Pharmaco-Toxicological Effects of Atypical Synthetic Cathinone Mephtetramine (MTTA) in Mice: Possible Reasons for Its Brief Appearance over NPSs Scene
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Preparation and Dose Selection
2.3. Behavioural Tests
2.3.1. Evaluation of the Visual Response
2.3.2. Evaluation of Tactile Response
2.3.3. Evaluation of Acoustic Response
2.3.4. Evaluation of Breath Rate
2.3.5. Evaluation of Surface and Core Temperature
2.3.6. Motor Activity Assessment
2.3.7. Evaluation of Skeletal Muscle Strength (Grip Strength)
2.4. Biochemical Studies
2.4.1. Collection of Samples
2.4.2. The Scil Vet abc Plus+
2.4.3. Element RC
2.4.4. Aution Micro
2.5. Histological Studies
2.5.1. Collection of Tissue Samples
2.5.2. Histological Procedure
2.6. Statistical Analysis
3. Results
3.1. Behavioural Studies
3.1.1. Evaluation of the Sensorial Response
3.1.2. Evaluation of the Physiological Parameters
3.1.3. Evaluation of the Motor Response
3.2. Biochemical Studies
3.2.1. Complete Blood Count
3.2.2. Element RC
3.2.3. Urine Analysis
3.3. Histological Results
3.3.1. Kidney
3.3.2. Heart
3.3.3. Spleen
3.3.4. Liver
4. Discussion
4.1. Behavioural Changes
4.2. Biochemical and Histological Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2C-B | 2,5-dimethoxy-4-bromophenethylamine |
| 4-MCC | Mephedrone |
| A/G | Albumin/Globulin Ratio |
| ALB | Albumin |
| ALP | Alkaline Phosphatase Level |
| ALT | Alanine Aminotransferase Level |
| AMY | Amylase |
| BIL | Bilirubin |
| BLD | Blood |
| BUN | Blood Urea Nitrogen |
| Crea | Creatinine |
| EOS | Eosinophils |
| GGT | Gamma Glutamyltransferase |
| GLOB | Globulin |
| GLU | Glucose |
| GRA | Granulocytes |
| HCT | Haematocrit test |
| HGB | Haemoglobin |
| KET | Ketones |
| LEU | Leucocytes |
| LSD | lysergic acid diethylamide |
| LYM | Lymphocytes |
| MABP | Buphedrone |
| MCH | Mean Corpuscular Haemoglobin |
| MCHC | Mean Corpuscular Haemoglobin Concentration |
| MCV | Mean Corpuscular Volume |
| MDMA | 3,4-methylenedioxy-N-methylamphetamine |
| MDPV | 3,4-methylenedioxypyrovalerone |
| MON | Monocytes |
| MPV | Mean Platelet Volume |
| MTTA | Mephtetramine |
| NIT | Nitrates |
| NPS | Novel Psychoactive Substances |
| PHOS | Phosphates |
| PLT | Platelets |
| PRO | Proteins |
| RBC | Red Blood Cells |
| RDW | Red Cell Distribution Width |
| S.G. | Specific Gravity |
| SCs | Synthetic Cathinones |
| TB | Total Bilirubin |
| tCO2 | Total CO2 |
| TG | Triglycerides |
| TP | Total Protein |
| URO | Urobilinogen |
| WBC | White Blood Cells |
References
- Griffiths, P.; Evans-Brown, M.; Sedefov, R. Getting up to speed with the public health and regulatory challenges posed by new psychoactive substances in the information age. Addiction 2013, 108, 1700–1703. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime, Current NPS Threats, UNODC Laboratory and Scientific Service Portals. 2020. Available online: https://www.unodc.org/unodc/en/scientists/current-nps-threats.html (accessed on 5 December 2022).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report 2020: Trends and Developments; Publications Office of the European Union: Lisbon, Portugal, 2021. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Impact of COVID-19 on Drug Markets, Use, Harms and Drug Services in the Community and Prisons: Results from an EMCDDA Trendspotter Study; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Miliano, C.; Serpelloni, G.; Rimondo, C.; Mereu, M.; Marti, M.; De Luca, M.A. Neuropharmacology of New Psychoactive Substances (NPS): Focus on the Rewarding and Reinforcing Properties of Cannabimimetics and Amphetamine-Like Stimulants. Front. Neurosci. 2016, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Hondebrink, L.; Zwartsen, A.; Westerink, R.H.S. Effect fingerprinting of new psychoactive substances (NPS): What can we learn from in vitro data? Pharmacol. Ther. 2018, 182, 193–224. [Google Scholar] [CrossRef] [PubMed]
- De Mello-Sampayo, C.; Vaz, A.R.; Henriques, S.C.; Fernandes, A.; Paradinha, F.; Florindo, P.; Faria, P.; Moreira, R.; Brites, D.; Lopes, A. Designer Cathinones N-Ethylhexedrone and Buphedrone Show Different In Vitro Neurotoxicity and Mice Behaviour Impairment. Neurotox. Res. 2021, 39, 392–412. [Google Scholar] [CrossRef] [PubMed]
- Measham, F.; Moore, K.; Newcombe, R.; Welch, Z. Tweaking, bombing, dabbing and stockpiling: The emergence of mephedrone and the perversity of prohibition. Drugs Alcohol Today 2021, 10, 14–21. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; King, L.A.; Nutt, D.J. A web-based survey on mephedrone. Drug Alcohol Depend. 2011, 118, 19–22. [Google Scholar] [CrossRef]
- Corkery, J.; Guirguis, A.; Papanti, D.; Orsolini, L.; Schifano, F. Synthetic cathinones—Prevalence and motivations for use. In Synthetic Cathinones: Novel Addiction and Stimulatory Psychoactive Substances; Current Topics in Neurotoxicity; Zawilska, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 12, pp. 153–189. [Google Scholar] [CrossRef]
- Soares, J.; Costa, V.M.; Bastos, M.L.; Carvalho, F.; Capela, J.P. An updated review on synthetic cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Rothman, R.B.; Goldberg, S.R.; Lupica, C.R.; Sitte, H.H.; et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 2013, 38, 552–562. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime, Early Warning Advisory on NPS, UNODC Laboratory and Scientific Service Portals. 2014. Available online: https://www.unodc.org/LSS/Page/NPS/Resources (accessed on 5 December 2022).
- United Nations Office on Drugs and Crime. The Challenge of Synthetic Drugs in East and South-East Asia and Oceania. Trends and Patterns of Amphetamine-Type Stimulants and New Psychoactive Substances, UNODC Laboratory and Scientific Service Portals. 2015. Available online: https://www.unodc.org/documents/scientific/Trends_and_Patterns_of_ATS_and_NPS_2017.pdf (accessed on 5 December 2022).
- Dolengevich-Segal, H.; Rodríguez-Salgado, B.; Gómez-Arnau, J.; Sánchez-Mateos, D. An approach to the new psychoactive drugs phenomenon. Salud Ment. 2017, 40, 71–82. [Google Scholar] [CrossRef]
- Simmler, L.D.; Buser, T.A.; Donzelli, M.; Schramm, Y.; Dieu, L.H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef]
- Simmler, L.D.; Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 2014, 79, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M. Novel psychoactive substances (designer drugs): Overview and pharmacology of modulators of monoamine signaling. Swiss Med. Wkly. 2015, 145, w14043. [Google Scholar] [CrossRef] [PubMed]
- Odoardi, S.; Mestria, S.; Biosa, G.; Arfè, R.; Tirri, M.; Marti, M.; Strano Rossi, S. Metabolism study and toxicological determination of mephtetramine in biological samples by liquid chromatography coupled with high-resolution mass spectrometry. Drug Test. Anal. 2021, 13, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Abuse (EMCDDA)—Europol. Annual Report on the Implementation of Council Decision; 2005/387/JHA; Publications Office of the European Union: Luxemoburg, 2013. [Google Scholar]
- Strano Rossi, S.; Odoardi, S.; Gregori, A.; Peluso, G.; Ripani, L.; Ortar, G.; Serpelloni, G.; Romolo, F.S. An analytical approach to the forensic identification of different classes of new psychoactive substances (NPSs) in seized materials. Rapid Commun. Mass Spectrom. 2014, 28, 1904–1916. [Google Scholar] [CrossRef]
- Odoardi, S.; Romolo, F.S.; Strano-Rossi, S. A snapshot on NPS in Italy: Distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013–2015. Forensic Sci. Int. 2016, 265, 116–120. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA)–Europol. European Drug Report 2017: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2017. [Google Scholar]
- Fabregat-Safont, D.; Carbón, X.; Ventura, M.; Fornís, I.; Hernández, F.; Ibáñez, M. Characterization of a recently detected halogenated aminorex derivative: Para-fluoro-4-methylaminorex (4′F-4-MAR). Sci. Rep. 2019, 9, 8314. [Google Scholar] [CrossRef] [PubMed]
- Bluelight. Available online: https://bluelight.org/xf/threads/mtta-and-meop-waste-or-useful-if-combinated-with-something.777781/#post-13369987 (accessed on 18 December 2022).
- Bluelight. Available online: https://bluelight.org/xf/threads/new-and-less-new-rcs-alphabet-soup.715035/page-53#post-13296946 (accessed on 18 December 2022).
- Land der Träume. Available online: https://www.land-der-traeume.de/forum.php?t=35537 (accessed on 18 December 2022).
- Tuv, S.S.; Krabseth, H.; Karinen, R.; Olsen, K.M.; Øiestad, E.L.; Vindenes, V. Prevalence of synthetic cannabinoids in blood samples from Norwegian drivers suspected of impaired driving during a seven weeks period. Accid. Anal. Prev. 2014, 62, 26–31. [Google Scholar] [CrossRef]
- Rajotte, J.W.; Palmentier, J.F.P.; Wallage, H.R. Drug recognition evaluation and chemical confirmation of a 25C-NBOMe-impaired driver. J. Forensic Sci. 2017, 62, 1410–1413. [Google Scholar] [CrossRef]
- Busardo, F.P.; Pichini, S.; Pellegrini, M.; Montana, A.; Lo Faro, A.F.; Zaami, S.; Graziano, S. Correlation between blood and oral fluid psychoactive drug concentrations and cognitive impairment in driving under the influence of drugs. Curr. Neuropharmacol. 2017, 16, 84–96. [Google Scholar] [CrossRef]
- Ji Kwon, N.; Han, E. A review of drug abuse in recently reported cases of driving under the influence of drugs (DUID) in Asia, USA, and Europe. Forensic Sci. Int. 2019, 302, 109854. [Google Scholar] [CrossRef]
- Bilel, S.; Giorgetti, A.; Tirri, M.; Arfè, R.; Cristofori, V.; Marchetti, B.; Corli, G.; Caruso, L.; Zauli, G.; Giorgetti, R.; et al. Sensorimotor Alterations Induced by Novel Fentanyl Analogs in Mice: Possible Impact on Human Driving Performances. Curr. Neuropharmacol. 2022. Epub ahead of printing. [Google Scholar] [CrossRef]
- Tirri, M.; Bilel, S.; Arfè, R.; Corli, G.; Marchetti, B.; Bernardi, T.; Boccuto, F.; Serpelloni, G.; Botrè, F.; De-Giorgio, F.; et al. Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison With the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs. Front. Psychiatry 2022, 13, 875722. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Vigolo, A.; Ossato, A.; Trapella, C.; Vincenzi, F.; Rimondo, C.; Seri, C.; Varani, K.; Serpelloni, G.; Marti, M. Novel halogenated derivates of JWH-018: Behavioral and binding studies in mice. Neuropharmacology 2015, 95, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Ossato, A.; Vigolo, A.; Trapella, C.; Seri, C.; Rimondo, C.; Serpelloni, G.; Marti, M. JWH-018 impairs sensorimotor functions in mice. Neuroscience 2015, 300, 174–188. [Google Scholar] [CrossRef]
- Ossato, A.; Bilel, S.; Gregori, A.; Talarico, A.; Trapella, C.; Gaudio, R.M.; De Giorgio, F.; Tagliaro, F.; Neri, M.; Fattore, L.; et al. Neurological, sensorimotor and cardiorespiratory alterations induced by methoxetamine, ketamine and phencyclidine in mice. Neuropharmacology 2018, 141, 167–180. [Google Scholar] [CrossRef]
- Bilel, S.; Azevedo, N.; Arfè, R.; Tirri, M.; Gregori, A.; Serpelloni, G.; De-Giorgio, F.; Frisoni, P.; Neri, M.; Calò, G.; et al. In vitro and in vivo pharmacological characterization of the synthetic opioid MT-45. Neuropharmacology 2020, 171, 108110. [Google Scholar] [CrossRef]
- Arfè, R.; Bilel, S.; Tirri, M.; Frisoni, P.; Serpelloni, G.; Neri, M.; Boccuto, F.; Bernardi, T.; Foti, F.; De-Giorgio, F.; et al. Comparison of N-methyl-2-pyrrolidone (NMP) and the "date rape" drug GHB: Behavioral toxicology in the mouse model. Psychopharmacology 2021, 238, 2275–2295. [Google Scholar] [CrossRef]
- Bilel, S.; Azevedo Neto, J.; Arfè, R.; Tirri, M.; Gaudio, R.M.; Fantinati, A.; Bernardi, T.; Boccuto, F.; Marchetti, B.; Corli, G.; et al. In vitro and in vivo pharmaco-dynamic study of the novel fentanyl derivatives: Acrylfentanyl, Ocfentanyl and Furanylfentanyl. Neuropharmacology 2022, 209, 109020. [Google Scholar] [CrossRef]
- Corli, G.; Tirri, M.; Arfè, R.; Bilel, S.; Marchetti, B.; Gregori, A.; Di Rosa, F.; Vincenzi, F.; De-Giorgio, F.; Borea, P.A.; et al. Behavioral and binding studies on the quinolinyl ester indoles 5F-PB22 (5F-QUPIC) and BB-22 (QUCHIC) in the mouse model. Emerg. Trends Drugs Addict. Health 2022, 2, 100039. [Google Scholar] [CrossRef]
- Marti, M.; Neri, M.; Bilel, S.; Di Paolo, M.; La Russa, R.; Ossato, A.; Turillazzi, E. MDMA alone affects sensorimotor and prepulse inhibition responses in mice and rats: Tips in the debate on potential MDMA unsafety in human activity. Forensic Toxicol. 2019, 37, 132–144. [Google Scholar] [CrossRef]
- Chieffi, C.; Camuto, C.; De-Giorgio, F.; de la Torre, X.; Diamanti, F.; Mazzarino, M.; Trapella, C.; Marti, M.; Botrè, F. Metabolic profile of the synthetic drug 4,4′-dimethylaminorex in urine by LC–MS-based techniques: Selection of the most suitable markers of its intake. Forensic Toxicol. 2021, 39, 89–100. [Google Scholar] [CrossRef]
- Camuto, C.; Pellegrini, S.; De-Giorgio, F.; de la Torre, X.; Marti, M.; Mazzarino, M.; Botrè, F. Urinary excretion profile of methiopropamine in mice following intraperitoneal administration: A liquid chromatography-tandem mass spectrometry investigation. Drug Test. Anal. 2021, 13, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Mestria, S.; Odoardi, S.; Federici, S.; Bilel, S.; Tirri, M.; Marti, M.; Strano Rossi, S. Metabolism Study of N-Methyl 2-Aminoindane (NM2AI) and Determination of Metabolites in Biological Samples by LC-HRMS. J. Anal. Toxicol. 2021, 45, 475–483. [Google Scholar] [CrossRef]
- Van de Water, E.; Oosterlinck, M.; Duchateau, L.; Pille, F. Agreement of manual cell counts and automated counts of the scil Vet abc Plus(+) hematology analyzer for analysis of equine synovial fluid. Res. Vet. Sci. 2016, 106, 62–65. [Google Scholar] [CrossRef]
- Banks, M.L.; Worst, T.J.; Rusyniak, D.E.; Sprague, J.E. Synthetic cathinones ("bath salts"). J. Emerg. Med. 2014, 46, 632–642. [Google Scholar] [CrossRef]
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef]
- Baumann, M.H.; Walters, H.M.; Niello, M.; Sitte, H.H. Neuropharmacology of Synthetic Cathinones. Handb. Exp. Pharmacol. 2018, 252, 113–142. [Google Scholar] [CrossRef]
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085–1133. [Google Scholar] [CrossRef]
- Baumann, M.H.; Ayestas, M.A., Jr.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef]
- De-Giorgio, F.; Bilel, S.; Tirri, M.; Arfè, R.; Trapella, C.; Camuto, C.; Foti, F.; Frisoni, P.; Neri, M.; Botrè, F.; et al. Methiopropamine and its acute behavioral effects in mice: Is there a gray zone in new psychoactive substances users? Int. J. Leg. Med. 2020, 134, 1695–1711. [Google Scholar] [CrossRef] [PubMed]
- Tirri, M.; Frisoni, P.; Bilel, S.; Arfè, R.; Trapella, C.; Fantinati, A.; Corli, G.; Marchetti, B.; De-Giorgio, F.; Camuto, C.; et al. Worsening of the Toxic Effects of (±)Cis-4,4′-DMAR Following Its Co-Administration with (±)Trans-4,4′-DMAR: Neuro-Behavioural, Physiological, Immunohistochemical and Metabolic Studies in Mice. Int. J. Mol. Sci. 2021, 22, 8771. [Google Scholar] [CrossRef] [PubMed]
- Wakita, R.; Tanabe, S.; Tabei, K.; Funaki, A.; Inoshita, T.; Hirano, T. Differential regulations of vestibulo-ocular reflex and optokinetic response by β- and α2-adrenergic receptors in the cerebellar flocculus. Sci. Rep. 2017, 7, 3944. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.E. The vestibular system: Multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012, 35, 185–196. [Google Scholar] [CrossRef]
- Etienne, A.S.; Maurer, R.; Séguinot, V. Path integration in mammals and its interaction with visual landmarks. J. Exp. Biol. 1996, 199, 201–209. [Google Scholar] [CrossRef]
- Felix, R.A., II; Elde, C.J.; Nevue, A.A.; Portfors, C.V. Serotonin modulates response properties of neurons in the dorsal cochlear nucleus of the mouse. Hear. Res. 2017, 344, 13–23. [Google Scholar] [CrossRef]
- Winstock, A.; Mitcheson, L.; Ramsey, J.; Davies, S.; Puchnarewicz, M.; Marsden, J. Mephedrone: Use, subjective effects and health risks. Addiction 2011, 106, 1991–1996. [Google Scholar] [CrossRef]
- Blumenfeld, H.; Rivera, M.; Vasquez, J.G.; Shah, A.; Ismail, D.; Enev, M.; Zaveri, H.P. Neocortical and thalamic spread of amygdala kindled seizures. Epilepsia 2007, 48, 254–262. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Gehlbach, B.K.; Kreple, C.J.; Kawasaki, H.; Oya, H.; Buzza, C.; Granner, M.A.; Welsh, M.J.; Howard, M.A.; Wemmie, J.A.; et al. Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation. J. Neurosci. 2015, 35, 10281–10289. [Google Scholar] [CrossRef]
- Rhone, A.E.; Kovach, C.K.; Harmata, G.I.; Sullivan, A.W.; Tranel, D.; Ciliberto, M.A.; Howard, M.A.; Richerson, G.B.; Steinschneider, M.; Wemmie, J.A.; et al. A human amygdala site that inhibits respiration and elicits apnea in pediatric epilepsy. JCI Insight 2020, 5, e134852. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.L.; Mitchell, G.S.; Nattie, E.E. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci. 2003, 26, 239–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Kim, E.J.; Callaway, E.M.; Feldman, J.L. Monosynaptic Projections to Excitatory and Inhibitory preBötzinger Complex Neurons. Front. Neuroanat. 2020, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Boulant, J.A. Hypothalamic neurons. Mechanisms of sensitivity to temperature. Ann. N. Y. Acad. Sci. 1998, 856, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1207–R1228. [Google Scholar] [CrossRef]
- Mallick, B.N.; Alam, M.N. Different types of norepinephrinergic receptors are involved in preoptic area mediated independent modulation of sleep-wakefulness and body temperature. Brain Res. 1992, 591, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.; Lee, T.F. Evidence for an endogenous dopamine-mediated hypothermia in the rat. Br. J. Pharmacol. 1979, 67, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.L.; Creehan, K.M.; Angrish, D.; Barlow, D.J.; Houseknecht, K.L.; Dickerson, T.J.; Taffe, M.A. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone). Drug Alcohol Depend. 2013, 127, 248–253. [Google Scholar] [CrossRef]
- Myles, B.J.; Sabol, K.E. The effects of methamphetamine on core body temperature in the rat--part 2: An escalating regimen. Psychopharmacology 2008, 198, 313–322. [Google Scholar] [CrossRef]
- Aarde, S.M.; Creehan, K.M.; Vandewater, S.A.; Dickerson, T.J.; Taffe, M.A. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: Self-administration and locomotor stimulation in male rats. Psychopharmacology 2015, 232, 3045–3055. [Google Scholar] [CrossRef]
- López-Arnau, R.; Martínez-Clemente, J.; Pubill, D.; Escubedo, E.; Camarasa, J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: Butylone, mephedrone and methylone. Br. J. Pharmacol. 2012, 167, 407–420. [Google Scholar] [CrossRef]
- Oh, J.H.; Hwang, J.Y.; Hong, S.I.; Ma, S.X.; Seo, J.Y.; Lee, S.Y.; Kim, H.C.; Jang, C.G. The new designer drug buphedrone produces rewarding properties via dopamine D1 receptor activation. Addict. Biol. 2018, 23, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, J.; Andrzejczak, D.; Wojtas, A.; Gołembiowska, K.; Zawilska, J.B. Methcathinone and 3-Fluoromethcathinone Stimulate Spontaneous Horizontal Locomotor Activity in Mice and Elevate Extracellular Dopamine and Serotonin Levels in the Mouse Striatum. Neurotox. Res. 2019, 35, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, J.; Kuczyńska, K.; Zawilska, J.B. Four Synthetic Cathinones: 3-Chloromethcathinone, 4-Chloromethcathinone, 4-Fluoro-α-Pyrrolidinopentiophenone, and 4-Methoxy-α-Pyrrolidinopentiophenone Produce Changes in the Spontaneous Locomotor Activity and Motor Performance in Mice with Varied Profiles. Neurotox. Res. 2020, 38, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.M.; Andén, N.E.; Dahlström, A. A functional effect of dopamine in the nucleus accumbens and in some other dopamine-rich parts of the rat brain. Psychopharmacologia 1975, 45, 139–149. [Google Scholar] [CrossRef]
- Ljungberg, T.; Ungerstedt, U. A rapid and simple behavioural screening method for simultaneous assessment of limbic and striatal blocking effects of neuroleptic drugs. Pharmacol. Biochem. Behav. 1985, 23, 479–485. [Google Scholar] [CrossRef]
- Zolkowska, D.; Jain, R.; Rothman, R.B.; Partilla, J.S.; Roth, B.L.; Setola, V.; Prisinzano, T.E.; Baumann, M.H. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J. Pharmacol. Exp. Ther. 2009, 329, 738–746. [Google Scholar] [CrossRef]
- Baumann, M.H.; Clark, R.D.; Woolverton, W.L.; Wee, S.; Blough, B.E.; Rothman, R.B. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J. Pharmacol. Exp. Ther. 2011, 337, 218–225. [Google Scholar] [CrossRef]
- Luethi, D.; Kolaczynska, K.E.; Docci, L.; Krähenbühl, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology 2018, 134, 4–12. [Google Scholar] [CrossRef]
- Aurbach, K.; Spindler, M.; Haining, E.J.; Bender, M.; Pleines, I. Blood collection, platelet isolation and measurement of platelet count and size in mice-a practical guide. Platelets 2019, 30, 698–707. [Google Scholar] [CrossRef]
- Grindem, C.B. Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Teng, W.F.; Sun, W.M.; Shi, L.F.; Hou, D.D.; Liu, H. Effects of restraint stress on iron, zinc, calcium, and magnesium whole blood levels in mice. Biol. Trace Elem. Res. 2008, 121, 243–248. [Google Scholar] [CrossRef]
- Yip, P.M.; Chan, M.K.; Zielinski, N.; Adeli, K. Heparin interference in whole blood sodium measurements in a pediatric setting. Clin. Biochem. 2006, 39, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Liashenko, V.P.; Lukashov, S.M. Effect of stress on calcium homeostasis in muscular tissues. Fiziolohichnyi Zhurnal 2003, 49, 76–81. [Google Scholar] [PubMed]
- Joëls, M.; Velzing, E.; Nair, S.; Verkuyl, J.M.; Karst, H. Acute stress increases calcium current amplitude in rat hippocampus: Temporal changes in physiology and gene expression. Eur. J. Neurosci. 2003, 18, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Takase, B.; Akima, T.; Uehata, A.; Ohsuzu, F.; Kurita, A. Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin. Cardiol. 2004, 27, 223–227. [Google Scholar] [CrossRef]
- Yuan, G.; Dai, S.; Yin, Z.; Lu, H.; Jia, R.; Xu, J.; Song, X.; Li, L.; Shu, Y.; Zhao, X. Toxicological assessment of combined lead and cadmium: Acute and sub-chronic toxicity study in rats. Food Chem. Toxicol. 2014, 65, 260–268. [Google Scholar] [CrossRef]
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Shemesh, E.; Zafrir, B. Hypertriglyceridemia-Related Pancreatitis in Patients With Type 2 Diabetes: Links And Risks. Diabetes Metab. Syndr. Obes. 2019, 12, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Raimi, T.H.; Dele-Ojo, B.F.; Dada, S.A.; Fadare, J.O.; Ajayi, D.D.; Ajayi, E.A.; Ajayi, O.A. Triglyceride-Glucose Index and Related Parameters Predicted Metabolic Syndrome in Nigerians. Metab. Syndr. Relat. Disord. 2021, 19, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Nemoto, T.; Muneyuki, T.; Kakei, M.; Fuchigami, H.; Munakata, H. Low serum amylase in association with metabolic syndrome and diabetes: A community-based study. Cardiovasc. Diabetol. 2011, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Ban, S. Urinalysis. In Contemporary Practice in Clinical Chemistry, 4th ed.; Clarke, W., Mark, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Ngashangva, L.; Bachu, V.; Goswami, P. Development of new methods for determination of bilirubin. J. Pharm. Biomed. Anal. 2019, 162, 272–285. [Google Scholar] [CrossRef]
- D’Amico, G.; Bazzi, C. Pathophysiology of proteinuria. Kidney Int. 2003, 63, 809–825. [Google Scholar] [CrossRef]
- Marsden, J.; Pickering, D. Urine testing for diabetic analysis. Community Eye Health 2015, 28, 77. [Google Scholar] [PubMed]
- Greaves, P. Hemopoietic and Lymphatic Systems. In Histopathology of Preclinical Toxicity Studies, 4th ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Baumann, M.H.; Bukhari, M.O.; Lehner, K.R.; Anizan, S.; Rice, K.C.; Concheiro, M.; Huestis, M.A. Neuropharmacology of 3,4-Methylenedioxypyrovalerone (MDPV), Its Metabolites, and Related Analogs. Curr. Top. Behav. Neurosci. 2017, 32, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, A.J.; Nagarajan, S.; Wolfrum, K.M.; Reed, J.F.; Swanson, T.L.; Nilsen, A.; Janowsky, A. Structure-activity relationships of bath salt components: Substituted cathinones and benzofurans at biogenic amine transporters. Psychopharmacology 2019, 236, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Bluelight. Available online: https://bluelight.org/xf/threads/mephtetramine-methoxypiperamide.668456/ (accessed on 18 December 2022).
- Land der Träume. Available online: https://www.land-der-traeume.de/forum.php?t=35538 (accessed on 18 December 2022).

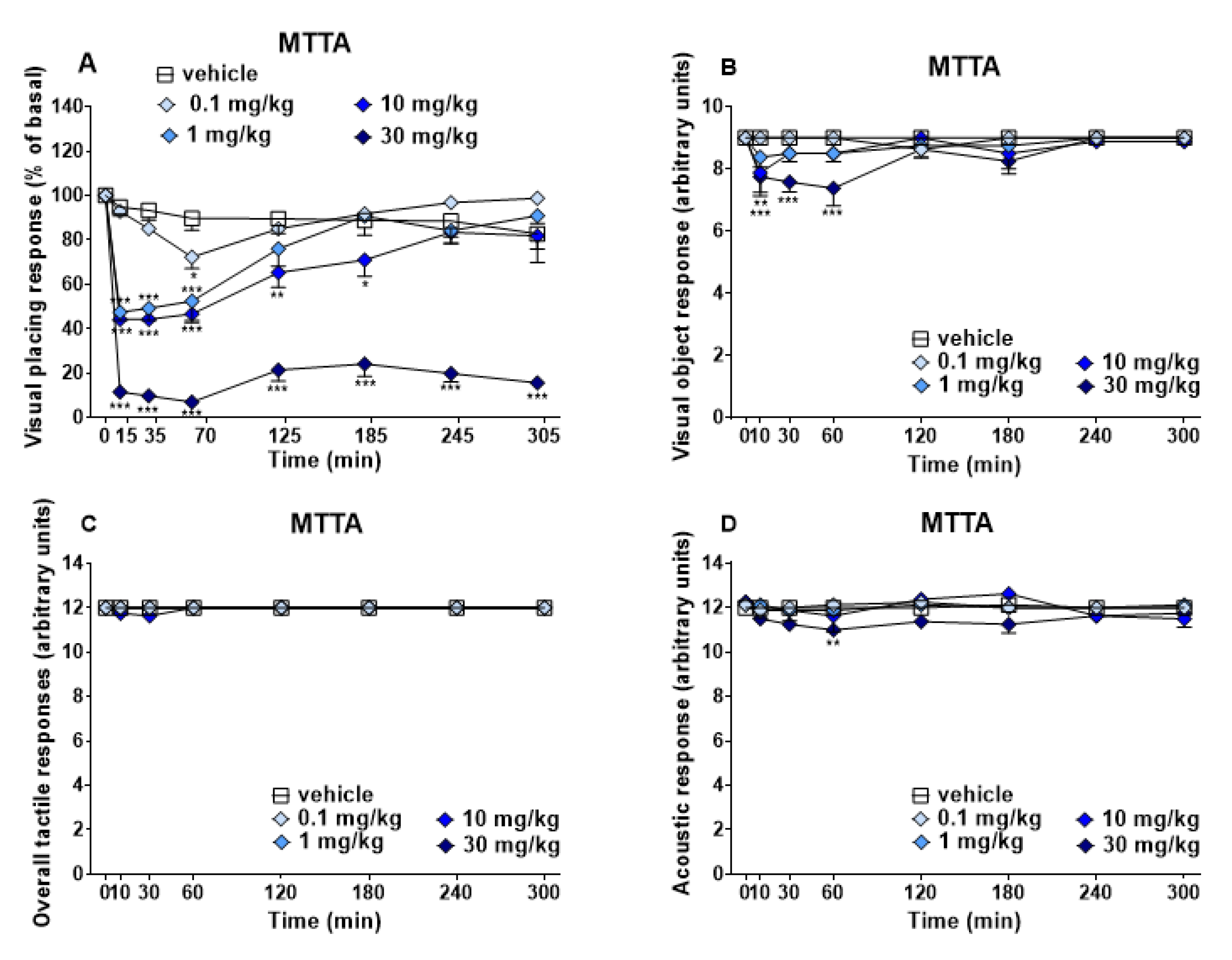
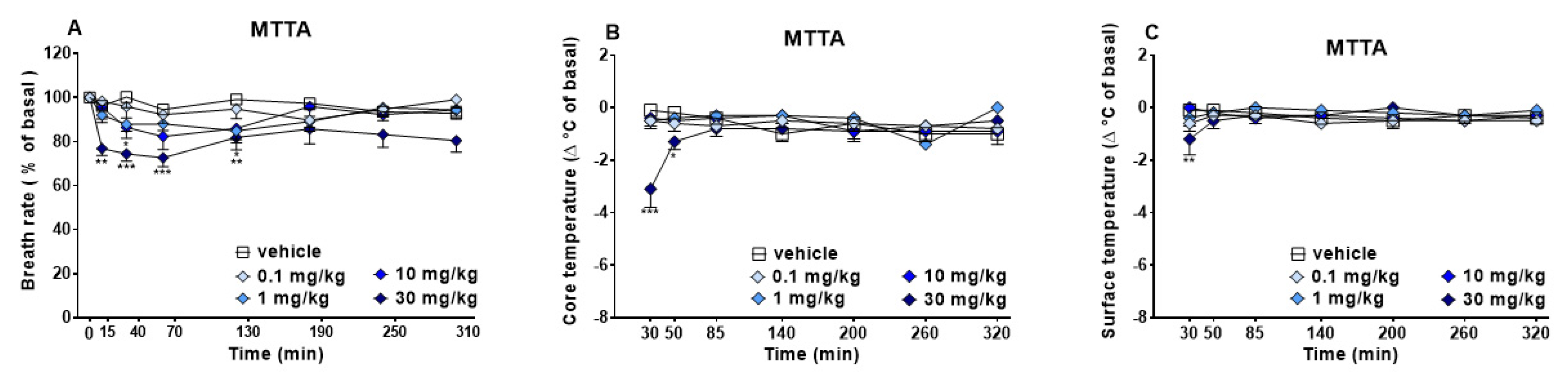


| Blood Count Parameters | Vehicle | MTTA | Variations |
|---|---|---|---|
| WBC (103/mm3) | 5.02 ± 0.234 | 3.30 ± 0.16 *** | ⇓⇓ |
| LYM# (103/mm3) | 2.68 ± 0.279 | 1.63 ± 0.13 *** | ⇓⇓ |
| MON# (103/mm3) | 0.26 ± 0.055 | 0.29 ± 0.02 | - |
| GRA# (103/mm3) | 1.38 ± 0.259 | 1.39 ± 0.11 | - |
| EOS# (103/mm3) | 0.36 ± 0.10 | 0.31 ± 0.10 | - |
| LYM% (%) | 58.12 ± 2.52 | 38.55 ± 2.13 *** | ⇓⇓ |
| MON% (%) | 6.12 ± 0.34 | 6.75 ± 0.75 * | ⇑ |
| GRA% (%) | 24.64 ± 2.31 | 29.52 ± 2.01 ** | ⇑ |
| EOS% (%) | 6.51 ± 1.35 | 5.54 ± 1.69 | - |
| RBC (106/mm3) | 8.03 ± 0.17 | 6.25 ± 0.28 *** | ⇓ |
| HGB (g/dL) | 15.92 ± 0.27 | 10.75 ± 0.50 *** | ⇓⇓ |
| HCT (%) | 45.40 ± 1.23 | 34.19 ± 1.57 *** | ⇓ |
| MCV (μm3) | 50.11 ± 0.38 | 42.90 ± 0.10 *** | ⇓ |
| MCH (pg) | 17.69 ± 0.29 | 13.62 ± 0.09 *** | ⇓ |
| MCHC (g/dL) | 31.50 ± 0.68 | 24.87 ± 0.18 *** | ⇓ |
| RDW (%) | 12.64 ± 0.23 | 11.34 ± 0.27 *** | ⇓ |
| PLT (103/mm3) | 583.78 ± 27.38 | 605.69 ± 84.97 | - |
| MPV(μm3) | 6.09 ± 0.08 | 4.88 ± 0.13 *** | ⇓ |
| Electrolyte Parameters | Vehicle | MTTA | Variations |
|---|---|---|---|
| tCO2 (mmol/L) | 12.2 ± 0.27 | 16.8 ± 0.53 *** | ⇑⇑ |
| Ca (mg/dL) | 8.9 ± 0.10 | 8.24 ± 0.05 *** | ⇓ |
| PHOS (mg/dL) | 12.8 ± 0.89 | 8.30 ± 0.07 *** | ⇓⇓ |
| Mg (mg/dL) | 2.1 ± 0.10 | 1.62 ± 0.12 *** | ⇓ |
| K+ (mmol/L) | 6.6 ± 0.21 | 6.7 ± 0.20 | - |
| Na+ (mmol/L) | 120.0 ± 1.28 | 118.7 ± 0.92 * | ⇓ |
| Cl- (mmol/L) | 101.0 ± 0.86 | 103.6 ± 4.35 | - |
| Comprehensive Parameters | Vehicle | MTTA | Variations |
|---|---|---|---|
| ALB (g/dL) | 3.29 ± 0.12 | 3.13 ± 0.05 ** | ⇓ |
| TP (g/dL) | 5.22 ± 0.19 | 4.67 ± 0.08 *** | ⇓ |
| GLOB (g/dL) | 1.58 ± 0.33 | 1.61 ± 0.37 | - |
| A/G | 1.19 ± 0.13 | 1.04 ± 0.08 * | ⇓ |
| TB (mg/L) | 0.06 ± 0.00 | 0.14 ± 0.00 *** | ⇑⇑⇑ |
| GGT (U/L) | 1.78 ± 0.00 | 2.11 ± 0.26 ** | ⇑ |
| ALT (U/L) | 109.44 ± 18.87 | 49.66 ± 9.11 *** | ⇓⇓ |
| ALP (U/L) | 101.00 ± 3.62 | 46.33 ± 10.52 *** | ⇓⇓ |
| AMY (U/L) | 3175 ± 18.30 | 2510 ± 521.07 *** | ⇓ |
| Crea (mg/L) | 0.11 ± 0.02 | 0.66 ± 0.48 ** | ⇑⇑⇑ |
| TC (mg/L) | 0.00 ± 0.00 | 102.11 ± 19.95 *** | ⇑⇑⇑ |
| GLU (mg/L) | 100.25 ± 10.67 | 203.92 ± 22.50 *** | ⇑⇑⇑ |
| Ca(mg/L) | 8.37 ± 0.27 | 6.24 ± 1.15 ** | ⇓ |
| PHOS (mg/L) | 6.49 ± 0.53 | 5.74 ± 1.22 | - |
| BUN/CREA | 0.00 ± 0.00 | 0.00 ± 0.00 | - |
| BUN (mg/L) | 20.67 ± 0.56 | 21.03 ± 0.65 | - |
| Urine Parameters | Vehicle | MTTA | Variations |
|---|---|---|---|
| GLU (mg/dL) | 0.00 ± 0.00 | 0.00 ± 0.00 | - |
| PRO (mg/dL) | 73.8 ± 12.81 | 141.25 ± 35.68 ** | ⇑⇑ |
| BIL (mg/dL) | 0.00 ± 0.00 | 0.00 ± 0.00 | - |
| URO (mg/dL) | 0.60 ± 0.21 | 5.75 ± 0.88 *** | ⇑⇑⇑ |
| pH | 6.4 ± 0.16 | 6.6 ± 0.15 * | ⇑ |
| S.G. | 1.0 ± 0.00 | 1.0 ± 0.00 | - |
| BLD (mg/dL) | 0.0 ± 0.01 | 0.1 ± 0.03 *** | ⇑ |
| KET (mg/dL) | 5.6 ± 2.20 | 24.37 ± 8.84 *** | ⇑⇑⇑ |
| NIT | 0.00 ± 0.00 | 0.00 ± 0.00 | - |
| LEU(Leu/uL) | 9.4 ± 4.57 | 134.37 ± 34.38 *** | ⇑⇑⇑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corli, G.; Tirri, M.; Arfè, R.; Marchetti, B.; Bernardi, T.; Borsari, M.; Odoardi, S.; Mestria, S.; Strano-Rossi, S.; Neri, M.; et al. Pharmaco-Toxicological Effects of Atypical Synthetic Cathinone Mephtetramine (MTTA) in Mice: Possible Reasons for Its Brief Appearance over NPSs Scene. Brain Sci. 2023, 13, 161. https://doi.org/10.3390/brainsci13020161
Corli G, Tirri M, Arfè R, Marchetti B, Bernardi T, Borsari M, Odoardi S, Mestria S, Strano-Rossi S, Neri M, et al. Pharmaco-Toxicological Effects of Atypical Synthetic Cathinone Mephtetramine (MTTA) in Mice: Possible Reasons for Its Brief Appearance over NPSs Scene. Brain Sciences. 2023; 13(2):161. https://doi.org/10.3390/brainsci13020161
Chicago/Turabian StyleCorli, Giorgia, Micaela Tirri, Raffaella Arfè, Beatrice Marchetti, Tatiana Bernardi, Martina Borsari, Sara Odoardi, Serena Mestria, Sabina Strano-Rossi, Margherita Neri, and et al. 2023. "Pharmaco-Toxicological Effects of Atypical Synthetic Cathinone Mephtetramine (MTTA) in Mice: Possible Reasons for Its Brief Appearance over NPSs Scene" Brain Sciences 13, no. 2: 161. https://doi.org/10.3390/brainsci13020161
APA StyleCorli, G., Tirri, M., Arfè, R., Marchetti, B., Bernardi, T., Borsari, M., Odoardi, S., Mestria, S., Strano-Rossi, S., Neri, M., Gaudio, R. M., Bilel, S., & Marti, M. (2023). Pharmaco-Toxicological Effects of Atypical Synthetic Cathinone Mephtetramine (MTTA) in Mice: Possible Reasons for Its Brief Appearance over NPSs Scene. Brain Sciences, 13(2), 161. https://doi.org/10.3390/brainsci13020161









