive Neuronal Component: A Case Report and Considerations of Fluorescence-Guided Surgery. Surg. Neurol. Int. 2020, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, D.A.A.; Havrda, M.C.; Lee, M.C.; Evans, L.; Ran, C.; Qian, D.C.; Harrington, L.X.; Valdes, P.A.; Cheng, C.; Amos, C.I.; et al. Characterizing the Heterogeneity in 5-Aminolevulinic Acid-Induced Fluorescence in Glioblastoma. J. Neurosurg. 2020, 132, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Omoto, K.; Matsuda, R.; Nakagawa, I.; Motoyama, Y.; Nakase, H. Case Report False—Positive Inflammatory Change Mimicking Glioblastoma Multiforme under 5—Aminolevulinic Acid—Guided Surgery: A Case Report. Surg. Neuron. Int. 2018, 9, 49. [Google Scholar] [CrossRef]
- della Puppa, A.; Munari, M.; Gardiman, M.P.; Volpin, F. Combined Fluorescence Using 5-Aminolevulinic Acid and Fluorescein Sodium at Glioblastoma Border: Intraoperative Findings and Histopathologic Data About 3 Newly Diagnosed Consecutive Cases. World Neurosurg. 2019, 122, e856–e863. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Nakayama, N.; Ohe, N.; Miwa, K.; Shinoda, J.; Iwama, T. Pathological Analysis of the Surgical Margins of Resected Glioblastomas Excised Using Photodynamic Visualization with Both 5-Aminolevulinic Acid and Fluorescein Sodium. J. Neurooncol. 2017, 133, 389–397. [Google Scholar] [CrossRef]
- Coburger, J.; Hagel, V.; Wirtz, C.R.; König, R. Surgery for Glioblastoma: Impact of the Combined Use of 5-Aminolevulinic Acid and Intraoperative MRI on Extent of Resection and Survival. PLoS ONE 2015, 10, e0131872. [Google Scholar] [CrossRef]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High-Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188.e7. [Google Scholar] [CrossRef]
- Wang, L.M.; Banu, M.A.; Canoll, P.; Bruce, J.N. Rationale and Clinical Implications of Fluorescein-Guided Supramarginal Resection in Newly Diagnosed High-Grade Glioma. Front. Oncol. 2021, 11, 666734. [Google Scholar] [CrossRef]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-gadol, A.A. Study of the Biodistribution of Fluorescein in Glioma-Infiltrated Mouse Brain and Histopathological Correlation of Intraoperative Findings in High-Grade Gliomas Resected under Fluorescein Fluorescence Guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Shamim, M.S. Sodium Fluorescein Guided Resection of Malignant Glioma. J. Pak. Med. Assoc. 2018, 68, 968–970. [Google Scholar] [PubMed]
- Schipmann, S.; Schwake, M.; Suero Molina, E.; Stummer, W. Markers for Identifying and Targeting Glioblastoma Cells during Surgery. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2019, 80, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Schebesch, K.M.; Höhne, J.; Cavallo, C.; de Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Katsevman, G.A.; Turner, R.C.; Urhie, O.; Voelker, J.L.; Bhatia, S. Utility of Sodium Fluorescein for Achieving Resection Targets in Glioblastoma: Increased Gross- or near-Total Resections and Prolonged Survival. J. Neurosurg. 2019, 132, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Kutlay, M.; Durmaz, O.; Ozer, İ.; Kırık, A.; Yasar, S.; Kural, C.; Temiz, Ç.; Tehli, Ö.; Ezgu, M.C.; Daneyemez, M.; et al. Fluorescein Sodium-Guided Neuroendoscopic Resection of Deep-Seated Malignant Brain Tumors: Preliminary Results of 18 Patients. Oper Neurosurg. 2021, 20, 206–218. [Google Scholar] [CrossRef]
- Fan, C.; Jiang, Y.; Liu, R.; Wu, G.; Wu, G.; Xu, K.; Miao, Z. Safety and Feasibility of Low-Dose Fl Uorescein-Guided Resection of Glioblastoma. Clin. Neurol. Neurosurg. 2018, 175, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Höhne, J.; Schebesch, K.M.; de Laurentis, C.; Akçakaya, M.O.; Pedersen, C.B.; Brawanski, A.; Poulsen, F.R.; Kiris, T.; Cavallo, C.; Broggi, M.; et al. Fluorescein Sodium in the Surgical Treatment of Recurrent Glioblastoma Multiforme. World Neurosurg. 2019, 125, e158–e164. [Google Scholar] [CrossRef]
- Bowden, S.G.; Neira, J.A.; Gill, B.J.A.; Ung, T.H.; Englander, Z.K.; Zanazzi, G.; Chang, P.D.; Samanamud, J.; Grinband, J.; Sheth, S.A.; et al. Sodium Fluorescein Facilitates Guided Sampling of Diagnostic Tumor Tissue in Nonenhancing Gliomas. Neurosurgery 2018, 82, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Schebesch, K.-M.; Höhne, J.; Rosengarth, K.; Noeva, E.; Schmidt, N.O.; Proescholdt, M. Fluorescein-Guided Resection of Newly Diagnosed High-Grade Glioma: Impact on Extent of Resection and Outcome. Brain Spine 2022, 2, 101690. [Google Scholar] [CrossRef]
- Willems, P.W.A.; Taphoorn, M.J.B.; Burger, H.; van der Sprenkel, J.W.B.; Tulleken, C.A.F. Effectiveness of Neuronavigation in Resecting Solitary Intracerebral Contrast-Enhancing Tumors: A Randomized Controlled Trial. J. Neurosurg. 2006, 104, 360–368. [Google Scholar] [CrossRef]
- Orringer, D.A.; Golby, A.; Jolesz, F. Neuronavigation in the Surgical Management of Brain Tumors: Current and Future Trends. Expert Rev. Med. Devices 2012, 9, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Kuhn, S.A.; Waschke, A.; Kalff, R.; Ewald, C. Operative Treatment of Subcortical Metastatic Tumours in the Central Region. J. Neurooncol. 2011, 103, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Kubben, P.L.; Scholtes, F.; Schijns, O.E.M.G.; ter Laak-Poort, M.P.; Teernstra, O.P.M.; Kessels, A.G.H.; van Overbeeke, J.J.; Martin, D.H.; van Santbrink, H. Intraoperative Magnetic Resonance Imaging versus Standard Neuronavigation for the Neurosurgical Treatment of Glioblastoma: A Randomized Controlled Trial. Surg. Neurol. Int. 2014, 5, 70. [Google Scholar] [CrossRef]
- Castellano, A.; Bello, L.; Michelozzi, C.; Gallucci, M.; Fava, E.; Iadanza, A.; Riva, M.; Casaceli, G.; Falini, A. Role of Diffusion Tensor Magnetic Resonance Tractography in Predicting the Extent of Resection in Glioma Surgery. Neuro Oncol. 2012, 14, 192–202. [Google Scholar] [CrossRef]
- Toescu, S.M.; Hales, P.W.; Tisdall, M.M.; Aquilina, K.; Clark, C.A. Neurosurgical Applications of Tractography in the UK. Br. J. Neurosurg. 2021, 35, 424–429. [Google Scholar] [CrossRef]
- Gulati, S.; Berntsen, E.M.; Solheim, O.; Kvistad, K.A.; Håberg, A.; Selbekk, T.; Torp, S.H.; Unsgaard, G. Surgical Resection of High-Grade Gliomas in Eloquent Regions Guided by Blood Oxygenation Level Dependent Functional Magnetic Resonance Imaging, Diffusion Tensor Tractography, and Intraoperative Navigated 3D Ultrasound. Minim Invasive Neurosurg. 2009, 52, 17–24. [Google Scholar] [CrossRef]
- Vassal, F.; Schneider, F.; Sontheimer, A.; Lemaire, J.J.; Nuti, C. Intraoperative Visualisation of Language Fascicles by Diffusion Tensor Imaging-Based Tractography in Glioma Surgery. Acta Neurochir. 2013, 155, 437–448. [Google Scholar] [CrossRef]
- Wu, J.-S.; Mao, Y.; Zhou, L.-F.; Tang, W.-J.; Hu, J.; Song, Y.-Y.; Hong, X.-N.; Du, G.-H. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: A prospective, controlled study in patients with gliomas involving pyramidal tracts clinical studies. Neurosurgery 2007, 61, 935. [Google Scholar] [CrossRef] [PubMed]
- Zhylka, A.; Sollmann, N.; Kofler, F.; Radwan, A.; de Luca, A.; Gempt, J.; Wiestler, B.; Menze, B.; Krieg, S.M.; Zimmer, C.; et al. Tracking the Corticospinal Tract in Patients with High-Grade Glioma: Clinical Evaluation of Multi-Level Fiber Tracking and Comparison to Conventional Deterministic Approaches. Front. Oncol. 2021, 11, 761169. [Google Scholar] [CrossRef] [PubMed]
- Sparacia, G.; Parla, G.; lo Re, V.; Cannella, R.; Mamone, G.; Carollo, V.; Midiri, M.; Grasso, G. Resting-State Functional Connectome in Patients with Brain Tumors Before and After Surgical Resection. World Neurosurg. 2020, 141, e182–e194. [Google Scholar] [CrossRef] [PubMed]
- Sparacia, G.; Parla, G.; Mamone, G.; Caruso, M.; Torregrossa, F.; Grasso, G. Resting-State Functional Magnetic Resonance Imaging for Surgical Neuro-Oncology Planning: Towards a Standardization in Clinical Settings. Brain Sci. 2021, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Sparacia, G.; Parla, G.; Cannella, R.; Perri, A.; lo Re, V.; Mamone, G.; Miraglia, R.; Torregrossa, F.; Grasso, G. Resting-State Functional Magnetic Resonance Imaging for Brain Tumor Surgical Planning: Feasibility in Clinical Setting. World Neurosurg. 2019, 131, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Morales, H. Current and Future Challenges of Functional MRI and Diffusion Tractography in the Surgical Setting: From Eloquent Brain Mapping to Neural Plasticity. Semin. Ultrasound CT MRI 2021, 42, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Unsgaard, G.; Gronningsaeter, A.; Ommedal, S.; Nagelhus Hernes, T.A.; Schramm, J.; van Roost, D.; Chandler, W.F.; Langmoen, I.A.; Kelly, P.J. Brain Operations Guided by Real-Time Two-Dimensional Ultrasound: New Possibilities as a Result of Improved Image Quality. Neurosurgery 2002, 51, 402–412. [Google Scholar] [CrossRef] [PubMed]
- del Bene, M.; Perin, A.; Casali, C.; Legnani, F.; Saladino, A.; Mattei, L.; Vetrano, I.G.; Saini, M.; DiMeco, F.; Prada, F. Advanced Ultrasound Imaging in Glioma Surgery: Beyond Gray-Scale B-Mode. Front. Oncol. 2018, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Perin, A.; Martegani, A.; Aiani, L.; Solbiati, L.; Lamperti, M.; Casali, C.; Legnani, F.; Mattei, L.; Saladino, A.; et al. Intraoperative Contrast-Enhanced Ultrasound for Brain Tumor Surgery. Neurosurgery 2014, 74, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Mattei, L.; del Bene, M.; Aiani, L.; Saini, M.; Casali, C.; Filippini, A.; Legnani, F.G.; Perin, A.; Saladino, A.; et al. Intraoperative Cerebral Glioma Characterization with Contrast Enhanced Ultrasound. Biomed. Res. Int. 2014, 2014, 484261. [Google Scholar] [CrossRef]
- Prada, F.; del Bene, M.; Fornaro, R.; Vetrano, I.G.; Martegani, A.; Aiani, L.; Sconfienza, L.M.; Mauri, G.; Solbiati, L.; Pollo, B.; et al. Identification of Residual Tumor with Intraoperative Contrast-Enhanced Ultrasound during Glioblastoma Resection. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef]
- Prada, F.; Vitale, V.; del Bene, M.; Boffano, C.; Sconfienza, L.M.; Pinzi, V.; Mauri, G.; Solbiati, L.; Sakas, G.; Kolev, V.; et al. Contrast-Enhanced MR Imaging versus Contrast-Enhanced US: A Comparison in Glioblastoma Surgery by Using Intraoperative Fusion Imaging. Radiology 2017, 285, 242–249. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Ba, Y.M.; Yang, Y.L.; Gao, G.D.; Wang, L.; Duan, Y.Y. Effect of sonographically guided cerebral glioma surgery on survival time. J. Ultrasound Med. 2012, 31, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; del Bene, M.; Mattei, L.; Lodigiani, L.; Debeni, S.; Kolev, V.; Vetrano, I.; Solbiati, L.; Sakas, G.; Dimeco, F. Preoperative Magnetic Resonance and Intraoperative Ultrasound Fusion Imaging for Real-Time Neuronavigation in Brain Tumor Surgery. Ultraschall Der Med. 2014, 9, 174–186. [Google Scholar] [CrossRef]
- Prada, F.; del Bene, M.; Mattei, L.; Casali, C.; Filippini, A.; Legnani, F.; Mangraviti, A.; Saladino, A.; Perin, A.; Richetta, C.; et al. Fusion Imaging for Intra-Operative Ultrasound-Based Navigation in Neurosurgery. J. Ultrasound 2014, 17, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Bal, J.; Camp, S.J.; Nandi, D. The Use of Ultrasound in Intracranial Tumor Surgery. Acta Neurochir. 2016, 158, 1179–1185. [Google Scholar] [PubMed]
- Unsgaard, G.; Rygh, O.M.; Selbekk, T.; Müller, T.B.; Kolstad, F.; Lindseth, F.; Hernes, T.A.N. Intra-Operative 3D Ultrasound in Neurosurgery. Acta Neurochir. 2006, 148, 235–253. [Google Scholar]
- Mahboob, S.; McPhillips, R.; Qiu, Z.; Jiang, Y.; Meggs, C.; Schiavone, G.; Button, T.; Desmulliez, M.; Demore, C.; Cochran, S.; et al. Intraoperative Ultrasound-Guided Resection of Gliomas: A Meta-Analysis and Review of the Literature. World Neurosurg. 2016, 92, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Barak, T.; Vetsa, S.; Nadar, A.; Jin, L.; Gupte, T.P.; Fomchenko, E.I.; Miyagishima, D.F.; Yalcin, K.; Vasandani, S.; Gorelick, E.; et al. Surgical Strategies for Older Patients with Glioblastoma. J. Neurooncol. 2021, 155, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.M.; Jones, P.S.; Weinberg, J.S. Intraoperative MRI for Brain Tumors. J. Neurooncol. 2021, 151, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Ille, S.; Schroeder, A.; Wagner, A.; Negwer, C.; Kreiser, K.; Meyer, B.; Krieg, S.M. Intraoperative MRI–Based Elastic Fusion for Anatomically Accurate Tractography of the Corticospinal Tract: Correlation with Intraoperative Neuromonitoring and Clinical Status. Neurosurg. Focus 2021, 50, E9. [Google Scholar] [CrossRef] [PubMed]
- Bander, E.D.; Magge, R.; Ramakrishna, R. Advances in Glioblastoma Operative Techniques. World Neurosurg. 2018, 116, 529–538. [Google Scholar] [CrossRef]
- Noh, T.; Mustroph, M.; Golby, A.J. Intraoperative Imaging for High-Grade Glioma Surgery. Neurosurg. Clin. N. Am. 2021, 32, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hatiboglu, M.A.; Weinberg, J.S.; Suki, D.; Rao, G.; Prabhu, S.S.; Shah, K.; Jackson, E.; Sawaya, R. Impact of Intraoperative High-Field Magnetic Resonance Imaging Guidance on Glioma Surgery: A Prospective Volumetric Analysis. Neurosurgery 2009, 64, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Vaz, G.; Lawson, T.M.; Docquier, M.A.; van Maanen, A.; Duprez, T.; Raftopoulos, C. Glioblastoma Surgery with and without Intraoperative MRI at 3.0T. Neurochirurgie 2014, 60, 143–150. [Google Scholar] [CrossRef]
- Golub, D.; Hyde, J.; Dogra, S.; Nicholson, J.; Kirkwood, K.A.; Gohel, P.; Loftus, S.; Schwartz, T.H. Intraoperative MRI versus 5-ALA in High-Grade Glioma Resection: A Network Meta-Analysis. J. Neurosurg. 2021, 134, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Waqas, M.; Shamim, M.S. Role of Intra-Operative MRI (IMRI) in Improving Extent of Resection and Survival in Patients with Glioblastoma Multiforme. J. Pak. Med. Assoc. 2017, 67, 1121–1123. [Google Scholar]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bauer, M.; Buchfelder, M.; Nimsky, C. Correlation of the Extent of Tumor Volume Resection and Patient Survival in Surgery of Glioblastoma Multiforme with High-Field Intraoperative MRI Guidance. Neuro. Oncol. 2011, 13, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Staartjes, V.E.; Togni-Pogliorini, A.; Stumpo, V.; Serra, C.; Regli, L. Impact of Intraoperative Magnetic Resonance Imaging on Gross Total Resection, Extent of Resection, and Residual Tumor Volume in Pituitary Surgery: Systematic Review and Meta-Analysis. Pituitary 2021, 24, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Heßelmann, V.; Mager, A.-K.; Goetz, C.; Detsch, O.; Theisgen, H.-K.; Friese, M.; Schwindt, W.; Gottschalk, J.; Kremer, P. Accuracy of High-Field Intraoperative MRI in the Detectability of Residual Tumor in Glioma Grade IV Resections. RöFo Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2017, 189, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman Spectroscopy for Chemical Biology Research. J. Am. Chem. Soc. 2022, 144, 19651–19667. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, B.; Tseng, J.; Kast, R.; Noh, T.; Brusatori, M.; Kalkanis, S.N.; Auner, G.W. Shining Light on Neurosurgery Diagnostics Using Raman Spectroscopy. J. Neurooncol. 2016, 130, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Livermore, L.J.; Isabelle, M.; Bell, I.M.; Edgar, O.; Voets, N.L.; Stacey, R.; Ansorge, O.; Vallance, C.; Plaha, P. Raman Spectroscopy to Differentiate between Fresh Tissue Samples of Glioma and Normal Brain: A Comparison with 5-ALA–Induced Fluorescence-Guided Surgery. J. Neurosurg. 2021, 135, 469–479. [Google Scholar] [CrossRef]
- le Reste, P.; Pilalis, E.; Aubry, M.; McMahon, M.; Cano, L.; Etcheverry, A.; Chatziioannou, A.; Chevet, E.; Fautrel, A. Integration of Raman Spectra with Transcriptome Data in Glioblastoma Multiforme Defines Tumour Subtypes and Predicts Patient Outcome. J. Cell. Mol. Med. 2021, 25, 10846–10856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, C.-H.; Wu, B.; Yu, X.; Cheng, G.; Zhu, K.; Wang, K.; Zhang, C.; Zhao, M.; Zong, R.; et al. Optical Biopsy Identification and Grading of Gliomas Using Label-Free Visible Resonance Raman Spectroscopy. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz-Rodríguez, N.; de Pasquale, D.; Fiaschi, P.; Ciofani, G. Discrimination of Glioma Patient-Derived Cells from Healthy Astrocytes by Exploiting Raman Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 269, 120773. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.; Auner, G.; Yurgelevic, S.; Broadbent, B.; Raghunathan, A.; Poisson, L.M.; Mikkelsen, T.; Rosenblum, M.L.; Kalkanis, S.N. Identification of Regions of Normal Grey Matter and White Matter from Pathologic Glioblastoma and Necrosis in Frozen Sections Using Raman Imaging. J. Neurooncol. 2015, 125, 287–295. [Google Scholar] [CrossRef]
- Grasso, G.; Torregrossa, F. Magnetic Resonance Spectrobiopsy for Prediction of Isocitrate Dehydrogenase Mutation in Glioma. World Neurosurg. 2020, 134, 187–189. [Google Scholar] [CrossRef]
- Hollon, T.; Lewis, S.; Freudiger, C.W.; Sunney Xie, X.; Orringer, D.A. Improving the Accuracy of Brain Tumor Surgery via Raman-Based Technology. Neurosurg. Focus 2016, 40, E9. [Google Scholar] [CrossRef]
- Villard, A.; Breuskin, I.; Casiraghi, O.; Asmandar, S.; Laplace-Builhe, C.; Abbaci, M.; Moya Plana, A. Confocal Laser Endomicroscopy and Confocal Microscopy for Head and Neck Cancer Imaging: Recent Updates and Future Perspectives. Oral Oncol. 2022, 127, 105826. [Google Scholar] [CrossRef]
- Kakaletri, I.; Linxweiler, M.; Ajlouni, S.; Charalampaki, P. Development, Implementation and Application of Confocal Laser Endomicroscopy in Brain, Head and Neck Surgery—A Review. Diagnostics 2022, 12, 2697. [Google Scholar] [CrossRef] [PubMed]
- Restelli, F.; Pollo, B.; Vetrano, I.G.; Cabras, S.; Broggi, M.; Schiariti, M.; Falco, J.; de Laurentis, C.; Raccuia, G.; Ferroli, P.; et al. Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications. J. Clin. Med. 2021, 10, 2035. [Google Scholar] [CrossRef]
- Höhne, J.; Schebesch, K.-M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative Imaging of Brain Tumors with Fluorescein: Confocal Laser Endomicroscopy in Neurosurgery. Clinical and User Experience. Neurosurg. Focus 2021, 50, E19. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.S.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective Evaluation of the Utility of Intraoperative Confocal Laser Endomicroscopy in Patients with Brain Neoplasms Using Fluorescein Sodium: Experience with 74 Cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef]
- Abramov, I.; Park, M.T.; Belykh, E.; Dru, A.B.; Xu, Y.; Gooldy, T.C.; Scherschinski, L.; Farber, S.H.; Little, A.S.; Porter, R.W.; et al. Intraoperative Confocal Laser Endomicroscopy: Prospective in Vivo Feasibility Study of a Clinical-Grade System for Brain Tumors. J. Neurosurg. 2022, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Cavallo, C.; Gandhi, S.; Zhao, X.; Veljanoski, D.; Izady Yazdanabadi, M.; Martirosyan, N.L.; Byvaltsev, V.A.; Eschbacher, J.; Preul, M.C.; et al. Utilization of Intraoperative Confocal Laser Endomicroscopy in Brain Tumor Surgery. J. Neurosurg. Sci. 2018, 62, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Restelli, F.; Mathis, A.M.; Höhne, J.; Mazzapicchi, E.; Acerbi, F.; Pollo, B.; Quint, K. Confocal Laser Imaging in Neurosurgery: A Comprehensive Review of Sodium Fluorescein-Based CONVIVO Preclinical and Clinical Applications. Front. Oncol. 2022, 12, 998384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).