Reelin Signaling and Synaptic Plasticity in Schizophrenia
Abstract
1. Introduction
2. Physiological Significance of Reelin in CNS
3. Reelin Signaling Pathway
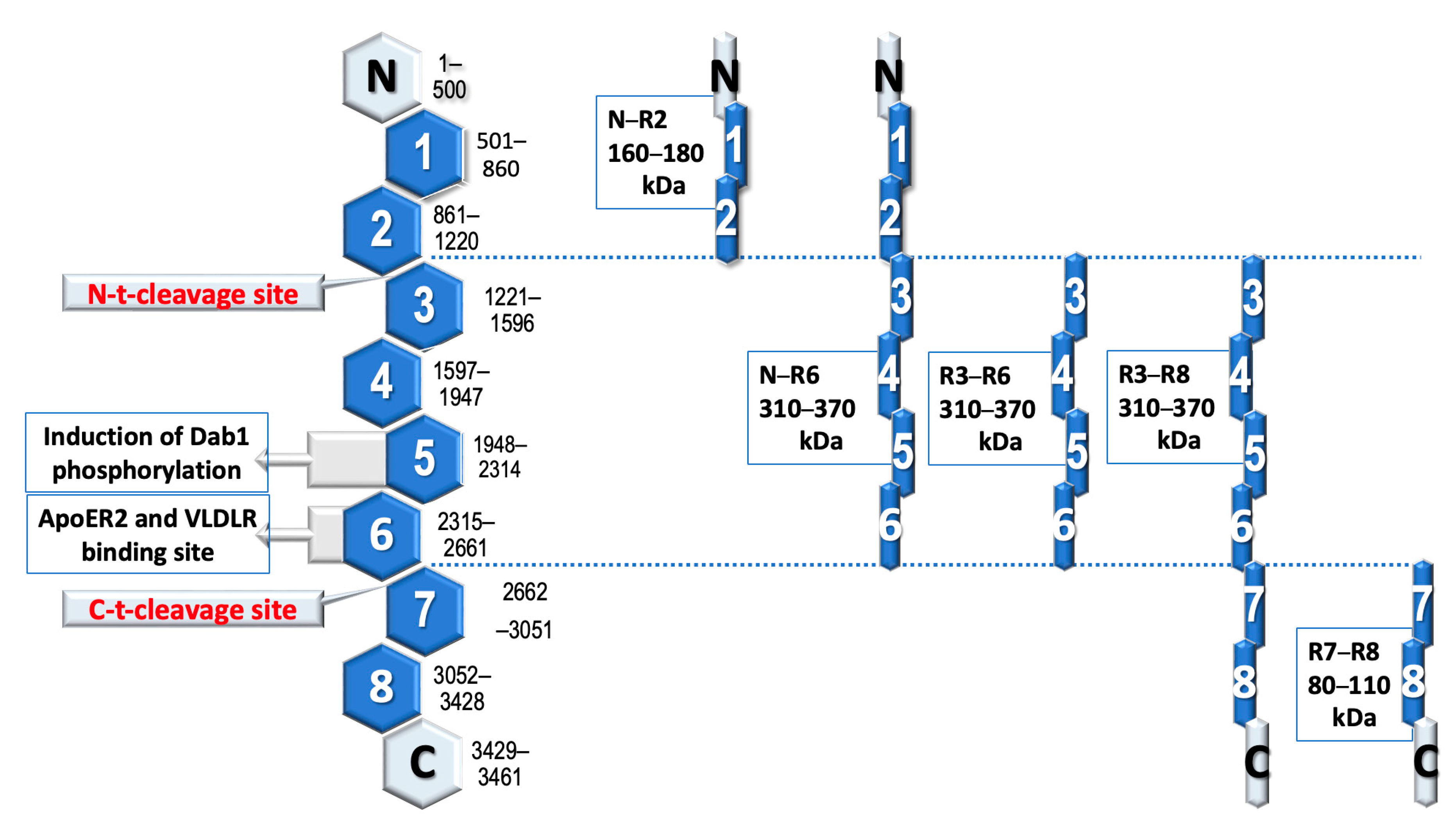
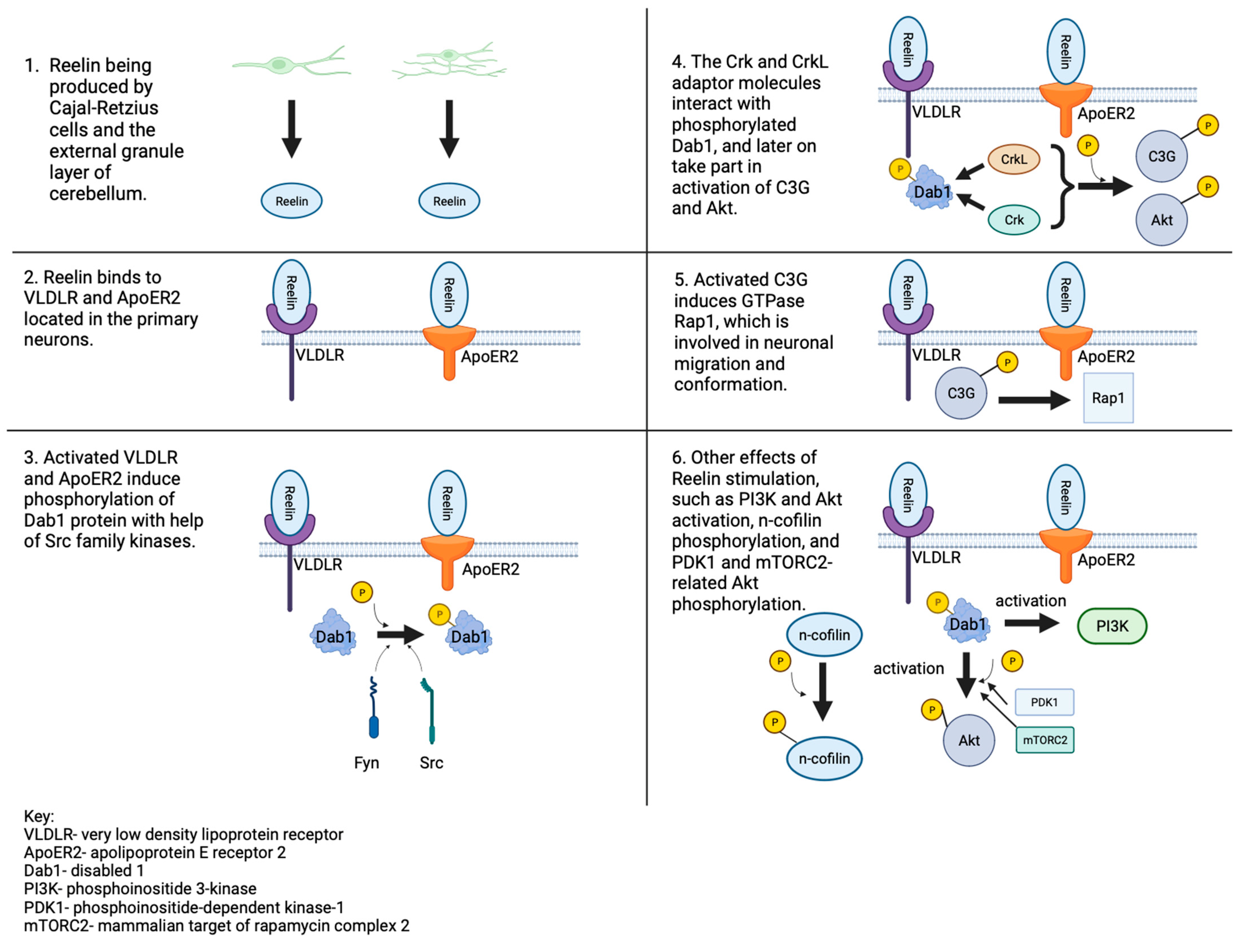
3.1. The Role of Reelin Protein
3.2. Receptor Binding
3.3. Navigating the Second Path
3.4. Abnormalities in the Signaling Pathway
3.5. Possible Research Regarding the Reelin Signaling Pathway
4. Reelin Functions in Neuronal Migration
5. Reelin Expression and Schizophrenia
5.1. Neurodevelopmental Hypothesis
5.2. Reelin Expression
5.3. Epigenetic Hypermethylation
5.4. Reelin and Its Circulating Isoforms
5.5. Reelin: Whole-Body Balance
5.6. Unlocking the Antipsychotic Potential of Reelin
5.7. Exploring Alternative Splicing
5.8. Unraveling the Genetic Connection: Reelin and Predisposition to Schizophrenia
6. Conclusions and Future Directions
- An increased level of methylation of the RELN gene in patients diagnosed with schizophrenia causes a multiple decrease in reelin expression [43,89,90]. Monitoring the level of methylation can be used as a marker in assessing the severity of symptoms occurring in schizophrenia [43,62,89,90,91,92,93].
- To our knowledge, no systematic studies have been conducted so far linking the clinical effects of treatment with changes in the reelin signaling system as the primary target of antipsychotic therapy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hirota, Y.; Nakajima, K. Control of neuronal migration and aggregation by Reelin signaling in the developing cerebral cortex. Front. Cell Dev. Biol. 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Vílchez-Acosta, A.; Manso, Y.; Cárdenas, A.; Elias-Tersa, A.; Martínez-Losa, M.; Pascual, M.; Álvarez-Dolado, M.; Nairn, A.C.; Borrell, V.; Soriano, E. Specific contribution of Reelin expressed by cajal-retzius cells or GABAergic interneurons to cortical lamination. Proc. Natl. Acad. Sci. USA 2022, 119, e2120079119. [Google Scholar] [CrossRef] [PubMed]
- Pujadas, L.; Gruart, A.; Bosch, C.; Delgado, L.; Teixeira, C.M.; Rossi, D.; de Lecea, L.; Martínez, A.; Delgado-García, J.M.; Soriano, E. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J. Neurosci. 2010, 30, 4636–4649. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.M.; Kron, M.M.; Masachs, N.; Zhang, H.; Lagace, D.C.; Martinez, A.; Reillo, I.; Duan, X.; Bosch, C.; Pujadas, L.; et al. Cell-autonomous inactivation of the Reelin pathway impairs adult neurogenesis in the hippocampus. J. Neurosci. 2012, 32, 12051–12065. [Google Scholar] [CrossRef] [PubMed]
- Bosch, C.; Masachs, N.; Exposito-Alonso, D.; Martínez, A.; Teixeira, C.M.; Fernaud, I.; Pujadas, L.; Ulloa, F.; Comella, J.X.; DeFelipe, J.; et al. Reelin regulates the maturation of dendritic spines, synaptogenesis and glial ensheathment of newborn granule cells. Cereb. Cortex 2016, 26, 4282–4298. [Google Scholar] [CrossRef]
- González-Billault, C.; Del Río, J.A.; Ureña, J.M.; Jiménez-Mateos, E.M.; Barallobre, M.J.; Pascual, M.; Pujadas, L.; Simó, S.; Torre, A.L.; Gavin, R.; et al. A role of MAP1B in Reelin-dependent neuronal migration. Cereb. Cortex 2005, 15, 1134–1145. [Google Scholar] [CrossRef]
- Simó, S.; Pujadas, L.; Segura, M.F.; La Torre, A.; Del Río, J.A.; Ureña, J.M.; Comella, J.X.; Soriano, E. Reelin induces the detachment of postnatal subventricular zone cells and the expression of the Egr-1 through Erk1/2 activation. Cereb. Cortex 2007, 17, 294–303. [Google Scholar] [CrossRef][Green Version]
- Simó, S.; Jossin, Y.; Cooper, J.A. Cullin 5 regulates cortical layering by modulating the speed and duration of Dab1-dependent neuronal migration. J. Neurosci. 2010, 30, 5668–5676. [Google Scholar] [CrossRef]
- Yasui, N.; Nogi, T.; Takagi, J. Structural basis for specific recognition of Reelin by its receptors. Structure 2010, 18, 320–331. [Google Scholar] [CrossRef]
- Molnár, Z.; Clowry, G.J.; Šestan, N.; Alzu’bi, A.; Bakken, T.; Hevner, R.F.; Hüppi, P.S.; Kostović, I.; Rakic, P.; Anton, E.S.; et al. New insights into the development of the human cerebral cortex. J. Anat. 2019, 235, 432–451. [Google Scholar] [CrossRef]
- Quattrocchi, C.C.; Wannenes, F.; Persico, A.M.; Ciafré, S.A.; D’Arcangelo, G.; Farace, M.G.; Keller, F. Reelin is a serine protease of the extracellular matrix. J. Biol. Chem. 2002, 277, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Hattori, M. Re-evaluation of protease activity of reelin. Biol. Pharm. Bull. 2010, 33, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, G.; Shifman, S. The genetic variation of RELN expression in schizophrenia and bipolar disorder. PLoS ONE 2011, 6, e19955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hong, Y.; Zou, L.; Zhong, R.; Zhu, B.; Shen, N.; Chen, W.; Lou, J.; Ke, J.; Zhang, T.; et al. Reelin gene variants and risk of autism spectrum disorders: An integrated meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014, 165, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.T.; Copeland, B.; Yun, E.-J.; Kwon, S.K.; Guemez-Gamboa, A.; Schaffer, A.E.; Kim, S.; Kang, H.C.; Song, S.; Mathern, G.W.; et al. An AKT3-FOXG1-Reelin network underlies defective migration in human focal malformations of cortical development. Nat. Med. 2015, 21, 1445–1454. [Google Scholar] [CrossRef]
- Lammert, D.B.; Howell, B.W. RELN mutations in autism spectrum disorder. Front. Cell. Neurosci. 2016, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Marrone, M.C.; Marinelli, S.; Biamonte, F.; Keller, F.; Sgobio, C.A.; Ammassari-Teule, M.; Bernardi, G.; Mercuri, N.B. Altered cortico-striatal synaptic plasticity and related behavioral impairments in reeler mice. Eur. J. Neurosci. 2006, 24, 2061–2070. [Google Scholar] [CrossRef]
- Ammassari-Teule, M.; Sgobio, C.; Biamonte, F.; Marrone, C.; Mercuri, N.B.; Keller, F. Reelin haploinsufficiency reduces the density of PV+ neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology 2009, 204, 511–521. [Google Scholar] [CrossRef]
- Folsom, T.; Fatemi, S. The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology 2013, 68, 122–135. [Google Scholar] [CrossRef]
- Zhao, S.; Chai, X.; Frotscher, M. Balance between neurogenesis and gliogenesis in the adult hippocampus: Role of reelin. Dev. Neurosci. 2006, 29, 84–90. [Google Scholar] [CrossRef]
- Niu, S.; Yabut, O.; D’Arcangelo, G. The reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 2008, 28, 10339–10348. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends Neurosci. 2008, 31, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bolam, J.P.; Hanley, J.J.; Booth, P.A.; Bevan, M.D. Synaptic organization of the basal ganglia. J. Anat. 2000, 196, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, J.R.; Graybiel, A.M. Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front. Neuroanat. 2011, 5, 59. [Google Scholar] [CrossRef]
- de Guglielmo, G.; Iemolo, A.; Nur, A.; Turner, A.; Montilla-Perez, P.; Martinez, A.; Crook, C.; Roberts, A.; Telese, F. Reelin deficiency exacerbates cocaine-induced hyperlocomotion by enhancing neuronal activity in the dorsomedial striatum. Genes Brain Behav. 2022, 21, e12828. [Google Scholar] [CrossRef] [PubMed]
- Impagnatiello, F.; Guidotti, A.R.; Pesold, C.; Dwivedi, Y.; Caruncho, H.; Pisu, M.G.; Uzunov, D.P.; Smalheiser, N.R.; Davis, J.M.; Pandey, G.N.; et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc. Natl. Acad. Sci. USA 1998, 95, 15718–15723. [Google Scholar] [CrossRef]
- Matigian, N.; Abrahamsen, G.; Sutharsan, R.; Cook, A.L.; Vitale, A.M.; Nouwens, A.; Bellette, B.; An, J.; Anderson, M.; Beckhouse, A.G.; et al. Disease-specific, neurosphere-derived cells as models for brain disorders. Dis. Model. Mech. 2010, 3, 785–798. [Google Scholar] [CrossRef]
- Tee, J.Y.; Sutharsan, R.; Fan, Y.; Mackay-Sim, A. Schizophrenia patient-derived olfactory neurosphere-derived cells do not respond to extracellular reelin. NPJ Schizophr. 2016, 2, 16027. [Google Scholar] [CrossRef]
- Sturm, L.; van Elst, L.T.; Fiebich, B.; Wolkewitz, M.; Hornig, T. Intra-day variations of blood reelin levels in healthy individuals. Arch. Med. Sci. 2019, 16, 118–123. [Google Scholar] [CrossRef]
- Lidón, L.; Urrea, L.; Llorens, F.; Gil, V.; Alvarez, I.; Diez-Fairen, M.; Aguilar, M.; Pastor, P.; Zerr, I.; Alcolea, D.; et al. Disease-specific changes in reelin protein and mRNA in neurodegenerative diseases. Cells 2020, 9, 1252. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakamura, K.; Iwata, Y.; Sekine, Y.; Kawai, M.; Sugihara, G.; Tsuchiya, K.J.; Suda, S.; Matsuzaki, H.; Takei, N.; et al. Decreased expression of reelin receptor VLDLR in peripheral lymphocytes of drug-naive schizophrenic patients. Schizophr. Res. 2008, 98, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lu, Y.; Yu, S.; Dai, Z.; Zhang, F.; Yuan, J. Exploring the mRNA expression level of RELN in peripheral blood of schizophrenia patients before and after antipsychotic treatment. Hereditas 2020, 157, 43. [Google Scholar] [CrossRef]
- Lee, G.H.; D’Arcangelo, G. New sights into reelin-mediated signaling pathways. Front. Cell. Neurosci. 2016, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Kohno, T.; Nakano, Y.; Suzuki, K.; Ishii, M.; Tagami, H.; Baba, A.; Hattori, M. Mechanism, and significance of specific proteolytic cleavage of reelin. Biochem. Biophys. Res. Commun. 2009, 380, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.M.; Qiu, S.; Weeber, E.J. The role of reelin in adult synaptic function and the genetic and epigenetic regulation of the reelin gene. Biochim. Et Biophys. Acta (BBA) Gene Regul. Mech. 2008, 1779, 422–431. [Google Scholar] [CrossRef]
- Soda, T.; Nakashima, R.; Watanabe, D.; Nakajima, K.; Pastan, I.; Nakanishi, S. Segregation and coactivation of developing neocortical layer 1 neurons. J. Neurosci. 2004, 23, 6272–6279. [Google Scholar] [CrossRef]
- Ko, J.; Humbert, S.; Bronson, R.T.; Takahashi, S.; Kulkarni, A.B.; Li, E.; Tsai, L.H. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J. Neurosci. 2001, 21, 6758–6771. [Google Scholar] [CrossRef]
- Amstrong, N.C.; Anderson, R.C.; McDermott, K.W. Reelin: Diverse roles in central nervous system development, health, and disease. Int. J. Biochem. Cell Biol. 2019, 112, 72–75. [Google Scholar] [CrossRef]
- Scala, M.; Grasso, E.A.; Di Cara, G.; Riva, A.; Striano, P.; Verrotti, A. The pathophysiological link between reelin and autism: Overview and new sights. Front. Genet. 2022, 12, 869002. [Google Scholar] [CrossRef]
- Jossin, Y. Reelin functions, mechanisms of action and signaling pathways during brain development and maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef]
- Khialeeva, E.; Carpenter, E.M. Nonneuronal roles for the reelin signaling pathway. Dev. Dyn. 2017, 246, 217–226. [Google Scholar] [CrossRef]
- Stein, T.; Cosimo, E.; Yu, X.; Smith, P.R.; Simon, R.; Cottrell, L.; Pringle, M.A.; Bell, A.K.; Lattanzio, L.; Sauter, G.; et al. Loss of reelin expression in breast cancer is epigenetically controlled and associated with poor prognosis. Am. J. Pathol. 2010, 177, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Fikri, R.M.N.; Norlelawati, A.T.; El-Huda, A.R.N.; Hanisah, M.N.; Kartini, A.; Noesidah, K.; Zamizla, A.N. Reelin (RELN) DNA methylation in the peripheral blood of schizophrenia. J. Psychiatr. Res. 2017, 88, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.I.K.; Jbara, A.; Rabaya, O.; Petrova, P.; Daoud, S.; Melliti, N.; Meseke, M.; Lutz, D.; Petrasch-Parwez, E.; Schwitalla, J.C.; et al. Reelin signaling modulates GABAB receptor function in the neocortex. J. Neurochem. 2021, 156, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, A.; Auta, J.; Davis, J.M.; Gerevini, V.D.; Dwivedi, Y.; Grayson, D.R.; Impagnatiello, F.; Pandey, G.; Pesold, C.; Sharma, R.; et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: A postmortem brain study. Arch. Gen. Psychiatry 2000, 57, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Anstotz, M.; Quattrocolo, G.; Maccaferri, G. Cajal-Retzius cells and GABAergic interneurons of the developing hippocampus: Close electrophysiological encounters of the third kind. Brain Res. 2018, 1679, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Herz, J.; Calvier, L. Reelin through the years: From brain development to inflammation. Cell Rep. 2023, 42, 1–20. [Google Scholar] [CrossRef]
- Dlugosz, P.; Nimpf, J. The reelin receptors apolipoprotein E receptor 2 (ApoER2) and VLDL receptor. Int. J. Mol. Sci. 2018, 19, 3090. [Google Scholar] [CrossRef]
- Lane-Donovan, C.; Herz, J. The ApoE receptors Vldlr and Apoer2 in central nervous system function and disease. J. Lipid Res. 2017, 58, 1036–1043. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Zamora, D.; Horowitz, M.S.; Jahanipour, J.; Keyes, G.S.; Li, X.; Murray, H.C.; Curtis, M.A.; Faull, R.M.; Sedlock, A.; et al. ApoER2-Dab1 disruption as the origin of pTau-related neurodegeneration in sporadic Alzheimer’s disease. medRxiv, 2023; preprint. [Google Scholar] [CrossRef]
- Di Donato, N.; Guerrini, R.; Billington, C.J.; Barkovich, A.J.; Dinkel, P.; Freri, E.; Heide, M.; Gershon, E.S.; Gertler, T.S.; Hopkin, R.J.; et al. Monoallelic and biallelic mutations in RELN underlie a graded series of neurodevelopmental disorders. Brain 2022, 145, 3274–3287. [Google Scholar] [CrossRef] [PubMed]
- Tsuneura, Y.; Nakai, T.; Mizoguchi, H.; Yamada, K. New strategies for the treatment of neuropsychiatric disorders based on reelin dysfunction. Int. J. Mol. Sci. 2022, 23, 1829. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hidalgo, A.C.; Martín-Cuevas, C.; Crespo-Facorro, B.; Garrido-Torres, N. Reelin alterations, behavioral phenotypes, and brain anomalies in schizophrenia: A systematic review of insights from rodent models. Front. Neuroanat. 2022, 16, 844737. [Google Scholar] [CrossRef]
- Imai, H.; Shoji, H.; Ogata, M.; Kagawa, Y.; Owada, Y.; Miyakawa, T.; Sakimura, K.; Terashima, T.; Katsuyama, Y. Dorsal forebrain-specific deficiency of reelin-Dab1 signal causes behavioral abnormalities related to psychiatric disorders. Cereb. Cortex 2017, 27, 3485–3501. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Kubo, K.I.; Nakajima, K. Reelin and neuropsychiatric disorders. Front. Cell Neurosci. 2016, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-de-Oliveira, J.P.; Hallak, J.; Howland, J.G. An overview of animal models related to schizophrenia. Can. J. Psychiatry 2019, 64, 5–17. [Google Scholar] [CrossRef]
- Ang, M.J.; Lee, S.; Kim, J.C.; Kim, S.H.; Moon, C. Behavioral tasks evaluating schizophrenia-like symptoms in animal models: A recent update. Curr. Neuropharmacol. 2021, 19, 641–664. [Google Scholar] [CrossRef]
- Pardo, M.; Gregorio, S.; Montalban, E.; Pujadas, L.; Elias-Tersa, A.; Masachs, N.; Vílchez-Acosta, A.; Parent, A.; Auladell, C.; Girault, J.A.; et al. Adult-specific Reelin expression alters striatal neuronal organization: Implications for neuropsychiatric disorders. Front. Cell. Neurosci. 2023, 17, 1143319. [Google Scholar] [CrossRef]
- Sawahata, M.; Asano, H.; Nagai, T.; Ito, N.; Kohno, T.; Nabeshima, T.; Hattori, M.; Yamada, K. Microinjection of Reelin into the mPFC prevents MK-801-induced recognition memory impairment in mice. Pharmacol. Res. 2021, 173, 105832. [Google Scholar] [CrossRef]
- Nie, F.; Zhang, Q.; Ma, J.; Wang, P.; Gu, R.; Han, J.; Zhang, R. Schizophrenia risk candidate EGR3 is a novel transcriptional regulator of RELN and regulates neurite outgrowth via the Reelin signal pathway in vitro. J. Neurochem. 2021, 157, 1745–1758. [Google Scholar] [CrossRef]
- Ibi, D.; Nakasai, G.; Koide, N.; Sawahata, M.; Kohno, T.; Takaba, R.; Nagai, T.; Hattori, M.; Nabeshima, T.; Yamada, K.; et al. Reelin supplementation into the hippocampus rescues abnormal behavior in a mouse model of neurodevelopmental disorders. Front. Cell. Neurosci. 2020, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Kho, S.H.; Yee, J.Y.; Puang, S.J.; Han, L.; Chiang, C.; Rapisarda, A.; Goh, W.W.B.; Lee, J.; Sng, J.C.G. DNA methylation levels of RELN promoter region in ultra-high risk, first episode and chronic schizophrenia cohorts of schizophrenia. Schizophrenia 2022, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Marckx, A.T.; Fritschle, K.E.; Calvier, L.; Herz, J. Reelin changes hippocampal learning in aging and Alzheimer’s disease. Behav. Brain Res. 2021, 414, 113482. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.R.; Di Fazio, C.; Battaglia, S. Activated tryptophan-kynurenine metabolic system in the human brain is associated with learned fear. Front. Mol. Neurosci. 2023, 16, 1217090. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial impairment: A common motif in neuropsychiatric presentation? The link to the tryptophan-kynurenine metabolic system. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Abo El Fotoh, W.M.M.; Bayomy, N.R.; Kasemy, Z.A.; Barain, A.M.; Shalaby, B.M.; Abd El Naby, S.A. Genetic variants, and haplotypes of tryptophan hydroxylase 2 and reelin genes may be linked with attention deficit hyperactivity disorder in Egyptian children. ACS Chem. Neurosci. 2020, 11, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.I.K.; Petrova, P.; Daoud, S.; Rabaya, O.; Jbara, A.; Melliti, N.; Leifeld, J.; Jakovčevski, I.; Reiss, G.; Herz, J.; et al. Reelin restricts dendritic growth of interneurons in the neocortex. Development 2021, 148, dev199718. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, G. Reelin in the years: Controlling neuronal migration and maturation in the mammalian brain. Adv. Neurosci. 2014, 2014, 597395. [Google Scholar] [CrossRef]
- Chai, X.; Frotscher, M. How does Reelin signaling regulate the neuronal cytoskeleton during migration? Neurogenesis 2016, 3, e1242455. [Google Scholar] [CrossRef]
- Kohno, T.; Ishii, K.; Hirota, Y.; Honda, T.; Makino, M.; Kawasaki, T.; Nakjima, K.; Hattori, M. Reelin-Nrp1 interaction regulates neocortical dendrite development in a context-specific manner. J. Neurosci. 2020, 40, 8248–8261. [Google Scholar] [CrossRef]
- Tissir, F.; Lambert de Reuvroit, C.; Goffinet, A.M. The role of reelin in the development and evolution of the cerebral cortex. Braz. J. Med. Biol. Res. 2002, 35, 1473–1484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bock, H.H.; May, P. Canonical and non-canonical reelin signaling. Front. Cell. Neurosci. 2016, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Frotscher, M. Go or stop? Divergent roles of reelin in radial neuronal migration. Neuroscientist 2010, 16, 330–474. [Google Scholar] [CrossRef] [PubMed]
- de Rouvroit, C.L.; Goffinet, A.M. Neuronal migration, mechanisms of development. Mech. Dev. 2001, 105, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.D. Chapter three-platelet-activating factor acetylhydrolase and brain development. In The Enzymes; Inoue, K., Stafforini, D.M., Tamanoi, F., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 38, pp. 37–42. [Google Scholar] [CrossRef]
- Assadi, A.H.; Zhang, G.; McNeil, R.; Clark, G.D.; D’Arcangelo, G. Pafah1b2 mutations suppress the development of hydrocephalus in compound Pafah1b1; Reln and Pafah1b1; Dab1 mutant mice. Neurosci. Lett. 2008, 439, 100–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Bennison, S.A.; Robinson, L.; Toyo-oka, K. Responsible genes for neuronal migration in the chromosome 17p13.3: Beyond Pafah1b1(Lis1), Crk and Ywhae(14-3-3ε). Brain Sci. 2022, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, J.; Bock, H.H. Reelin modulates cytoskeletal organization by regulating Rho GTPases. Commun. Integr. Biol. 2011, 4, 254–257. [Google Scholar] [CrossRef][Green Version]
- Santana, J.; Marzolo, M.P. The functions of Reelin in membrane trafficking and cytoskeletal dynamics: Implications for neuronal migration, polarization, and differentiation. Biochem. J. 2017, 474, 3137–3165. [Google Scholar] [CrossRef]
- Joly-Amado, A.; Kulkarni, N.; Nask, K.R. Reelin signaling in neurodevelopmental disorders and neurodegenerative disorders. Brain Sci. 2023, 13, 1479. [Google Scholar] [CrossRef]
- Negrón-Oyarzo, I.; Lara-Vásquez, A.; Palacios-García, I.; Fuentealba, P.; Aboitiz, F. Schizophrenia and reelin: A model based on prenatal stress to study epigenetics, brain development and behavior. Biol. Res. 2016, 49, 16. [Google Scholar] [CrossRef]
- Owen, M.J.; O’Donovan, M.C.; Thapar, A.; Craddock, N. Neurodevelopmental hypothesis of schizophrenia. Br. J. Psychiatry 2011, 198, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Knable, M.B.; Torrey, E.F.; Webster, M.J.; Bartko, J.J. Multivariate analysis of prefrontal cortical data from the Stanley Foundation Neuropathology Consortium. Brain Res. Bull. 2001, 55, 651–659. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Pesold, C.; Liu, W.S.; Kriho, V.; Guidotti, A.; Pappas, G.D.; Costa, E. Colocalization of integrin receptors and reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex. Proc. Natl. Acad. Sci. USA 2000, 97, 3550–3555. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Guidotti, A.; Grayson, D.R.; Costa, E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc. Natl. Acad. Sci. USA 2007, 104, 4676–4681. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Agis-Balboa, R.C.; Simonini, M.V.; Grayson, D.R.; Costa, E.; Guidotti, A. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc. Natl. Acad. Sci. USA 2005, 102, 12578–12583. [Google Scholar] [CrossRef]
- Pollin, W.; Cardon, P.V.; Kety, S.S., Jr. Effects of amino acid feedings in schizophrenic patients treated with iproniazid. Science 1961, 133, 104–105. [Google Scholar] [CrossRef]
- Brymer, K.J.; Johnston, J.; Botterill, J.J.; Romay-Tallon, R.; Mitchell, M.A.; Allen, J.; Pinna, G.; Caruncho, H.J.; Kalynchuk, L.E. Fast-acting antidepressant-like effects of Reelin evaluated in the repeated-corticosterone chronic stress paradigm. Neuropsychopharmacology 2020, 45, 1707–1716. [Google Scholar] [CrossRef]
- Ruzicka, W.B.; Zhubi, A.; Veldic, M.; Grayson, D.R.; Costa, E.; Guidotti, A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol. Psychiatry 2007, 12, 385–397. [Google Scholar] [CrossRef]
- Guidotti, A.; Ruzicka, W.; Grayson, D.R.; Veldic, M.; Pinna, G.; Davis, J.M.; Costa, E. S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. Neuroreport 2007, 18, 57–60. [Google Scholar] [CrossRef]
- Fazio, C.; Andreoli, V.; Agnoli, A.; Casacchia, M.; Cerbo, R.; Pinzello, A. Therapy of schizophrenia and depressive disorders with S-adenosyl-L-methionine. Intern. Res. Comm. Syst. (IRCS) Clin. Pharmacol. Ther. 1974, 2, 1015. [Google Scholar]
- Sharma, A.; Gerbarg, P.; Bottiglieri, T.; Massoumi, L.; Carpenter, L.L.; Lavretsky, H.; Muskin, P.R.; Brown, R.P.; Mischoulon, D. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: A clinician-oriented review of research. J. Clin. Psychiatry 2017, 78, e656–e667. [Google Scholar] [CrossRef]
- Fukumoto, K.; Ito, K.; Saer, B.; Taylor, G.; Ye, S.; Yamano, M.; Toriba, Y.; Hayes, A.; Okamura, H.; Fustin, J.M. Excess S-adenosylmethionine inhibits methylation via catabolism to adenine. Commun. Biol. 2022, 5, 313. [Google Scholar] [CrossRef] [PubMed]
- Hornig, T.; Haas, C.; Sturm, L.; Fiebich, B.; van Elst, T.L. Increased blood-reelin-levels in first episode schizophrenia. PLoS ONE 2015, 10, e0134671. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, E.; Ogino, H.; Shigenobu, T.; Yamakage, Y.; Tsuiji, H.; Oishi, H.; Kohno, T.; Hattori, M. Physiological significance of proteolytic processing of Reelin revealed by cleavage-resistant Reelin knock-in mice. Sci. Rep. 2020, 10, 4471. [Google Scholar] [CrossRef] [PubMed]
- Botella-López, A.; Burgaya, F.; Gavín, R.; García-Ayllón, M.S.; Gómez-Tortosa, E.; Peña-Casanova, J.; Ureña, J.M.; Del Río, J.A.; Blesa, R.; Soriano, E.; et al. Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Brummelte, S.; Galea, L.A.; Devlin, A.M.; Oberlander, T.F. Antidepressant use during pregnancy and serotonin transporter genotype (SLC6A4) affect newborn serum reelin levels. Dev. Psychobiol. 2013, 55, 518–529. [Google Scholar] [CrossRef]
- Magnani, A.; Pattacini, L.; Boiardi, L.; Casali, B.; Salvarani, C. Reelin levels are increased in synovial fluid of patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2010, 28, 546–548. [Google Scholar]
- Mansy, S.S.; Nosseir, M.M.; Zoheiry, M.A.; Hassanein, M.H.; Guda, M.F.; Othman, M.M.; Abu Talab, H. Value of reelin for assessing hepatic fibrogenesis in a group of Egyptian HCV infected patients. Clin. Chem. Lab. Med. 2014, 52, 1319–1328. [Google Scholar] [CrossRef]
- Botella-Lopez, A.; de Madaria, E.; Jover, R.; Bataller, R.; Sancho-Bru, P.; Candela, A.; Compañ, A.; Pérez-Mateo, M.; Martinez, S.; Sáez-Valero, J. Reelin is overexpressed in the liver and plasma of bile duct ligated rats and its levels and glycosylation are altered in plasma of humans with cirrhosis. Int. J. Biochem. Cell Biol. 2008, 40, 766–775. [Google Scholar] [CrossRef]
- Dazzo, E.; Fanciulli, M.; Serioli, E.; Minervini, G.; Pulitano, P.; Binelli, S.; Di Bonaventura, C.; Luisi, C.; Pasini, E.; Striano, S.; et al. Heterozygous reelin mutations cause autosomal-dominant lateral temporal epilepsy. Am. J. Hum. Genet. 2015, 96, 992–1000. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Stary, J.M.; Egan, E.A. Reduced blood levels of reelin as a vulnerability factor in pathophysiology of autistic disorder. Cell Mol. Neurobiol. 2002, 22, 139–152. [Google Scholar] [CrossRef]
- Smalheiser, N.R.; Costa, E.; Guidotti, A.; Impagnatiello, F.; Auta, J.; Lacor, P.; Kriho, V.; Pappas, G.D. Expression of reelin in adult mammalian blood, liver, pituitary pars intermedia, and adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D. Chronic psychotropic drug treatment causes differential expression of Reelin signaling system in frontal cortex of rats. Schizophr. Res. 2009, 111, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Beffert, U.; Weeber, E.J.; Durudas, A.; Qiu, S.; Masiulis, I.; Sweatt, J.D.; Li, W.P.; Adelmann, G.; Frotscher, M.; Hammer, R.E.; et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 2005, 47, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Howell, B.W.; Gertler, F.B.; Cooper, J.A. Mouse disabled (mDab1): A Src binding protein implicated in neuronal development. EMBO J. 1997, 16, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Shifman, S.; Johannesson, M.; Bronstein, M.; Chen, S.X.; Collier, D.A.; Craddock, N.J.; Kendler, K.S.; Li, T.; O’Donovan, M.; O’Neill, F.A.; et al. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 2008, 4, e28. [Google Scholar] [CrossRef]
- Wedenoja, J.; Loukola, A.; Tuulio-Henriksson, A.; Paunio, T.; Ekelund, J.; Silander, K.; Varilo, T.; Heikkilä, K.; Suvisaari, J.; Partonen, T.; et al. Replication of linkage on chromosome 7q22 and association of the regional Reelin gene with working memory in schizophrenia families. Mol. Psychiatry 2008, 13, 673–684. [Google Scholar] [CrossRef]
- Erbel-Sieler, C.; Dudley, C.; Zhou, Y.; Wu, X.; Estill, S.J.; Han, T.; Diaz-Arrastia, R.; Brunskill, E.W.; Potter, S.S.; McKnight, S.L. Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors. Proc. Natl. Acad. Sci. USA 2004, 101, 13648–13653. [Google Scholar] [CrossRef]
- Kamnasaran, D.; Muir, W.J.; Ferguson-Smith, M.A.; Cox, D.W. Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J. Med. Genet. 2003, 40, 325–332. [Google Scholar] [CrossRef]
- Wang, G.S.; Hong, C.J.; Yen, T.Y.; Huang, H.Y.; Ou, Y.; Huang, T.N.; Jung, W.G.; Kuo, T.Y.; Sheng, M.; Wang, T.F.; et al. Transcriptional modification by a CASK-interacting nucleosome assembly protein. Neuron 2004, 42, 113–128. [Google Scholar] [CrossRef]
- Chen, Z.; Schwahn, B.C.; Wu, Q.; He, X.; Rozen, R. Postnatal cerebellar defects in mice deficient in methylenetetrahydrofolate reductase. Int. J. Dev. Neurosci. 2005, 23, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Groc, L.; Choquet, D.; Stephenson, F.A.; Verrier, D.; Manzoni, O.J.; Chavis, P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J. Neurosci. 2007, 27, 10165–10175. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, X.; Xiao, S. Evaluating the relationship between reelin gene variants (rs7341475 and rs262355) and schizophrenia: A meta-analysis. Neurosci. Lett. 2015, 609, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Tsuneura, Y.; Sawahata, M.; Itoh, N.; Miyajima, R.; Mori, D.; Kohno, T.; Hattori, M.; Sobue, A.; Nagai, T.; Mizoguchi, H.; et al. Analysis of Reelin signaling and neurodevelopmental trajectory in primary cultured cortical neurons with RELN deletion identified in schizophrenia. Neurochem. Int. 2021, 144, 104954. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Martín, E.D.; Sahún, I.; Masachs, N.; Pujadas, L.; Corvelo, A.; Bosch, C.; Rossi, D.; Martinez, A.; Maldonado, R.; et al. Overexpression of Reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology 2011, 36, 2395–2405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markiewicz, R.; Markiewicz-Gospodarek, A.; Borowski, B.; Trubalski, M.; Łoza, B. Reelin Signaling and Synaptic Plasticity in Schizophrenia. Brain Sci. 2023, 13, 1704. https://doi.org/10.3390/brainsci13121704
Markiewicz R, Markiewicz-Gospodarek A, Borowski B, Trubalski M, Łoza B. Reelin Signaling and Synaptic Plasticity in Schizophrenia. Brain Sciences. 2023; 13(12):1704. https://doi.org/10.3390/brainsci13121704
Chicago/Turabian StyleMarkiewicz, Renata, Agnieszka Markiewicz-Gospodarek, Bartosz Borowski, Mateusz Trubalski, and Bartosz Łoza. 2023. "Reelin Signaling and Synaptic Plasticity in Schizophrenia" Brain Sciences 13, no. 12: 1704. https://doi.org/10.3390/brainsci13121704
APA StyleMarkiewicz, R., Markiewicz-Gospodarek, A., Borowski, B., Trubalski, M., & Łoza, B. (2023). Reelin Signaling and Synaptic Plasticity in Schizophrenia. Brain Sciences, 13(12), 1704. https://doi.org/10.3390/brainsci13121704