Cervico-Ocular and Vestibulo-Ocular Reflexes in Subclinical Neck Pain and Healthy Individuals: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Participants
2.3. Questionnaires: Neck Pain Characteristics
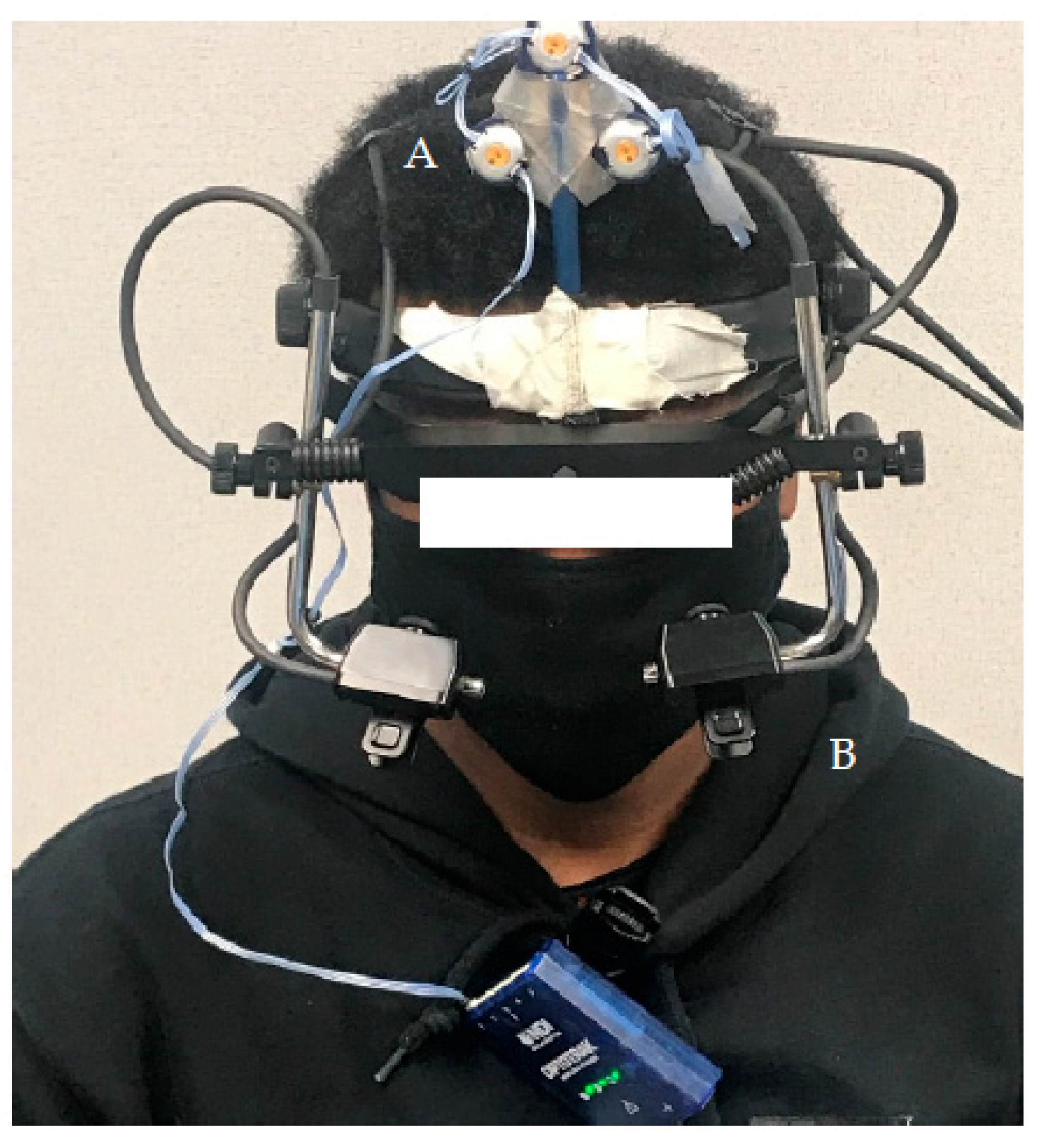
2.4. Instrumentation and Data Acquistion
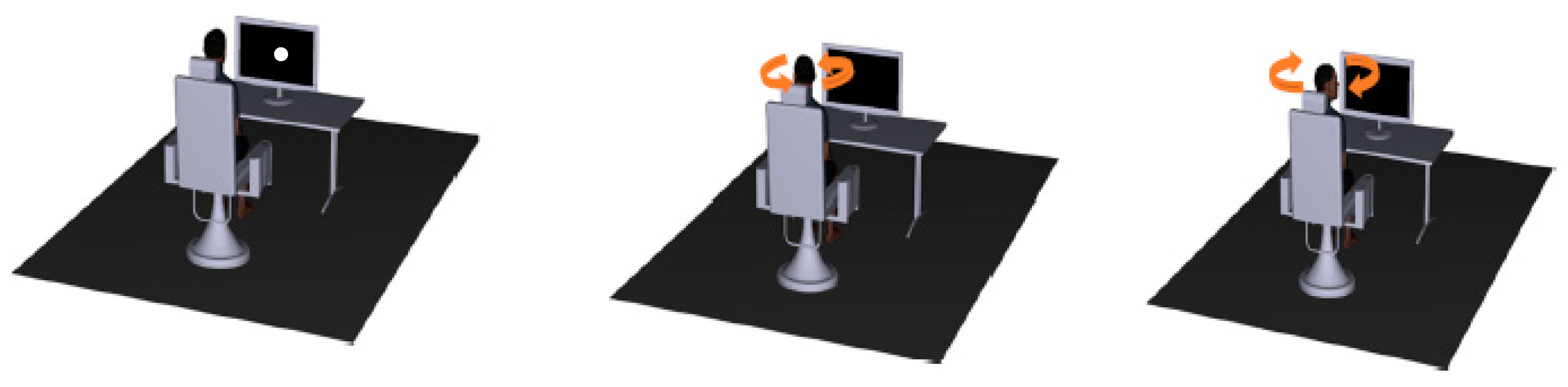
2.5. Experimental Flow
2.5.1. COR
2.5.2. VOR
2.6. Data Analysis
2.6.1. COR Analysis
2.6.2. VOR Analysis
VOR Analysis—Measurement Error
2.7. Statistical Analysis
3. Results
3.1. Demographic and Neck Pain Characteristics
3.2. COR Gain
3.3. VOR Gain
3.3.1. Head Peak Velocity
3.3.2. Measurement Error
Constant Error
Variable Error
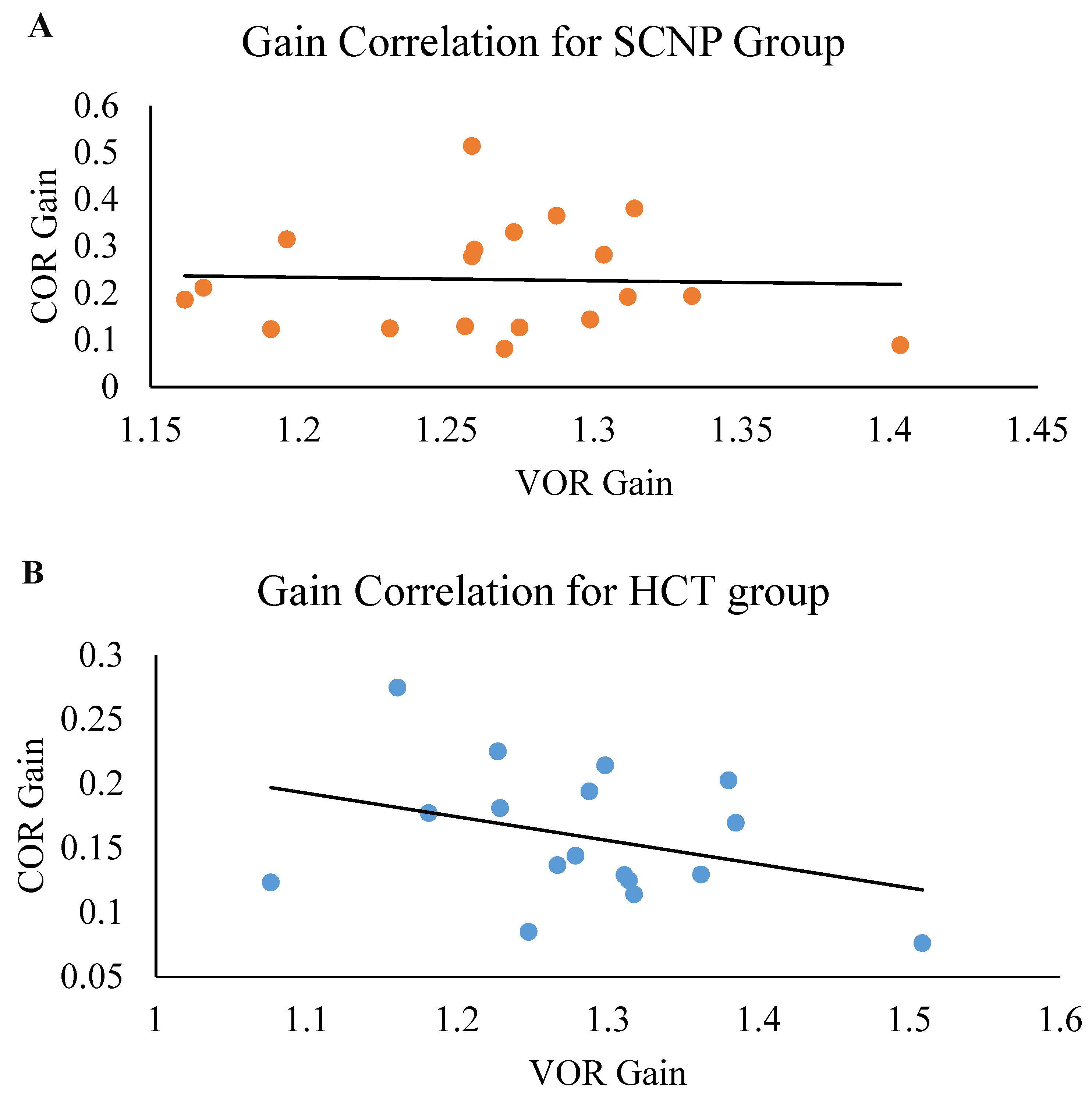
3.4. Correlation of COR Gain and VOR Gain
4. Discussion
4.1. COR Gain
4.2. COR and VOR Gain
4.3. VOR Gain
4.4. Limitations
4.5. Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knierim, J.; Byrne, J.; Dafny, N. Neuroscience online: An electronic textbook for the neurosciences. In Department of Neurobiology and Anatomy; The University of Texas Medical School at Houston: Houston, TX, USA, 1997. [Google Scholar]
- Gdowski, G.T.; Belton, T.; McCrea, R.A. The neurophysiological substrate for the cervico-ocular reflex in the squirrel monkey. Exp. Brain Res. 2001, 140, 253–264. [Google Scholar] [CrossRef]
- Ranjbaran, M.; Galiana, H.L. Hybrid model of the context dependent vestibulo-ocular reflex: Implications for vergence-version interactions. Front. Comput. Neurosci. 2015, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Ischebeck, B.K.; Voogt, L.P.; Janssen, M.; Frens, M.A.; Kleinrensink, G.-J.; van der Geest, J.N. Cervico-ocular reflex is increased in people with nonspecific neck pain. Phys. Ther. 2016, 96, 1190–1195. [Google Scholar] [CrossRef]
- Gdowski, G.T.; McCrea, R.A. Neck proprioceptive inputs to primate vestibular nucleus neurons. Exp. Brain Res. 2000, 135, 511–526. [Google Scholar] [CrossRef]
- Ito, M. Cerebellar learning in the vestibulo–ocular reflex. Trends Cogn. Sci. 1998, 2, 313–321. [Google Scholar] [CrossRef]
- Gray, L. Vestibular System: Structure and Function. In Neuroscience Online: An Electronic Textbook for the Neurosciences; McGovern Medical School at UTHealth: Houston, TX, USA, 2020. [Google Scholar]
- Gray, L. Vestibular System: Pathways and Reflexes. In Neuroscience Online: An Electronic Textbook for the Neurosciences; McGovern Medical School at UTHealth: Houston, TX, USA, 2020. [Google Scholar]
- Ischebeck, B.K.; de Vries, J.; Janssen, M.; van Wingerden, J.P.; Kleinrensink, G.-J.; van der Geest, J.N.; Frens, M.A. Eye stabilization reflexes in traumatic and non-traumatic chronic neck pain patients. Musculoskelet. Sci. Pract. 2017, 29, 72–77. [Google Scholar] [CrossRef]
- Fadaee, S.B.; Migliaccio, A.A. The effect of retinal image error update rate on human vestibulo-ocular reflex gain adaptation. Exp. Brain Res. 2016, 234, 1085–1094. [Google Scholar] [CrossRef]
- Kelders, W.; Kleinrensink, G.; Geest, J.V.D.; Schipper, I.; Feenstra, L.; Zeeuw, C.D.; Frens, M. The cervico-ocular reflex is increased in whiplash injury patients. J. Neurotrauma 2005, 22, 133–137. [Google Scholar] [CrossRef]
- Montfoort, I.; Van Der Geest, J.N.; Slijper, H.P.; De Zeeuw, C.I.; Frens, M.A. Adaptation of the cervico-and vestibulo-ocular reflex in whiplash injury patients. J. Neurotrauma 2008, 25, 687–693. [Google Scholar] [CrossRef]
- Hellmann, D.; Giannakopoulos, N.N.; Schmitter, M.; Lenz, J.; Schindler, H.J. Anterior and posterior neck muscle activation during a variety of biting tasks. Eur. J. Oral Sci. 2012, 120, 326–334. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Biochemistry, D.o.; Jessell, M.B.T.; Siegelbaum, S.; Hudspeth, A. Principles of neural science; McGraw-hill New York: New York, NY, USA, 2000; Volume 4. [Google Scholar]
- Zamysłowska-Szmytke, E.; Adamczewski, T.; Ziąber, J.; Majak, J.; Kujawa, J.; Śliwińska-Kowalska, M. Cervico-ocular reflex upregulation in dizzy patients with asymmetric neck pathology. Int. J. Occup. Med. Environ. Health 2019, 32, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Tabak, S.; Collewijn, H.; Boumans, L.; Van der Steen, J. Gain and delay of human vestibulo-ocular reflexes to oscillation and steps of the head by a reactive torque helmet: I. Normal subjects. Acta Oto-Laryngol. 1997, 117, 785–795. [Google Scholar] [CrossRef]
- Kelders, W.; Kleinrensink, G.; Van der Geest, J.; Feenstra, L.; De Zeeuw, C.; Frens, M. Compensatory increase of the cervico-ocular reflex with age in healthy humans. J. Physiol. 2003, 553, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Nicholson, L.L.; Adams, R.D.; Bae, S.-S. Proprioception and rotation range sensitization associated with subclinical neck pain. Spine 2005, 30, E60–E67. [Google Scholar] [CrossRef]
- Haavik, H.; Murphy, B. Subclinical neck pain and the effects of cervical manipulation on elbow joint position sense. J. Manip. Physiol. Ther. 2011, 34, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Zabihhosseinian, M.; Holmes, M.W.R.; Howarth, S.; Ferguson, B.; Murphy, B. Neck muscle fatigue differentially alters scapular and humeral kinematics during humeral elevation in subclinical neck pain participants versus healthy controls. J. Electromyogr. Kinesiol. 2017, 33, 73–82. [Google Scholar] [CrossRef]
- Paulus, I.; Brumagne, S. Altered interpretation of neck proprioceptive signals in persons with subclinical recurrent neck pain. J. Rehabil. Med. 2008, 40, 426–432. [Google Scholar] [CrossRef]
- Daligadu, J.; Haavik, H.; Yielder, P.C.; Baarbe, J.; Murphy, B. Alterations in cortical and cerebellar motor processing in subclinical neck pain patients following spinal manipulation. J. Manip. Physiol. Ther. 2013, 36, 527–537. [Google Scholar] [CrossRef]
- Baarbé, J.K.; Yielder, P.; Haavik, H.; Holmes, M.W.; Murphy, B.A. Subclinical recurrent neck pain and its treatment impacts motor training-induced plasticity of the cerebellum and motor cortex. PLoS ONE 2018, 13, e0193413. [Google Scholar] [CrossRef]
- Andrew, D.; Yielder, P.; Haavik, H.; Murphy, B. The effects of subclinical neck pain on sensorimotor integration following a complex motor pursuit task. Exp. Brain Res. 2018, 236, 1–11. [Google Scholar] [CrossRef]
- Ambalavanar, U.; Yielder, P.; McCracken, H.; Tabbert, H.; Murphy, B. Subclinical neck pain leads to differential changes in early and middle-latency somatosensory evoked potentials and motor performance in response to a novel force matching tracking task. J. Integr. Neurosci. 2023; in press. [Google Scholar]
- Von Korff, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain 1992, 50, 133–149. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef]
- McCarthy, M.J.H.; Grevitt, M.P.; Silcocks, P.; Hobbs, G. The reliability of the Vernon and Mior neck disability index, and its validity compared with the short form-36 health survey questionnaire. Eur. Spine J. 2007, 16, 2111–2117. [Google Scholar] [CrossRef]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Goh, G.S.; Yue, W.-M.; Guo, C.-M.; Tan, S.-B.; Chen, J.L. Defining threshold values on the neck disability index corresponding to a patient acceptable symptom state in patients undergoing elective surgery for degenerative disorders of the cervical spine. Spine J. 2020, 20, 1316–1326. [Google Scholar] [CrossRef]
- Bijur, P.E.; Silver, W.; Gallagher, E.J. Reliability of the visual analog scale for measurement of acute pain. Acad. Emerg. Med. 2001, 8, 1153–1157. [Google Scholar] [CrossRef]
- Begum, M.R.; Hossain, M.A. Validity and reliability of visual analogue scale (VAS) for pain measurement. J. Med. Case Rep. Rev. 2019, 2, 394–402. [Google Scholar]
- Dimitriadis, Z.; Strimpakos, N.; Kapreli, E.; Oldham, J. Validity of visual analog scales for assessing psychological states in patients with chronic neck pain. J. Musculoskelet. Pain 2014, 22, 242–246. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Deloach, J.; Porucznik, C.A.; Powell, A.P. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elb. Surg 2009, 18, 927–932. [Google Scholar] [CrossRef]
- Schubert, M.C.; Della Santina, C.C.; Shelhamer, M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp. Brain Res. 2008, 191, 435–446. [Google Scholar] [CrossRef]
- Sanmugananthan, P.; Nguyen, N.; Murphy, B.; Hosseini, A. Design and Development of a Rotating Chair to Measure the Cervico-Ocular Reflex. Cureus 2021, 13, e19099. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Ischebeck, B.K.; de Vries, J.; van Wingerden, J.P.; Kleinrensink, G.J.; Frens, M.A.; van der Geest, J.N. The influence of cervical movement on eye stabilization reflexes: A randomized trial. Exp. Brain Res. 2018, 236, 297–304. [Google Scholar] [CrossRef]
- Montfoort, I.; Kelders, W.P.; Van Der Geest, J.N.; Schipper, I.B.; Feenstra, L.; de Zeeuw, C.I.; Frens, M.A. Interaction between ocular stabilization reflexes in patients with whiplash injury. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2881–2884. [Google Scholar] [CrossRef]
- Seibold, P. Sine fitting. MATLAB Central File Exchange. 2022. Available online: https://www.mathworks.com/matlabcentral/fileexchange/66793-sine-fitting (accessed on 7 January 2022).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Simes, R.J. An improved Bonferroni procedure for multiple tests of significance. Biometrika 1986, 73, 751–754. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2009; Volume 2. [Google Scholar]
- Schubert, M.C.; Das, V.; Tusa, R.J.; Herdman, S.J. Cervico-ocular reflex in normal subjects and patients with unilateral vestibular hypofunction. Otol. Neurotol. 2004, 25, 65–71. [Google Scholar] [CrossRef]
- Bakker, D.; Richmond, F.; Abrahams, V. Central projections from cat suboccipital muscles: A study using transganglionic transport of horseradish peroxidase. J. Comp. Neurol. 1984, 228, 409–421. [Google Scholar] [CrossRef]
- Edney, D.P.; Porter, J.D. Neck muscle afferent projections to the brainstem of the monkey: Implications for the neural control of gaze. J. Comp. Neurol. 1986, 250, 389–398. [Google Scholar] [CrossRef]
- Porter, J.D. Brainstem terminations of extraocular muscle primary afferent neurons in the monkey. J. Comp. Neurol. 1986, 247, 133–143. [Google Scholar] [CrossRef]
- Liu, J.-X.; Thornell, L.-E.; Pedrosa-Domellöf, F. Muscle spindles in the deep muscles of the human neck: A morphological and immunocytochemical study. J. Histochem. Cytochem. 2003, 51, 175–186. [Google Scholar] [CrossRef]
- Röijezon, U.; Clark, N.C.; Treleaven, J. Proprioception in musculoskeletal rehabilitation. Part 1: Basic science and principles of assessment and clinical interventions. Man. Ther. 2015, 20, 368–377. [Google Scholar] [CrossRef]
- Belton, T.; McCrea, R. Contribution of the cerebellar flocculus to gaze control during active head movements. J. Neurophysiol. 1999, 81, 3105–3109. [Google Scholar] [CrossRef]
- Baarbé, J.K.; Holmes, M.W.; Murphy, H.E.; Haavik, H.; Murphy, B.A. Influence of subclinical neck pain on the ability to perform a mental rotation task: A 4-week longitudinal study with a healthy control group comparison. J. Manip. Physiol. Ther. 2016, 39, 23–30. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Wang, J.-D.; Yao, G.; Wang, S.-F. Association between cervicocephalic kinesthetic sensibility and frequency of subclinical neck pain. Man. Ther. 2008, 13, 419–425. [Google Scholar] [CrossRef]
- Zabihhosseinian, M.; Yielder, P.; Holmes, M.W.R.; Murphy, B. Neck muscle fatigue affects performance of an eye-hand tracking task. J. Electromyogr. Kinesiol. 2019, 47, 1–9. [Google Scholar] [CrossRef]
- Jeka, J.J.; Oie, K.S.; Kiemel, T. Asymmetric adaptation with functional advantage in human sensorimotor control. Exp. Brain Res. 2008, 191, 453–463. [Google Scholar] [CrossRef]
- Bronstein, A.; Morland, A.; Ruddock, K.; Gresty, M. Recovery from bilateral vestibular failure: Implications for visual and cervico-ocular function. Acta Oto-Laryngol. 1995, 115, 405–407. [Google Scholar] [CrossRef]
- Treleaven, J.; Jull, G.; Sterling, M. Dizziness and unsteadiness following whiplash injury: Characteristic features and relationship with cervical joint position error. J. Rehabil. Med. 2003, 35, 36–43. [Google Scholar] [CrossRef]



| SCNP Group | Healthy Control Group | |||
| Biological Sex (F:M) | 11:9 | 10:7 | ||
| Age (years) | 21.80 ± 2.35 | 22.40 ± 3.66 | ||
| Von Korff CPGS | ||||
| Grade 0 | 0 | 17 | ||
| Grade I | 13 | 0 | ||
| Grade II | 6 | 0 | ||
| Grade III | 1 | 0 | ||
| Grade IV | 0 | 0 | ||
| SCNP Group | Healthy Control Group | p-value | ||
| * NDI Score (/50) | 7.95 ± 5.53 | 0.77 ± 1.09 | <0.001 | |
| * Pain VAS (/10 cm) | 1.47 ± 1.05 | 0.14 ± 0.18 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, D.; Murphy, B.A.; Burkitt, J.; La Delfa, N.; Sanmugananthan, P.; Ambalavanar, U.; Yielder, P. Cervico-Ocular and Vestibulo-Ocular Reflexes in Subclinical Neck Pain and Healthy Individuals: A Cross-Sectional Study. Brain Sci. 2023, 13, 1603. https://doi.org/10.3390/brainsci13111603
Campbell D, Murphy BA, Burkitt J, La Delfa N, Sanmugananthan P, Ambalavanar U, Yielder P. Cervico-Ocular and Vestibulo-Ocular Reflexes in Subclinical Neck Pain and Healthy Individuals: A Cross-Sectional Study. Brain Sciences. 2023; 13(11):1603. https://doi.org/10.3390/brainsci13111603
Chicago/Turabian StyleCampbell, Devonte, Bernadette Ann Murphy, James Burkitt, Nicholas La Delfa, Praveen Sanmugananthan, Ushani Ambalavanar, and Paul Yielder. 2023. "Cervico-Ocular and Vestibulo-Ocular Reflexes in Subclinical Neck Pain and Healthy Individuals: A Cross-Sectional Study" Brain Sciences 13, no. 11: 1603. https://doi.org/10.3390/brainsci13111603
APA StyleCampbell, D., Murphy, B. A., Burkitt, J., La Delfa, N., Sanmugananthan, P., Ambalavanar, U., & Yielder, P. (2023). Cervico-Ocular and Vestibulo-Ocular Reflexes in Subclinical Neck Pain and Healthy Individuals: A Cross-Sectional Study. Brain Sciences, 13(11), 1603. https://doi.org/10.3390/brainsci13111603







