Abstract
This systematic review assesses current molecular targeted therapies for glioblastoma multiforme (GBM), a challenging condition with limited treatment options. Using PRISMA methodology, 166 eligible studies, involving 2526 patients (61.49% male, 38.51% female, with a male-to-female ratio of 1.59/1), were analyzed. In laboratory studies, 52.52% primarily used human glioblastoma cell cultures (HCC), and 43.17% employed animal samples (mainly mice). Clinical participants ranged from 18 to 100 years, with 60.2% using combined therapies and 39.8% monotherapies. Mechanistic categories included Protein Kinase Phosphorylation (41.6%), Cell Cycle-Related Mechanisms (18.1%), Microenvironmental Targets (19.9%), Immunological Targets (4.2%), and Other Mechanisms (16.3%). Key molecular targets included Epidermal Growth Factor Receptor (EGFR) (10.8%), Mammalian Target of Rapamycin (mTOR) (7.2%), Vascular Endothelial Growth Factor (VEGF) (6.6%), and Mitogen-Activated Protein Kinase (MEK) (5.4%). This review provides a comprehensive assessment of molecular therapies for GBM, highlighting their varied efficacy in clinical and laboratory settings, ultimately impacting overall and progression-free survival in GBM management.
1. Introduction
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults, representing 45.2% of malignant brain and CNS tumors [1,2,3,4]. It is classified as a grade IV diffuse astrocytic glioma by the World Health Organization (WHO) due to its invasive growth and specific histopathological and immunohistochemical features [5]. Molecular targeted therapies have emerged as a promising avenue for addressing GBM’s complexity and limited treatment options [6,7,8,9,10,11]. Frequent genetic alterations, such as p53 mutations, EGFR amplification, CDKN2a deletion, and PTEN mutations, offer potential therapeutic targets [11,12,13,14,15,16,17,18,19,20,21]. Current treatments, including surgery, radiation, and chemotherapy, yield a median survival of only 15 months for GBM patients, with frequent aggressive recurrences [12]. Patients also contend with significant psychological challenges that impact their quality of life [14].
This systematic review is driven by the critical need to consolidate and analyze key advancements in the field of molecular targeted therapies for GBM. Despite ongoing efforts, the complex nature of GBM and limited treatment options emphasize the significance of evaluating current research directions. Our primary goal is to offer crucial insights to the scientific community and healthcare professionals, contributing to the quest for more effective molecular interventions and improved outcomes for GBM patients.
2. Materials and Methods
A comprehensive systematic analysis was conducted to assess the present status of molecular targeted treatments for gliomas, aimed at providing valuable insights for scientific advancement and steering progress in this research domain. The methodology adhered to the established PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [22]. This systematic review was registered in the Open Science Framework (OSF) registry under the identifier OSF-REGISTRATIONS-UBGYC-V1.
2.1. Search Strategy
In March 2023, a literature search of English-text articles was conducted using PubMed and Web of Science. Categories of concepts related to molecular targeted therapy were explored, focusing on Glioblastoma multiforme (GBM) and excluding other specific types. The search query used was (Glioblastoma multiforme OR GBM) AND (Molecular targeted therapy OR Protein Kinase Inhibitors OR Immunotherapy OR Apoptosis) from 2000 to 2022. Details about the search methodology are provided in Appendix A.
2.2. Inclusion and Exclusion Criteria
The screening and analysis process involved multiple authors to ensure rigor and accuracy. Initially, article titles and abstracts were assessed by four authors. Subsequently, the remaining articles underwent meticulous examination by a panel of five authors. To ensure the highest level of precision, the screening process was carried out in multiple stages. Initially, two authors evaluated article titles and abstracts for relevance, with a focus on removing any duplicate entries. Following this initial phase, the remaining articles underwent comprehensive full-text scrutiny by three authors.
The inclusion criteria were rigorously adhered to, encompassing studies that met the following criteria: (1) clinical studies, (2) laboratory studies, (3) molecular targeted therapies designed specifically for GBM, (4) studies involving adult participants, and (5) studies from 2000 to 2022. Exclusion criteria were applied as follows: (1) book or book chapters, (2) conference papers, (3) narrative and systematic reviews, (4) non-English literature, (5) studies lacking data of interest (including those related to other glial tumors or studies without predefined data for extraction), and (6) studies involving pediatric populations (Figure 1).

Figure 1.
PRISMA flowchart.
2.3. Data Extraction and Processing
In the systematic review, data extraction encompassed several key elements. These comprised the primary author’s name, year of publication, geographical location, study design, number of subjects (if applicable), molecular target, associated molecular pathway, as well as the approach used and principal discoveries. For the purposes of this study, categorization was performed based on the molecular mechanisms targeted by therapy. The classification is further detailed in Table 1.

Table 1.
Categorization based on target therapy/pathways.
2.4. Statistical Analysis and Graphical Elements
The statistical analysis was conducted using IBM SPSS Statistics (Version 27.0., International Business Machines Corporation, Armonk, NY, USA). The analysis encompassed the processing of categorical variables, with their presentation in the form of frequencies and percentages. Graphical representations were generated for research purposes in non-commercial platforms (Google Sheets and Google Drawings). Elements utilized for depicting molecular pathways were sourced from the non-commercial database, Servier Medical Art (SMART, Manila, Philippines).
3. Results
3.1. Global Research Trends
A total of 166 studies met the eligibility criteria for the systematic review [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182]. The research trends showed that the majority of the studies were conducted in the USA, with 63 studies (38.0%) (Figure 2). China had the second-highest number of studies, with 41 (24.7%), followed by Germany with 10 (6.0%), Italy with 9 (5.4%), and Japan with 8 (4.8%). Other countries with a significant number of studies include France (5; 3.0%), Canada (6; 3.6%), and Australia (3; 1.8%). The remaining countries had one or two studies each, with India, Iran, Korea, Luxembourg, Norway, Romania, Russia, Spain, Switzerland, Taiwan, Turkey, and the United Kingdom each having one study.
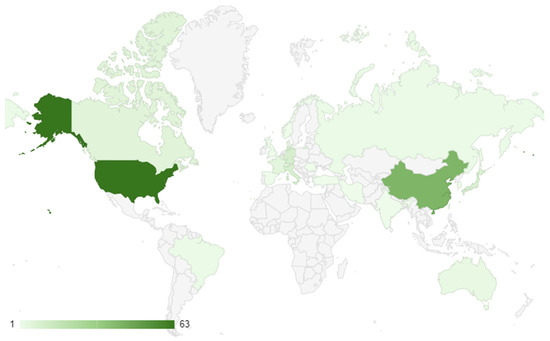
Figure 2.
Geographical distribution of research conduction.
The studies included in the review spanned from 2001 to 2022, with the majority of the studies conducted between 2013 and 2015, accounting for 11.4% and 12.0% of the total studies, respectively. The next highest number of studies took place in 2012, with 8.4% of the total studies. The years with the least number of studies were 2001, 2003, 2004, 2005, 2008, 2009, and 2017, each with only one study (Figure 3).
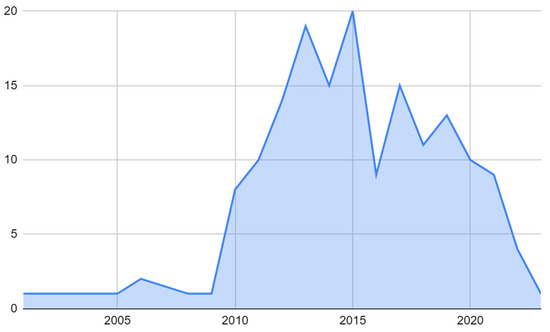
Figure 3.
Temporal distribution of research of molecular target therapy of GBMs.
3.2. Study Design, Type of Target Therapy, and Molecular Mechanisms
The comprehensive systematic review incorporated a total of 27 studies (constituting 16.3% of the total) focused on clinical applications, and a substantial majority of 139 studies (making up 83.7%) were conducted within controlled laboratory environments.
Within the domain of therapeutic modalities, a significant proportion of 100 studies (60.2%) embraced a multifaceted therapeutic approach, while a slightly smaller portion of 66 studies (39.8%) concentrated on mono-therapeutic strategies. In terms of mechanistic classification, 69 studies (41.6%) were categorized under the PKP mechanism, 30 studies (18.1%) were classified under CCRM, 33 studies (19.9%) were designated under Microenvironmental Targets (MT), 7 studies (4.2%) fell under IT, and 27 studies (16.3%) were attributed to OM (Figure 4).
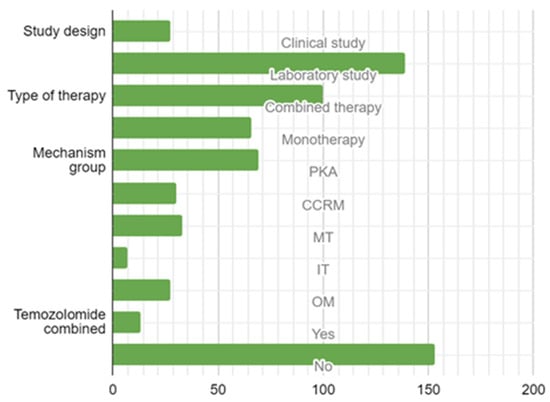
Figure 4.
Study design, type of targeted therapy, mechanism, and combination with temozolomide. Legend: PKP—Protein Kinase Pathway Group; CCRM—Cell Cycle-Related Mechanisms; MT—Microenvironmental Mechanisms; IT—Immunomodulatory Targets; OT—Other Targets.
The most frequently encountered molecular target was found to be the Epidermal Growth Factor Receptor (EGFR), accounting for a substantial 18 instances (10.8%). Following closely were the Mammalian Target of Rapamycin (mTOR) with 12 occurrences (7.2%), Vascular Endothelial Growth Factor (VEGF) with 11 instances (6.6%), and Mitogen-Activated Protein Kinase (MEK) with 9 cases (5.4%). Phosphoinositide 3-Kinase (PI3K) and B-Raf Proto-Oncogene (BRAF) exhibited an equal number of occurrences, each accounting for 8 cases (or 4.8%), while they were attributed to 5 cases (3.0%), respectively.
VEGF, known as Vascular Endothelial Growth Factor, induces an augmentation in the vascularization of GBM. Consequently, it is categorized within the Endothelial Targets (ET) group, despite subsequently activating the Protein Kinase Phosphorylation (PKP) mechanism, akin to EGFR. With respect to Immunological Targets (IT), it encompasses molecular targets such as Extracellular Matrix Metalloproteinase Inducer (EMMPRIN), Autotaxin (ATX), and Lysophosphatidic Acid (LPA), which are associated with the ATX–LPA pathway. This pathway eventually activates Beta Catenin, emerging as a significant avenue of interest in the context of targeted therapy for GBM (Figure 5).
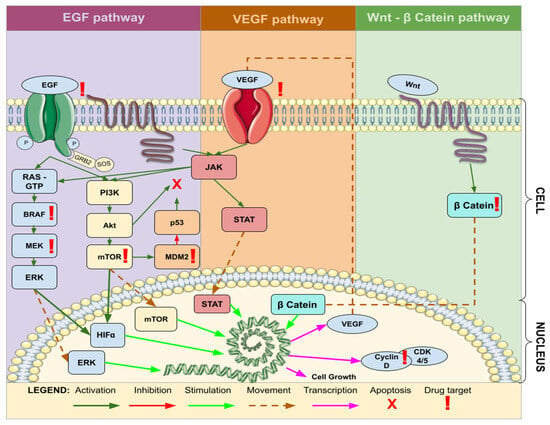
Figure 5.
Common molecular pathways associated with target therapy of GBM. Legend: EGF—Epidermal Growth Factor; VEGF—Vascular Endothelial Growth Factor; JAK—Janus Kinase; STAT—Signal Transducer and Activator of Transcription; Wnt—Wingless-Related Integration Site; Cyclin—Regulatory proteins involved in cell cycle progression; β Catenin—Beta-Catenin; RAS—Rat Sarcoma; GTP—Guanosine Triphosphate; BRAF—B-Raf Proto-Oncogene; MEK—Mitogen-Activated Protein Kinase Kinase; ERK—Extracellular Signal-Regulated Kinase; PI3K—Phosphatidylinositol 3-Kinase; Akt—Protein Kinase B; mTOR—Mammalian Target of Rapamycin; HIFa—Hypoxia-Inducible Factor alpha; CDK—Cyclin-Dependent Kinase; MDM2—Mouse Double Minute 2 Homolog.
3.3. Findings from Clinical Studies
The total number of patients involved in 27 clinical studies is 2526, with three studies not reporting gender distribution numbers (Table 2). Among the known gender distribution data for 1244 patients, 764 (61.49%) were male and 480 (38.51%) were female, resulting in a male-to-female ratio of 1.59/1. The lowest recorded median age was 49 years, while the highest was 90 years. Upon examining the interquartile ranges, it is observed that the youngest participant in these studies was 18 years old, while the oldest was 100 years old.

Table 2.
Overview of clinical studies.
In the context of GBM target therapy treatment, various therapeutic approaches and drug regimens have been explored, each yielding distinct success rates and outcomes. Notably, Imatinib exhibited no significant effect on GBM, with a median progression-free survival (mPFS) of 2.8 months (and control: 2.1 months), showing no statistical significance between the investigated and control groups [145]. In contrast, Nimotuzumab combined with temozolomide and radiation therapy resulted in similar survival times, boasting a median overall survival (mOS) of 15.9 months and a median progression-free survival (mPFS) of 10 months [165]. In the study by Desjardins et al. [54], the combination of bevacizumab with temozolomide showed activity and tolerance, with a median progression-free survival (mPFS) of 15.8 weeks. In the research conducted by Brown et al. [37], the combination of Bevacizumab with Cediranib and Gefitinib demonstrated improved progression-free survival, resulting in a progression-free survival (PFS) of 3.6 months. Additionally, Badruddoja et al. [29] found that bevacizumab, when combined with temozolomide, served as a salvage regimen for recurrent GBM, with an overall response rate from diagnosis of 51 weeks, a PFS-6 of 52%, and a median time to tumor progression of 5.5 months. Regorafenib demonstrated a survival benefit in recurrent GBM, with a survival of 24.8 months [109], while Pembrolizumab, with or without bevacizumab, proved ineffective in therapy, resulting in a progression-free survival rate of 26.0% and an overall survival of 8.8 months with bevacizumab, and a progression-free survival rate of 6.7% and an mOS of 10.3 months without bevacizumab [124]. These findings highlight the diverse landscape of therapeutic strategies and their associated outcomes in the management of GBM.
3.4. Findings from Laboratory Studies
Out of a total of 139 laboratory studies, the most common research samples were human GBM cell lines, specifically human cell cultures (HCC), accounting for 73 studies (52.52%). Subsequently, there were 60 studies (43.17%) that utilized animal samples, and 6 studies (4.32%) employed a combination of sample sources.
In animal studies, mice were predominantly used as the sample (52 studies), representing 37.41%.
Various drugs and treatment combinations demonstrated significant anti-glioma effects, including the inhibition of glioma proliferation, reduced invasion, enhanced apoptosis, and extended survival. Particular highlights include the effectiveness of O-acetyl GD2 ganglioside, Amb4269951, rSLURP-1, ILK inhibition, AAL881, and the combined mTOR1 and MEK1/2 inhibition in CDK4-dysregulated tumors. Moreover, the exploration of various molecular targets, such as EGFR, EGFRvIII, miRNAs, MET, and other signaling pathways, underscores the complex nature of glioma and the potential for targeted therapies.
3.4.1. Overview of In Vitro Laboratory Studies
The total number of in vitro studies included in the systematic review amounted to 42, constituting 25.3% of the overall study count. The GBM cell lines most frequently encountered in these studies were the U87 cell line (comprising 17 studies, or 40.5%), which featured prominently across various investigations. Following this, the U251 cell line (noted in 11 studies, or 26.2%) and the T98G cell line (present in 10 studies, or 23.8%) were also commonly employed.
Regarding potential drugs for the treatment of GBM, numerous compounds exhibited promise within the in vitro research. Particularly, Sorafenib, functioning as a multi-kinase inhibitor, showcased robust anti-glioma activity in both in vitro settings, as emphasized in the study by Siegelin et al. [132]. Furthermore, the combination of Metformin and Sorafenib was identified as an effective treatment strategy for TMZ-resistant GBM cells, as demonstrated in the investigation conducted by Aldea et al. [24]. The research by Paternot et al. [128] underscored the potential of Rapamycin and PD184352 as a combined therapeutic approach, effectively inhibiting DNA synthesis and pRb phosphorylation, especially in CDK4-dysregulated tumors (Table 3).

Table 3.
Overview of in vitro studies.
3.4.2. Overview of In Vivo Laboratory Studies
The systematic review encompassed a total of 62 in vivo studies, constituting 37.4% of the overall studies included in the analysis. Among these in vivo studies, the GBM cell line U87-MG was the most prominently observed (comprising 9.67% of the total), with GSC11 and U251-MG cell lines each being mentioned in two studies. Of these in vivo studies, the majority (87%) involved animal subjects, with a predominant focus on mouse samples (74.2%). Two studies (3.2%) reported human population involvement.
Regarding potential drugs for GBM treatment, the provided studies showcased several promising therapeutic approaches. For instance, AMB4269951, as elucidated in the investigation by Takano et al. [152], demonstrated remarkable anti-tumor effects against gliomas. Rslurp-1, as evidenced by the research conducted by Saito et al. [139], exhibited notable antitumor activity, resulting in increased survival rates. AA1881, explored in the study led by Sathorn-Sumetee et al. [143], targeted BRAF, CRAF, and VEGFR, yielding inhibition of glioma growth and an extension in median survival (Table 4).

Table 4.
Overview of in vivo studies.
3.4.3. Overview of Combined Laboratory Studies
Table 5 furnishes an overarching perspective on the amalgamation of in vivo and in vitro investigations pertaining to GBM, constituting a total of 32 combined studies (19.3%). One conspicuous facet of these studies is the breadth of molecular mechanisms and targets that they explore. For example, Kuan et al. [97] concentrate on receptor-based targeting strategies, with specific regard to TfR (transferrin receptor), while Guo et al. [71] delve into the realm of kinase inhibitors, particularly CDK 4/6 and PDGFRα. Moreover, various studies scrutinize molecular targets encompassing EZH2, FPR, JNK, and PI3K, thereby highlighting the intricate and multifaceted landscape of GBM.

Table 5.
Overview of combined (in vivo and in vitro) studies.
These investigations also shed light on the efficacy of the therapies, with numerous studies presenting encouraging outcomes in terms of extended survival and tumor regression. For instance, Rslurp-1, Dasatinib, GNE-317, and dual mTOR1/2 inhibition yield augmented survival rates, signifying their potential utility in GBM treatment. Furthermore, the juxtaposition of therapies such as TRAIL and TMZ or PDK1 and CHK1 inhibitors reveals synergistic effects in the inhibition of tumor growth (Table 5).
4. Discussion
4.1. Global and Research Trends of GBMs
The global incidence of CNS tumors in 2019 was reported at 347,992 cases, indicating a substantial 94.35% increase from the period spanning 1990 to 2019 [183]. Notably, the incidence of brain tumors exhibited significant regional variation, with the highest rates observed in North America and the lowest in Africa. This trend was found to correlate with increasing Gross Domestic Product (GDP) per capita [184].
Examining the temporal distribution of studies in this systematic review, a notable proportion were conducted between 2013 and 2015, collectively accounting for 23.4% of the total studies. This surge in research activity post-2000s appears to be closely linked to the escalating incidence of GBM. Grech et al.’s [185] research unveiled a significant increase in GBM incidence from 2010, accompanied by a noteworthy increase in incidence risk ratio, measured at 1.16 per additional year. Projections further anticipate a 72% surge in incidence by 2050, compared to figures from 2010 [186].
Within this systematic review, clinical studies constituted 27 (16.3%) of the studies, while laboratory studies comprised the majority, accounting for 139 (83.7%). This distribution reflects the inherent challenges associated with limited patient cohorts and abbreviated survival durations. Initially perceived as predominant in developed nations, oncological diseases like GBM are now assuming the role of a significant economic and health burden in low- and middle-income countries (LMICs) [187]. The management of GBM in these settings is hindered by escalating financial constraints, a shortage of clinical trials, and restricted access to first-line therapeutic agents. The scarcity of healthcare professionals and the suboptimal quality of care further exacerbate the treatment gap for GBM in these regions [187]. Consequently, GBM imposes a substantial financial strain on the healthcare systems of impoverished nations [188,189,190,191,192,193,194,195]
4.2. Current State of Targeted Molecular Therapy in GBM Treatment
The prevailing standard of care for GBM involves the maximal surgical removal of the tumor, followed by localized chemotherapy utilizing TMZ, a second-generation imidazotetrazine known for its DNA-alkylating properties [196]. Its ability to penetrate the blood-brain barrier makes it particularly potent in treating brain tumors [197]. However, alongside its benefits, TMZ is associated with significant side effects such as myelotoxicity, ulcers, nausea, vomiting, fatigue, and harmful DNA damage. Moreover, resistance to this drug is commonplace in GBM patients [198]. To enhance the effectiveness of initial GBM treatment, it may be worthwhile to investigate a more potent combination regimen [199]. The presented findings in this review pertain to the use of therapeutic methods and chemotherapeutic agents in the treatment of GBM. These results reveal that a substantial majority of studies (60.2%) advocated for a comprehensive therapeutic approach, while a slightly smaller portion (39.8%) focused on single-strategy treatments.
In terms of mechanistic categorization, 41.6% of studies fell into the PKP mechanism, 18.1% were classified as CCRM, 19.9% were designated as Microenvironmental Targets (MT), 4.2% were categorized as IT, and 16.3% were attributed to OM. Currently, the predominant chemotherapeutic compounds employed in the management of GBM are small molecules designed to intervene with specific aberrant signaling pathways within GBM cells, including receptor tyrosine kinase activity, the PI3K/AKT/mTOR cascade, the cellular response to DNA damage, TP53 function, and inhibitors of the cell cycle [200]. The disrupted regulation of numerous signaling pathways in GBM serves as the primary catalyst for the uncontrolled proliferation of both initial and recurring tumors. This underscores the critical importance of identifying the optimal combination of targeted therapeutics for GBM treatment. It is noteworthy that most GBMs do not exhibit a singularly aberrant pathway, rendering them less amenable to targeted therapeutic approaches. This is exemplified by the lack of success observed in late-stage clinical trials of various targeted agents for GBM [200]. The most recent molecular and genomic evidence highlights the presence of diverse genetic and molecular characteristics within and between tumors in GBM [200]. This leads to variations in the expression of therapeutic targets across different tumors and regions within a single tumor. This heterogeneity in GBM may elucidate the lack of success observed in targeted treatments aimed specifically at tumor biomarkers, including drugs like cetuximab, gefitinib, erlotinib (targeting EGFR), bevacizumab (targeting VEGF), and cilengitide (targeting integrin). It is recognized as the underlying cause of resistance to these therapies.
Temozolomide, akin to dacarbazine, is an imidazotetrazine derivative. It stands out as one of the rare drugs capable of exerting its effects within the central nervous system [201]. In the treatment of GBM, TMZ’s primary mechanism of action involves methylating the O6 positions of guanine. This modification hinders DNA replication during cellular proliferation and triggers programmed cell death, or apoptosis. Following its approval by the FDA in 2005 [202], TMZ, when administered alongside surgery and radiotherapy, has solidified its position as the established and pivotal standard of care for individuals with GBM. This marked a significant milestone, as it rose to prominence as the leading initial chemotherapeutic option for GBM treatment. Findings from this study revealed that TMZ was utilized in 28% of the studies as part of a treatment regimen in conjunction with other molecular targeted therapy drugs.
In contemporary practice, TMZ is administered alongside radiotherapy as the primary treatment for GBM and as a secondary option for other malignant gliomas in cases of relapse. However, the utilization of radiotherapy and chemotherapy comes with certain limitations, and the emergence of tumor drug resistance is a common outcome. Beyond the known factors contributing to TMZ resistance, such as uncontrolled signaling pathways, DNA repair mechanisms, the persistence of cancer stem cell (CSC) subpopulations, and the activation of self-defense mechanisms [203], it is worth delving into alternative approaches that may hold promise in addressing these challenges. Mesenchymal stem cells (MSCs) are gaining traction as a therapeutic avenue in the field of cancer immunotherapy [204]. The development of chemoresistance to TMZ may arise from genetic and epigenetic alterations induced by the drugs in cancerous cells. These changes encompass the induction and selection of genes that confer a survival advantage, or the preferential selection of pre-existing cell clones with resistance. Potential alterations encompass an upsurge in drug efflux facilitated by active membrane pumps, deactivation of intracellular drugs, heightened resilience to DNA damage, and modifications in genes linked to apoptosis. These adjustments hold substantial importance in extensively heterogeneous tumors such as GBM, as treatment interventions may inadvertently promote the survival of resistant cells, potentially culminating in tumor recurrence. Nevertheless, there is evidence suggesting that combining TMZ with other molecular targeted therapies has demonstrated an improved survival rate [199].
The acquired resistance pathways in GBM involve the Src tyrosine kinase pathway, which regulates actin dynamics and the invasion of malignant glial cells [205]. Src transmits signals from the extracellular matrix and interacts with various intracellular proteins, including integrins, Eph kinase, and growth factor receptors. GBM cells exhibit higher Src tyrosine kinase activity compared to normal brain cells [206,207]. In a study by Eom et al. [208], an Src tyrosine kinase inhibitor (PP2) was examined in combination with TMZ. The findings indicated that PP2 enhanced the in vitro radiosensitivity of malignant glioma cells and inhibited invasion and migration. However, in in vivo trials, the combination led to a statistically non-significant decrease in tumor volume. On a different note, other authors [79] discovered that suppressing Src family kinase signaling could impede bevacizumab-induced GBM cell invasion, suggesting a potential strategy for overcoming GBM treatment resistance. Certain studies propose that miRNA may serve as a predictive marker for the response to TMZ treatment in GBM patients. Certain researchers propose that when combined with specific drugs, standard-dose TMZ chemotherapy may lead to an improvement in progression-free survival. As an illustration, the administration of trans sodium crocetinate (TSC), a substance known for its ability to enhance oxygen delivery, alongside standard-dose TMZ and radiotherapy proved beneficial for 59 GBM patients in a phase I/II trial conducted by Gainer et al. [209]. The outcomes revealed that 36% of patients who received TSC were still alive after two years, in contrast to 27–30% of those who underwent the standard treatment. The authors proposed that administering TSC in conjunction with the standard treatment conferred an advantage in GBM therapy [209]. According to Vengoji et al. [158] the combination of afatinib with TMZ significantly postpones the progression of GBM. In a study by Sang-Soo et al. [93], a nanocomplex targeting MALAT1 was examined, and the authors suggested that silencing MALAT1, combined with TMZ, also provided a survival benefit. Other combinations involving TMZ, such as its combination with dual mTOR1/2 inhibition, have proven to be effective therapies for resistant GBM. Similarly, the combination of Metformin and sorafenib has yielded the same effect [210,211].
In this review, the most frequently targeted molecular entity was identified as the EGFR, accounting for a substantial proportion. Following closely were the mTOR, VEGF, and MEK. PI3K and BRAF exhibited an equal number of occurrences. EGFR amplification and mutation are the most prevailing genetic alterations, occurring in more than 50% of GBM [200,212]. EGFRvIII is the most common and highly oncogenic EGFR mutant in GBM, and imaging the status of EGFRvIII could be of great value in GBM treatment [212]. VEGF induces an augmentation in the vascularization of GBM and is categorized within the ET group, despite subsequently activating the PKP mechanism, akin to EGFR. VEGFR and PDGFR are overexpressed, amplified, and/or mutated in GBM, leading to uncontrolled cell proliferation, angiogenesis, migration, survival, and differentiation [213].
Different cell lines are widely used in scientific research as valuable tools for studying various biological processes and diseases, including GBM. In this systematic review, human GBM cell lines, specifically HCC, were the most commonly utilized research samples, comprising 52.52% of the included laboratory studies. The prominent use of cell lines in GBM research highlights their importance in providing a controlled and reproducible model system for investigating the molecular mechanisms underlying GBM development and testing potential therapeutic interventions. These cell lines, such as U87, U251, and T98G, have been extensively employed in numerous investigations, demonstrating their relevance and utility in advancing our understanding of GBM biology [63,69]. In vitro studies using GBM cell lines have contributed significantly to the identification and evaluation of potential drugs for GBM treatment. Within the systematic review, 25.3% of the included studies focused on in vitro research. Notably, the U87 cell line emerged as the most frequently encountered cell line in these studies, appearing in 40.5% of the investigations. This consistent utilization of the U87 cell line underscores its importance as a representative model for studying GBM in vitro [179].
4.3. Effectiveness of Targeted Therapy in GBM Treatment
Several drugs have shown promise in the context of GBM target therapy treatment, as indicated by various outcomes, including survival time, mPFS, PFS-6, and OS data from Table 2. For instance, AZD1775 demonstrated therapeutic concentrations and good tolerability [141]. Alectinib, Palbociclib, Temsirolimus, Idasanutlin, and Vismodegib were evaluated in the NCT Neuro Master Match trial, which utilizes GBM molecular signatures for treatment [169]. However, Imatinib did not show a significant effect on GBM, with an mPFS of 2.8 months in Arm A and 2.1 months in Arm B, along with corresponding mOS values of 5.0 and 6.5 months [145]. Nimotuzumab, when combined with temozolomide and radiation therapy, exhibited promising results, with an mOS of 15.9 months and an mPFS of 10 months [165]. Bevacizumab, used in various regimens, demonstrated diverse outcomes, from activity and tolerance [29,37,54] to serving as a salvage regimen for recurrent GBM [29]. Regorafenib presented a significant survival benefit in recurrent GBM, with an mOS of 24.8 months [109]. Conversely, pembrolizumab, with or without bevacizumab, did not prove effective, resulting in a PFS-6 of 26.0% and an mOS of 8.8 months with bevacizumab, and a PFS-6 of 6.7% and an mOS of 10.3 months without bevacizumab [124]. These findings not only highlight the potential of various therapies but also emphasize the importance of assessing survival times and progression-free intervals in evaluating treatment efficacy for GBM patients.
4.4. Promising Targeted Therapies for GBM Treatment
Various targeted therapies demonstrate promising GBM treatment potential. The Anti-GD2 antibody [36] specifically targets O-acetyl GD2 ganglioside, effectively preventing glioma proliferation. AMB4269951 [152] shows antitumor effects by targeting CTL1 and significantly improving mouse survival. rSLURP-1 [139] effectively inhibits GBM growth by targeting α7 nAChR. QLT0276 in DMSO [95] inhibits integrin-linked kinase (ILK), leading to decreased glioma cell invasiveness and down-regulated proliferation and invasion. AA1881 [143] targets BRAF, CRAF, and VEGFR, significantly increasing mouse survival. EF2-siRNA [175], targeting EF2-kinase, demonstrates increased survival in rats and inhibits cell migration. Furthermore, boronated EGFR MAB + Cetuximab [176] significantly enhances survival by targeting EGFR and EGFRvIII tumors. The combination of Rapamycin + PD184352 [128] offers promise in CDK4-dysregulated tumors by providing complete inhibition of DNA synthesis and pRb phosphorylation. Tamoxifen [61] induces apoptosis and presents potential therapeutic targets for GBM. PX-866 [96] inhibits PI3K/Akt and increases survival in mice. NVP-AEW541 + Dasatinib [151] through dual IGF1R and Src inhibition increases apoptosis in glioma cells. Sorafenib [132] exhibits potent in vivo and in vitro anti-GBM activity. Plumbagin [120] effectively inhibits glioma proliferation and induces apoptosis, especially when combined with radiation. T7-modified liposomes [97] effectively penetrate the blood-brain barrier (BBB). The combination of SB203580 + Rapamycin [51] significantly inhibits tumor growth by targeting SAPK2/p38 and mTORC1. Anti-bFGF siRNA [106] holds potential for glioma treatment by inducing apoptosis. Lenvatinib + Crenolanib + Abemaciclib + Palbociclib [71], targeting PDGFRα and CDK4/6 signaling, offers a potential GBM treatment. DMC nanoparticle-mediated EZH2-siRNA [161] decreases tumor size. Targeting ID2 with anti-ID2 siRNA [180] increases sensitivity and decreases glioma apoptosis. Finally, F2 procyanidins [146] downregulate FPR and exert cytotoxic effects in mouse models.
4.5. Advantages and Disadvantages in Molecular Targeted Therapy of GBM
Precision-targeted therapies are engineered to selectively target cancer cells, potentially mitigating the adverse effects of treatment [214]. This focused approach enhances therapeutic efficacy while minimizing collateral damage to healthy tissues. Furthermore, targeted therapies can synergize with complementary treatments like chemotherapy and radiation therapy, yielding improved outcomes for patients [215]. By tailoring these therapies to the specific genetic profile of the tumor, treatment effectiveness is optimized. Additionally, precise administration through controlled targeting enhances drug delivery to the tumor site, augmenting treatment efficacy while reducing systemic toxicity [216]. Also, by accumulating comprehensive data from large-scale studies on molecular targets, researchers can harness the power of artificial intelligence to develop predictive algorithms for patient outcomes and prognosis. This emerging field holds immense promise and aligns with the ongoing advancements in neurosurgery and medical technology [217].
While targeted therapies demonstrate remarkable efficacy against specific molecular targets, the emergence of resistance in tumors over time poses a significant challenge. These therapies may not be universally effective across all subtypes of GBM due to the tumor’s intrinsic heterogeneity, making the identification of reliable targets a complex endeavor [216,218]. Moreover, the cost associated with targeted therapies, coupled with potential insurance coverage limitations, may restrict patient access to these advanced treatments, especially in lower-middle-income countries. It is essential to note that, like many treatments, targeted therapies can also induce side effects, such as skin rash, diarrhea, and fatigue, which may impact the overall quality of life for patients undergoing treatment.
4.6. Limitations of the Study
The limitations of this systematic review primarily revolve around its inclusion criteria, which restricted the analysis to studies published in English, potentially excluding relevant research in other languages. Additionally, the presence of heterogeneity among the sampled studies, such as variations in patient populations, treatment approaches, and study designs, may introduce some degree of bias and make it challenging to draw uniform conclusions.
5. Conclusions
In conclusion, this systematic review provides insights into the global and research trends of GBM and the current state of targeted molecular therapy in GBM treatment. The increasing incidence of GBM, particularly in developed regions, presents a substantial healthcare and economic burden. The distribution of clinical and laboratory studies in this review reflects the challenges associated with limited patient cohorts and abbreviated survival durations, which are particularly pronounced in low- and middle-income countries. The standard of care for GBM primarily involves maximal surgical removal of the tumor and the use of TMZ. However, resistance to TMZ is common, and exploring more potent combination regimens is crucial for enhancing GBM treatment. The findings reveal that most studies advocate for a comprehensive therapeutic approach, and the mechanistic categorization shows the importance of targeting multiple pathways. The effectiveness of targeted therapy in GBM treatment varies, and promising therapies target various molecular entities. Precision-targeted therapies offer advantages in terms of efficacy and reduced collateral damage, but resistance, tumor heterogeneity, cost, and potential side effects remain significant challenges.
Author Contributions
Conceptualization, E.B., R.P., H.B. and M.P.; methodology, E.B., R.P., L.Č. and R.S.; software, E.B.; formal analysis, L.T.L., S.K.V. and E.S.; investigation, E.B.; resources, B.J.; data curation, E.B., R.P., A.Č., F.J.-B. and A.J.; writing—original draft preparation, E.B., A.Č., R.P., H.B. and L.T.L.; writing—review and editing, E.B., H.B., R.S., E.S., A.J. and M.P.; visualization, E.B.; supervision, H.B., R.S., E.S., F.J.-B. and M.P.; project administration, E.B., and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
| Search Strategy | |
| Search | (Glioblastoma multiforme OR GBM) AND (Molecular targeted therapy) |
| Filter | from 2000 to 2022 |
| Search details | ((“glioblastoma”[MeSH Terms] OR “glioblastoma”[All Fields] OR (“glioblastoma”[All Fields] AND “multiforme”[All Fields]) OR “glioblastoma multiforme”[All Fields] OR “GBM”[All Fields]) AND (“molecular targeted therapy”[MeSH Terms] OR (“molecular”[All Fields] AND “targeted”[All Fields] AND “therapy”[All Fields]) OR “molecular targeted therapy”[All Fields] OR (“protein kinase inhibitors”[Pharmacological Action] OR “protein kinase inhibitors”[MeSH Terms] OR (“protein”[All Fields] AND “kinase”[All Fields] AND “inhibitors”[All Fields]) OR “protein kinase inhibitors”[All Fields]) OR (“immunotherapy”[MeSH Terms] OR “immunotherapy”[All Fields] OR “immunotherapies”[All Fields] OR “immunotherapy s”[All Fields]) OR (“apoptosis”[MeSH Terms] OR “apoptosis”[All Fields]))) AND (2000:2022[pdat]) |
References
- Kanderi, T.; Gupta, V. Glioblastoma Multiforme. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Stoyanov, G.S.; Dzhenkov, D.L. On the Concepts and History of Glioblastoma Multiforme—Morphology, Genetics and Epigenetics. Folia Med. 2018, 60, 48–66. [Google Scholar] [CrossRef]
- McCutcheon, I.E.; Preul, M.C. Historical Perspective on Surgery and Survival with Glioblastoma: How Far Have We Come? World Neurosurg. 2021, 149, 148–168. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, A.; Proietti, G.; Sica, G.; Scicchitano, B.M. Pathological and Molecular Features of Glioblastoma and Its Peritumoral Tissue. Cancers 2019, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.-d.-M.; Bonavia, R.; Seoane, J. Glioblastoma Multiforme: A Look Inside Its Heterogeneous Nature. Cancers 2014, 6, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, B.W. Development of the WHO classification of tumors of the central nervous system: A historical perspective. Brain Pathol. 2009, 19, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, G.S.; Lyutfi, E.; Georgieva, R.; Georgiev, R.; Dzhenkov, D.L.; Petkova, L.; Ivanov, B.D.; Kaprelyan, A.; Ghenev, P. Reclassification of Glioblastoma Multiforme According to the 2021 World Health Organization Classification of Central Nervous System Tumors: A Single Institution Report and Practical Significance. Cureus 2022, 14, e21822. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Jain, K.K. A Critical Overview of Targeted Therapies for Glioblastoma. Front. Oncol. 2018, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- McBain, C.; Lawrie, T.A.; Rogozińska, E.; Kernohan, A.; Robinson, T.; Jefferies, S. Treatment options for progression or recurrence of glioblastoma: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 5, Cd013579. [Google Scholar] [CrossRef]
- Mofatteh, M.; Mashayekhi, M.S.; Arfaie, S.; Chen, Y.; Malhotra, A.K.; Alvi, M.A.; Sader, N.; Antonick, V.; Fatehi Hassanabad, M.; Mansouri, A.; et al. Suicidal Ideation and Attempts in Brain Tumor Patients and Survivors: A Systematic Review. Neuro-Oncol. Adv. 2023, 5, vdad058. [Google Scholar] [CrossRef]
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current Opinion on Molecular Characterization for GBM Classification in Guiding Clinical Diagnosis, Prognosis, and Therapy. Front. Mol. Biosci. 2020, 7, 562798. [Google Scholar] [CrossRef]
- Clark, K.; Voronovich, Z.; Horbinski, C. How molecular testing can help (and hurt) in the workup of gliomas. Am. J. Clin. Pathol. 2013, 139, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Trembath, D.G. Chapter 26—Molecular Testing for Glioblastoma. In Diagnostic Molecular Pathology; Coleman, W.B., Tsongalis, G.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 339–347. [Google Scholar]
- Hersh, A.M.; Gaitsch, H.; Alomari, S.; Lubelski, D.; Tyler, B.M. Molecular Pathways and Genomic Landscape of Glioblastoma Stem Cells: Opportunities for Targeted Therapy. Cancers 2022, 14, 3743. [Google Scholar] [CrossRef] [PubMed]
- Di Cintio, F.; Dal Bo, M.; Baboci, L.; De Mattia, E.; Polano, M.; Toffoli, G. The Molecular and Microenvironmental Landscape of Glioblastomas: Implications for the Novel Treatment Choices. Front. Neurosci. 2020, 14, 603647. [Google Scholar] [CrossRef] [PubMed]
- Wagle, N.; Nguyen, M.; Carrillo, J.; Truong, J.; Dobrawa, L.; Kesari, S. Characterization of molecular pathways for targeting therapy in glioblastoma. Chin. Clin. Oncol. 2020, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahim, S.A.; Dirkse, A.; Oudin, A.; Schuster, A.; Bohler, J.; Barthelemy, V.; Muller, A.; Vallar, L.; Janji, B.; Golebiewska, A. Regulation of hypoxia-induced autophagy in glioblastoma involves ATG9A. Br. J. Cancer 2017, 117, 813–825. [Google Scholar] [CrossRef]
- Aldea, M.D.; Petrushev, B.; Soritau, O.; Tomuleasa, C.I.; Berindan-Neagoe, I.; Filip, A.G.; Chereches, G.; Cenariu, M.; Craciun, L.; Tatomir, C.; et al. Metformin plus sorafenib highly impacts temozolomide resistant glioblastoma stem-like cells. J. Buon 2014, 19, 502–511. [Google Scholar]
- Angara, K.; Rashid, M.H.; Shankar, A.; Ara, R.; Iskander, A.; Borin, T.F.; Jain, M.; Achyut, B.R.; Arbab, A.S. Vascular mimicry in glioblastoma following anti-angiogenic and anti-20-HETE therapies. Histol. Histopathol. 2017, 32, 917. [Google Scholar]
- Anghileri, E.; Di Ianni, N.; Paterra, R.; Langella, T.; Zhao, J.; Eoli, M.; Patanè, M.; Pollo, B.; Cuccarini, V.; Iavarone, A. High tumor mutational burden and T-cell activation are associated with long-term response to anti-PD1 therapy in Lynch syndrome recurrent glioblastoma patient. Cancer Immunol. Immunother. 2021, 70, 831–842. [Google Scholar] [CrossRef]
- Arcella, A.; Biagioni, F.; Antonietta Oliva, M.; Bucci, D.; Frati, A.; Esposito, V.; Cantore, G.; Giangaspero, F.; Fornai, F. Rapamycin inhibits the growth of glioblastoma. Brain Res. 2013, 1495, 37–51. [Google Scholar] [CrossRef]
- Ariey-Bonnet, J.; Carrasco, K.; Le Grand, M.; Hoffer, L.; Betzi, S.; Feracci, M.; Tsvetkov, P.; Devred, F.; Collette, Y.; Morelli, X. In silico molecular target prediction unveils mebendazole as a potent MAPK14 inhibitor. Mol. Oncol. 2020, 14, 3083–3099. [Google Scholar] [CrossRef]
- Badruddoja, M.A.; Pazzi, M.; Sanan, A.; Schroeder, K.; Kuzma, K.; Norton, T.; Scully, T.; Mahadevan, D.; Ahmadi, M.M. Phase II study of bi-weekly temozolomide plus bevacizumab for adult patients with recurrent glioblastoma. Cancer Chemother. Pharmacol. 2017, 80, 715–721. [Google Scholar] [CrossRef]
- Bagca, B.G.; Ozates, N.P.; Asik, A.; Caglar, H.O.; Gunduz, C.; Avci, C.B. Temozolomide treatment combined with AZD3463 shows synergistic effect in glioblastoma cells. Biochem. Biophys. Res. Commun. 2020, 533, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Bähr, O.; Gross, S.; Harter, P.N.; Kirches, E.; Mawrin, C.; Steinbach, J.P.; Mittelbronn, M. ASA404, a vascular disrupting agent, as an experimental treatment approach for brain tumors. Oncol. Lett. 2017, 14, 5443–5451. [Google Scholar]
- Barbarisi, M.; Iaffaioli, R.V.; Armenia, E.; Schiavo, L.; De Sena, G.; Tafuto, S.; Barbarisi, A.; Quagliariello, V. Novel nanohydrogel of hyaluronic acid loaded with quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell. Physiol. 2018, 233, 6550–6564. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Sengupta, R.; Warrington, N.M.; Smith, E.; Wen, P.Y.; Brekken, R.A.; Romagnoli, B.; Douglas, G.; Chevalier, E.; Bauer, M.P. Combined VEGF and CXCR4 antagonism targets the GBM stem cell population and synergistically improves survival in an intracranial mouse model of glioblastoma. Oncotarget 2014, 5, 9811. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.; Hambardzumyan, D.; Penate-Medina, O.; Veach, D.R.; Pillarsetty, N.; Smith-Jones, P.; Phillips, E.; Ozawa, T.; Zanzonico, P.B.; Longo, V. Fluorine-labeled dasatinib nanoformulations as targeted molecular imaging probes in a PDGFB-driven murine glioblastoma model. Neoplasia 2012, 14, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Blanco, V.M.; Chu, Z.; Vallabhapurapu, S.D.; Sulaiman, M.K.; Kendler, A.; Rixe, O.; Warnick, R.E.; Franco, R.S.; Qi, X. Phosphatidylserine-selective targeting and anticancer effects of SapC-DOPS nanovesicles on brain tumors. Oncotarget 2014, 5, 7105. [Google Scholar] [CrossRef]
- Blank, M.; Weinschenk, T.; Priemer, M.; Schluesener, H. Systematic Evolution of a DNA Aptamer Binding to Rat Brain Tumor Microvessels: SELECTIVE TARGETING OF ENDOTHELIAL REGULATORY PROTEIN PIGPEN. J. Biol. Chem. 2001, 276, 16464–16468. [Google Scholar] [CrossRef]
- Brown, N.; McBain, C.; Nash, S.; Hopkins, K.; Sanghera, P.; Saran, F.; Phillips, M.; Dungey, F.; Clifton-Hadley, L.; Wanek, K. Multi-center randomized phase II study comparing cediranib plus gefitinib with cediranib plus placebo in subjects with recurrent/progressive glioblastoma. PLoS ONE 2016, 11, e0156369. [Google Scholar] [CrossRef] [PubMed]
- Butowski, N.; Chang, S.M.; Lamborn, K.R.; Polley, M.Y.; Parvataneni, R.; Hristova-Kazmierski, M.; Musib, L.; Nicol, S.J.; Thornton, D.E.; Prados, M.D. Enzastaurin plus temozolomide with radiation therapy in glioblastoma multiforme: A phase I study. Neuro-Oncology 2010, 12, 608–613. [Google Scholar] [CrossRef]
- Bychkov, M.; Shulepko, M.; Osmakov, D.; Andreev, Y.; Sudarikova, A.; Vasileva, V.; Pavlyukov, M.S.; Latyshev, Y.A.; Potapov, A.A.; Kirpichnikov, M. Mambalgin-2 induces cell cycle arrest and apoptosis in glioma cells via interaction with ASIC1a. Cancers 2020, 12, 1837. [Google Scholar] [CrossRef]
- Camorani, S.; Crescenzi, E.; Colecchia, D.; Carpentieri, A.; Amoresano, A.; Fedele, M.; Chiariello, M.; Cerchia, L. Aptamer targeting EGFRvIII mutant hampers its constitutive autophosphorylation and affects migration, invasion and proliferation of glioblastoma cells. Oncotarget 2015, 6, 37570. [Google Scholar] [CrossRef]
- Caruana, B.T.; Skoric, A.; Brown, A.J.; Lutze-Mann, L.H. Site-1 protease, a novel metabolic target for glioblastoma. Biochem. Biophys. Res. Commun. 2017, 490, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chen, P.-Y.; Lin, Y.-Y.; Feng, L.-Y.; Chen, S.-H.; Chen, C.-Y.; Huang, Y.-C.; Huang, C.-Y.; Jung, S.-M.; Chen, L.Y. Suppression of tumor growth via IGFBP3 depletion as a potential treatment in glioma. J. Neurosurg. 2019, 132, 168–179. [Google Scholar] [CrossRef]
- Chen, L.; Miao, W.; Tang, X.; Zhang, H.; Wang, S.; Luo, F.; Yan, J. Inhibitory effect of neuropilin-1 monoclonal antibody (NRP-1 MAb) on glioma tumor in mice. J. Biomed. Nanotechnol. 2013, 9, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, J.; Zhu, Y.; Li, Y.; Wang, Y.; Chen, H.; Wang, J.; Li, X.; Liu, Y.; Li, B. CD163, a novel therapeutic target, regulates the proliferation and stemness of glioma cells via casein kinase 2. Oncogene 2019, 38, 1183–1199. [Google Scholar] [CrossRef]
- Chen, W.; Wu, M.; Cui, S.-T.; Zheng, Y.; Liu, Z.; Luo, L.-S. CircRNA Circ-ITCH inhibits the proliferation and invasion of glioma cells through targeting the miR-106a-5p/SASH1 Axis. Cell Transplant. 2021, 30, 0963689720983785. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, X.; Georgakilas, A.G.; Chen, P.; Hu, H.; Yang, Y.; Tian, S.; Xia, L.; Zhang, J.; Cai, X.; et al. Tetramethylpyrazine (TMP) protects cerebral neurocytes and inhibits glioma by down regulating chemokine receptor CXCR4 expression. Cancer Lett. 2013, 336, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ren, Z.; Liu, Z.; Sun, X.; Qian, R.; Cao, C.; Liu, B.; Wang, J.; Wang, H.; Guo, Y. Cysteine cathepsin C: A novel potential biomarker for the diagnosis and prognosis of glioma. Cancer Cell Int. 2022, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, P.; Won, M.; Wen, P.Y.; Rojiani, A.M.; Wendland, M.; Dipetrillo, T.A.; Corn, B.W.; Mehta, M.P. RTOG 0913: A phase 1 study of daily everolimus (RAD001) in combination with radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, M.J.; Bu, Y.; Munich, S.A.; Teegarden, P.; Smolinski, M.P.; Clements, J.L.; Lau, J.Y.; Hangauer, D.G.; Fenstermaker, R.A. KX2-361: A novel orally bioavailable small molecule dual Src/tubulin inhibitor that provides long term survival in a murine model of glioblastoma. J. Neuro-Oncol. 2018, 140, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.L.; Molinaro, A.M.; Phillips, J.J.; Butowski, N.A.; Chang, S.M.; Perry, A.; Costello, J.F.; DeSilva, A.A.; Rabbitt, J.E.; Prados, M.D. A single-institution phase II trial of radiation, temozolomide, erlotinib, and bevacizumab for initial treatment of glioblastoma. Neuro-Oncology 2014, 16, 984–990. [Google Scholar] [CrossRef]
- Cloninger, C.; Bernath, A.; Bashir, T.; Holmes, B.; Artinian, N.; Ruegg, T.; Anderson, L.; Masri, J.; Lichtenstein, A.; Gera, J. Inhibition of SAPK2/p38 enhances sensitivity to mTORC1 inhibition by blocking IRES-mediated translation initiation in glioblastoma. Mol. Cancer Ther. 2011, 10, 2244–2256. [Google Scholar] [CrossRef]
- Colen, C.B.; Shen, Y.; Ghoddoussi, F.; Yu, P.; Francis, T.B.; Koch, B.J.; Monterey, M.D.; Galloway, M.P.; Sloan, A.E.; Mathupala, S.P. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: An in vivo study. Neoplasia 2011, 13, 620–632. [Google Scholar] [CrossRef]
- D’Alessandris, Q.G.; Montano, N.; Cenci, T.; Martini, M.; Lauretti, L.; Bianchi, F.; Larocca, L.M.; Maira, G.; Fernandez, E.; Pallini, R. Targeted therapy with bevacizumab and erlotinib tailored to the molecular profile of patients with recurrent glioblastoma. Preliminary experience. Acta Neurochir. 2013, 155, 33–40. [Google Scholar] [CrossRef]
- Desjardins, A.; Reardon, D.A.; Coan, A.; Marcello, J.; Herndon Ii, J.E.; Bailey, L.; Peters, K.B.; Friedman, H.S.; Vredenburgh, J.J. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer 2012, 118, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Reardon, D.A.; Peters, K.B.; Threatt, S.; Coan, A.D.; Herndon, J.E., 2nd; Friedman, A.H.; Friedman, H.S.; Vredenburgh, J.J. A phase I trial of the farnesyl transferase inhibitor, SCH 66336, with temozolomide for patients with malignant glioma. J. Neurooncol. 2011, 105, 601–606. [Google Scholar] [CrossRef]
- Di Stefano, A.L.; Fucci, A.; Frattini, V.; Labussiere, M.; Mokhtari, K.; Zoppoli, P.; Marie, Y.; Bruno, A.; Boisselier, B.; Giry, M. Detection, characterization, and inhibition of FGFR–TACC fusions in IDH wild-type glioma. Clin. Cancer Res. 2015, 21, 3307–3317. [Google Scholar] [CrossRef]
- Dominguez, C.L.; Floyd, D.H.; Xiao, A.; Mullins, G.R.; Kefas, B.A.; Xin, W.; Yacur, M.N.; Abounader, R.; Lee, J.K.; Wilson, G.M.; et al. Diacylglycerol Kinase α Is a Critical Signaling Node and Novel Therapeutic Target in Glioblastoma and Other Cancers. Cancer Discov. 2013, 3, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhou, J.-R.; Wang, D.-L.; Gong, K.; Zhang, Q.-J. Vitamin K1 enhances sorafenib-induced growth inhibition and apoptosis of human malignant glioma cells by blocking the Raf/MEK/ERK pathway. World J. Surg. Oncol. 2012, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Emlet, D.R.; Gupta, P.; Holgado-Madruga, M.; Del Vecchio, C.A.; Mitra, S.S.; Han, S.Y.; Li, G.; Jensen, K.C.; Vogel, H.; Xu, L.W.; et al. Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer Res. 2014, 74, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.J.; Matuszkiewicz, J.; Balakrishna, D.; Pandya, S.; Hixon, M.S.; Kamran, R.; Chu, S.; Lawson, J.D.; Okada, K.; Hori, A. MET tyrosine kinase inhibition enhances the antitumor efficacy of an HGF antibody. Mol. Cancer Ther. 2017, 16, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, J.; Ding, Y.; Xie, F.; Shen, X. Tamoxifen-induced apoptosis of rat C6 glioma cells via PI3K/Akt, JNK and ERK activation. Oncol. Rep. 2010, 24, 1561–1567. [Google Scholar] [CrossRef]
- Fleurence, J.; Cochonneau, D.; Fougeray, S.; Oliver, L.; Geraldo, F.; Terme, M.; Dorvillius, M.; Loussouarn, D.; Vallette, F.; Paris, F. Targeting and killing glioblastoma with monoclonal antibody to O-acetyl GD2 ganglioside. Oncotarget 2016, 7, 41172. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.G.; Moretti, I.F.; Marie, S.K. Mitochondria transcription factor a: A putative target for the effect of melatonin on U87MG malignant glioma cell line. Molecules 2018, 23, 1129. [Google Scholar] [CrossRef]
- Ge, Y.-F.; Sun, J.; Jin, C.-J.; Cao, B.-Q.; Jiang, Z.-F.; Shao, J.-F. AntagomiR-27a targets FOXO3a in glioblastoma and suppresses U87 cell growth in vitro and in vivo. Asian Pac. J. Cancer Prev. 2013, 14, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef]
- Genoud, V.; Espinoza, F.I.; Marinari, E.; Rochemont, V.; Dietrich, P.-Y.; McSheehy, P.; Bachmann, F.; Lane, H.A.; Walker, P.R. Treating ICB-resistant glioma with anti-CD40 and mitotic spindle checkpoint controller BAL101553 (lisavanbulin). JCI Insight 2021, 6, e142980. [Google Scholar] [CrossRef]
- Goswami, S.; Walle, T.; Cornish, A.E.; Basu, S.; Anandhan, S.; Fernandez, I.; Vence, L.; Blando, J.; Zhao, H.; Yadav, S.S. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat. Med. 2020, 26, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Grinshtein, N.; Rioseco, C.C.; Marcellus, R.; Uehling, D.; Aman, A.; Lun, X.; Muto, O.; Podmore, L.; Lever, J.; Shen, Y. Small molecule epigenetic screen identifies novel EZH2 and HDAC inhibitors that target glioblastoma brain tumor-initiating cells. Oncotarget 2016, 7, 59360. [Google Scholar] [CrossRef]
- Grossman, R.; Tyler, B.; Rudek, M.A.; Kim, E.; Zadnik, P.; Khan, U.; Blakeley, J.O.; Pathak, A.P.; Brem, H. Microdialysis measurement of intratumoral temozolomide concentration after cediranib, a pan-VEGF receptor tyrosine kinase inhibitor, in a U87 glioma model. Cancer Chemother. Pharmacol. 2013, 72, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, C.; Wang, X.; Ma, G.; Li, Y.; Cui, L.; Chen, Y.; Zhao, B.; Li, K. Efficient inhibition of human glioma development by RNA interference-mediated silencing of PAK5. Int. J. Biol. Sci. 2015, 11, 230. [Google Scholar] [CrossRef][Green Version]
- Guo, L.; Fan, L.; Pang, Z.; Ren, J.; Ren, Y.; Li, J.; Chen, J.; Wen, Z.; Jiang, X. TRAIL and doxorubicin combination enhances anti-glioblastoma effect based on passive tumor targeting of liposomes. J. Control. Release 2011, 154, 93–102. [Google Scholar] [CrossRef]
- Harford-Wright, E.; Andre-Gregoire, G.; Jacobs, K.A.; Treps, L.; Le Gonidec, S.; Leclair, H.M.; Gonzalez-Diest, S.; Roux, Q.; Guillonneau, F.; Loussouarn, D. Pharmacological targeting of apelin impairs glioblastoma growth. Brain 2017, 140, 2939–2954. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Tsuboi, A.; Kagawa, N.; Chiba, Y.; Izumoto, S.; Kinoshita, M.; Kijima, N.; Oka, Y.; Morimoto, S.; Nakajima, H. Wilms tumor 1 peptide vaccination combined with temozolomide against newly diagnosed glioblastoma: Safety and impact on immunological response. Cancer Immunol. Immunother. 2015, 64, 707–716. [Google Scholar] [CrossRef]
- Hasselbalch, B.; Eriksen, J.G.; Broholm, H.; Christensen, I.J.; Grunnet, K.; Horsman, M.R.; Poulsen, H.S.; Stockhausen, M.T.; Lassen, U. Prospective evaluation of angiogenic, hypoxic and EGFR-related biomarkers in recurrent glioblastoma multiforme treated with cetuximab, bevacizumab and irinotecan. APMIS 2010, 118, 585–594. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Jabouille, A.; Steri, V.; Johansson-Percival, A.; Michael, I.P.; Kotamraju, V.R.; Junckerstorff, R.; Nowak, A.K.; Hamzah, J.; Lee, G. Vascular targeting of LIGHT normalizes blood vessels in primary brain cancer and induces intratumoural high endothelial venules. J. Pathol. 2018, 245, 209–221. [Google Scholar] [CrossRef]
- He, H.; Yao, M.; Zhang, W.; Tao, B.; Liu, F.; Li, S.; Dong, Y.; Zhang, C.; Meng, Y.; Li, Y. MEK2 is a prognostic marker and potential chemo-sensitizing target for glioma patients undergoing temozolomide treatment. Cell. Mol. Immunol. 2016, 13, 658–668. [Google Scholar] [CrossRef]
- Hong, H.; Stastny, M.; Brown, C.; Chang, W.-C.; Ostberg, J.R.; Forman, S.J.; Jensen, M.C. Diverse solid tumors expressing a restricted epitope of L1-CAM can be targeted by chimeric antigen receptor redirected T lymphocytes. J. Immunother. 2014, 37, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy 2021, 17, 3592–3606. [Google Scholar] [CrossRef]
- Huveldt, D.; Lewis-Tuffin, L.J.; Carlson, B.L.; Schroeder, M.A.; Rodriguez, F.; Giannini, C.; Galanis, E.; Sarkaria, J.N.; Anastasiadis, P.Z. Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PLoS ONE 2013, 8, e56505. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, T.; Teduka, K.; Yamamoto, T.; Kawahara, K.; Matsuda, Y.; Naito, Z. Neuroepithelial stem cell marker nestin regulates the migration, invasion and growth of human gliomas. Oncol. Rep. 2011, 26, 91–99. [Google Scholar] [CrossRef]
- Jaszberenyi, M.; Schally, A.V.; Block, N.L.; Zarandi, M.; Cai, R.-Z.; Vidaurre, I.; Szalontay, L.; Jayakumar, A.R.; Rick, F.G. Suppression of the proliferation of human U-87 MG glioblastoma cells by new antagonists of growth hormone-releasing hormone in vivo and in vitro. Target. Oncol. 2013, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, H.; Zhu, J.; Tang, Y.; Zhou, Y.; Zhu, L.; Gao, C.; Li, W.; You, W.; Yu, B.; et al. Correlation of Nrf2 and HIF-1α in glioblastoma and their relationships to clinicopathologic features and survival. Neurol. Res. 2013, 35, 1044–1050. [Google Scholar] [CrossRef]
- Jin, R.; Nakada, M.; Teng, L.; Furuta, T.; Sabit, H.; Hayashi, Y.; Demuth, T.; Hirao, A.; Sato, H.; Zhao, G.; et al. Combination therapy using Notch and Akt inhibitors is effective for suppressing invasion but not proliferation in glioma cells. Neurosci. Lett. 2013, 534, 316–321. [Google Scholar] [CrossRef]
- Joel, M.; Mughal, A.A.; Grieg, Z.; Murrell, W.; Palmero, S.; Mikkelsen, B.; Fjerdingstad, H.B.; Sandberg, C.J.; Behnan, J.; Glover, J.C. Targeting PBK/TOPK decreases growth and survival of glioma initiating cells in vitro and attenuates tumor growth in vivo. Mol. Cancer 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Johanns, T.M.; Ferguson, C.J.; Grierson, P.M.; Dahiya, S.; Ansstas, G. Rapid clinical and radiographic response with combined dabrafenib and trametinib in adults with BRAF-mutated high-grade glioma. J. Natl. Compr. Cancer Netw. 2018, 16, 4–10. [Google Scholar] [CrossRef]
- Joshi, A.D.; Botham, R.C.; Schlein, L.J.; Roth, H.S.; Mangraviti, A.; Borodovsky, A.; Tyler, B.; Joslyn, S.; Looper, J.S.; Podell, M. Synergistic and targeted therapy with a procaspase-3 activator and temozolomide extends survival in glioma rodent models and is feasible for the treatment of canine malignant glioma patients. Oncotarget 2017, 8, 80124–80138. [Google Scholar] [CrossRef]
- Ju, R.-J.; Zeng, F.; Liu, L.; Mu, L.-M.; Xie, H.-J.; Zhao, Y.; Yan, Y.; Wu, J.-S.; Hu, Y.-J.; Lu, W.-L. Destruction of vasculogenic mimicry channels by targeting epirubicin plus celecoxib liposomes in treatment of brain glioma. Int. J. Nanomed. 2016, 11, 1131–1146. [Google Scholar]
- Junca, A.; Villalva, C.; Tachon, G.; Rivet, P.; Cortes, U.; Guilloteau, K.; Balbous, A.; Godet, J.; Wager, M.; Karayan-Tapon, L. Crizotinib targets in glioblastoma stem cells. Cancer Med. 2017, 6, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, H.; Zhao, H.-Y.; Jeon, R.; Ryu, J.-H.; Kim, W.-Y. Systemic approaches identify a garlic-derived chemical, Z-ajoene, as a glioblastoma multiforme cancer stem cell-specific targeting agent. Mol. Cells 2014, 37, 547. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, Y.; Natsumeda, M.; Okada, M.; Saito, R.; Kobayashi, D.; Eda, T.; Watanabe, J.; Saito, S.; Tsukamoto, Y.; Oishi, M. Dramatic response of BRAF V600E-mutant epithelioid glioblastoma to combination therapy with BRAF and MEK inhibitor: Establishment and xenograft of a cell line to predict clinical efficacy. Acta Neuropathol. Commun. 2019, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Kaneta, Y.; Ullrich, A. NEK9 depletion induces catastrophic mitosis by impairment of mitotic checkpoint control and spindle dynamics. Biochem. Biophys. Res. Commun. 2013, 442, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, D.; Takahashi, M.; Satomi, K.; Yamamuro, S.; Kobayashi, T.; Uchida, E.; Honda-Kitahara, M.; Narita, Y.; Iwadate, Y.; Ichimura, K. The ALK inhibitors, alectinib and ceritinib, induce ALK-independent and STAT3-dependent glioblastoma cell death. Cancer Sci. 2021, 112, 2442–2453. [Google Scholar] [CrossRef]
- Kim, S.-S.; Harford, J.B.; Moghe, M.; Rait, A.; Pirollo, K.F.; Chang, E.H. Targeted nanocomplex carrying siRNA against MALAT1 sensitizes glioblastoma to temozolomide. Nucleic Acids Res. 2018, 46, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ai, C.; Dong, F.; Xia, X.; Zhao, X.; Yang, C.; Kang, C.; Zhou, Y.; Zhao, Q.; Sun, X. Targeting of BMI-1 with PTC-209 inhibits glioblastoma development. Cell Cycle 2018, 17, 1199–1211. [Google Scholar] [CrossRef]
- Koul, D.; Shen, R.; Bergh, S.; Lu, Y.; de Groot, J.F.; Liu, T.J.; Mills, G.B.; Yung, W.A. Targeting integrin-linked kinase inhibits Akt signaling pathways and decreases tumor progression of human glioblastoma. Mol. Cancer Ther. 2005, 4, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Koul, D.; Shen, R.; Kim, Y.-W.; Kondo, Y.; Lu, Y.; Bankson, J.; Ronen, S.M.; Kirkpatrick, D.L.; Powis, G.; Yung, W.A. Cellular and in vivo activity of a novel PI3K inhibitor, PX-866, against human glioblastoma. Neuro-Oncology 2010, 12, 559–569. [Google Scholar] [CrossRef]
- Kuan, C.-T.; Wakiya, K.; Herndon, J.E.; Lipp, E.S.; Pegram, C.N.; Riggins, G.J.; Rasheed, A.; Szafranski, S.E.; McLendon, R.E.; Wikstrand, C.J. MRP3: A molecular target for human glioblastoma multiforme immunotherapy. BMC Cancer 2010, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Lamour, V.; Henry, A.; Kroonen, J.; Nokin, M.J.; von Marschall, Z.; Fisher, L.W.; Chau, T.L.; Chariot, A.; Sanson, M.; Delattre, J.Y. Targeting osteopontin suppresses glioblastoma stem-like cell character and tumorigenicity in vivo. Int. J. Cancer 2015, 137, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Lassen, U.; Chinot, O.L.; McBain, C.; Mau-Sørensen, M.; Larsen, V.A.; Barrie, M.; Roth, P.; Krieter, O.; Wang, K.; Habben, K. Phase 1 dose-escalation study of the antiplacental growth factor monoclonal antibody RO5323441 combined with bevacizumab in patients with recurrent glioblastoma. Neuro-Oncology 2015, 17, 1007–1015. [Google Scholar] [CrossRef][Green Version]
- Lee, C.; Fotovati, A.; Triscott, J.; Chen, J.; Venugopal, C.; Singhal, A.; Dunham, C.; Kerr, J.M.; Verreault, M.; Yip, S.; et al. Polo-like kinase 1 inhibition kills glioblastoma multiforme brain tumor cells in part through loss of SOX2 and delays tumor progression in mice. Stem Cells 2012, 30, 1064–1075. [Google Scholar] [CrossRef]
- Lee, C.H.; Alpert, B.O.; Sankaranarayanan, P.; Alter, O. GSVD comparison of patient-matched normal and tumor aCGH profiles reveals global copy-number alterations predicting glioblastoma multiforme survival. PLoS ONE 2012, 7, e30098. [Google Scholar] [CrossRef]
- Lescarbeau, R.S.; Lei, L.; Bakken, K.K.; Sims, P.A.; Sarkaria, J.N.; Canoll, P.; White, F.M. Quantitative phosphoproteomics reveals Wee1 kinase as a therapeutic target in a model of proneural glioblastoma. Mol. Cancer Ther. 2016, 15, 1332–1343. [Google Scholar] [CrossRef]
- Li, C.; Shen, J.; Wei, X.; Xie, C.; Lu, W. Targeted delivery of a novel palmitylated D-peptide for antiglioblastoma molecular therapy. J. Drug Target. 2012, 20, 264–271. [Google Scholar] [CrossRef]
- Lian, S.; Shi, R.; Bai, T.; Liu, Y.; Miao, W.; Wang, H.; Liu, X.; Fan, Y. Anti-miRNA-23a oligonucleotide suppresses glioma cells growth by targeting apoptotic protease activating factor-1. Curr. Pharm. Des. 2013, 19, 6382–6389. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, L.; Shi, S.; Wang, Y.; Ni, X.; Xiao, F.; Wang, S.; Li, P.; Ding, K. Oligosaccharide G19 inhibits U-87 MG human glioma cells growth in vitro and in vivo by targeting epidermal growth factor (EGF) and activating p53/p21 signaling. Glycobiology 2014, 24, 748–765. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, X.; Feng, X.; Zhang, B.; Wang, J. Adenovirus-mediated delivery of bFGF small interfering RNA reduces STAT3 phosphorylation and induces the depolarization of mitochondria and apoptosis in glioma cells U251. J. Exp. Clin. Cancer Res. 2011, 30, 80. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Shi, L.; Shan, Q.; Cao, Q.; Yue, C.; Li, H.; Li, S.; Wang, J.; Gao, S. The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 219. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chhipa, R.R.; Nakano, I.; Dasgupta, B. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol. Cancer Ther. 2014, 13, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Rudà, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef]
- Loskutov, Y.V.; Griffin, C.L.; Marinak, K.M.; Bobko, A.; Margaryan, N.V.; Geldenhuys, W.J.; Sarkaria, J.N.; Pugacheva, E.N. LPA signaling is regulated through the primary cilium: A novel target in glioblastoma. Oncogene 2018, 37, 1457–1471. [Google Scholar] [CrossRef]
- Luchman, H.A.; Stechishin, O.D.; Dang, N.H.; Blough, M.D.; Chesnelong, C.; Kelly, J.J.; Nguyen, S.A.; Chan, J.A.; Weljie, A.M.; Cairncross, J.G. An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro-Oncology 2011, 14, 184–191. [Google Scholar] [CrossRef]
- Luwor, R.; Morokoff, A.P.; Amiridis, S.; D’Abaco, G.; Paradiso, L.; Stylli, S.S.; Nguyen, H.P.; Tarleton, M.; Young, K.A.; O’Brien, T.J. Targeting glioma stem cells by functional inhibition of dynamin 2: A novel treatment strategy for glioblastoma. Cancer Investig. 2019, 37, 144–155. [Google Scholar] [CrossRef]
- Balkhi, H.M.; Gul, T.; Haq, E. Anti-neoplastic and calcium modulatory action of caffeic acid phenethyl ester and dasatinib in C6 glial cells: A therapeutic perspective. CNS Neurol. Disord.-Drug Targets Former. Curr. Drug Targets-CNS Neurol. Disord. 2016, 15, 54–63. [Google Scholar] [CrossRef]
- Ma, J.-W.; Zhang, Y.; Li, R.; Ye, J.-C.; Li, H.-Y.; Zhang, Y.-K.; Ma, Z.-L.; Li, J.-Y.; Zhong, X.-Y.; Yang, X. Tetrandrine suppresses human glioma growth by inhibiting cell survival, proliferation and tumour angiogenesis through attenuating STAT3 phosphorylation. Eur. J. Pharmacol. 2015, 764, 228–239. [Google Scholar] [CrossRef]
- Mao, P.; Hever-Jardine, M.P.; Rahme, G.J.; Yang, E.; Tam, J.; Kodali, A.; Biswal, B.; Fadul, C.E.; Gaur, A.; Israel, M.A. Serine/threonine kinase 17A is a novel candidate for therapeutic targeting in glioblastoma. PLoS ONE 2013, 8, e81803. [Google Scholar] [CrossRef]
- Mason, W.P.; MacNeil, M.; Kavan, P.; Easaw, J.; Macdonald, D.; Thiessen, B.; Urva, S.; Lwin, Z.; McIntosh, L.; Eisenhauer, E. A phase I study of temozolomide and everolimus (RAD001) in patients with newly diagnosed and progressive glioblastoma either receiving or not receiving enzyme-inducing anticonvulsants: An NCIC CTG study. Investig. New Drugs 2012, 30, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.-I.; Sato, A.; Okada, M.; Shibuya, K.; Seino, S.; Suzuki, K.; Watanabe, E.; Narita, Y.; Shibui, S.; Kayama, T. Targeting JNK for therapeutic depletion of stem-like glioblastoma cells. Sci. Rep. 2012, 2, 516. [Google Scholar] [CrossRef]
- Maxwell, M.J.; Arnold, A.; Sweeney, H.; Chen, L.; Lih, T.-S.M.; Schnaubelt, M.; Eberhart, C.G.; Rubens, J.A.; Zhang, H.; Clark, D.J. Unbiased proteomic and phosphoproteomic analysis identifies response signatures and novel susceptibilities after combined MEK and mTOR inhibition in BRAFV600E mutant glioma. Mol. Cell. Proteom. 2021, 20, 100123. [Google Scholar] [CrossRef]
- Merlino, F.; Daniele, S.; La Pietra, V.; Di Maro, S.; Di Leva, F.S.; Brancaccio, D.; Tomassi, S.; Giuntini, S.; Cerofolini, L.; Fragai, M. Simultaneous targeting of RGD-integrins and dual murine double minute proteins in glioblastoma multiforme. J. Med. Chem. 2018, 61, 4791–4809. [Google Scholar] [CrossRef]
- Michaud, K.; Solomon, D.A.; Oermann, E.; Kim, J.S.; Zhong, W.Z.; Prados, M.D.; Ozawa, T.; James, C.D.; Waldman, T. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010, 70, 3228–3238. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Pan, Y.; Joshi, K.; Purohit, D.; Hu, B.; Demir, H.; Mazumder, S.; Okabe, S.; Yamori, T.; Viapiano, M.; et al. Telomestatin impairs glioma stem cell survival and growth through the disruption of telomeric G-quadruplex and inhibition of the proto-oncogene, c-Myb. Clin Cancer Res 2012, 18, 1268–1280. [Google Scholar] [CrossRef]
- Mojarad-Jabali, S.; Farshbaf, M.; Hemmati, S.; Sarfraz, M.; Motasadizadeh, H.; Mojarrad, J.S.; Atyabi, F.; Zakeri-Milani, P.; Valizadeh, H. Comparison of three synthetic transferrin mimetic small peptides to promote the blood–brain barrier penetration of vincristine liposomes for improved glioma targeted therapy. Int. J. Pharm. 2022, 613, 121395. [Google Scholar] [CrossRef]
- Nandhu, M.S.; Behera, P.; Bhaskaran, V.; Longo, S.L.; Barrera-Arenas, L.M.; Sengupta, S.; Rodriguez-Gil, D.J.; Chiocca, E.A.; Viapiano, M.S. Development of a function-blocking antibody against fibulin-3 as a targeted reagent for glioblastoma. Clin. Cancer Res. 2018, 24, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C. Randomized phase II and biomarker study of pembrolizumab plus bevacizumab versus pembrolizumab alone for patients with recurrent glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef]
- Niu, M.; Cai, W.; Liu, H.; Chong, Y.; Hu, W.; Gao, S.; Shi, Q.; Zhou, X.; Liu, X.; Yu, R. Plumbagin inhibits growth of gliomas in vivo via suppression of FOXM1 expression. J. Pharmacol. Sci. 2015, 128, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Nonnenmacher, L.; Westhoff, M.A.; Fulda, S.; Karpel-Massler, G.; Halatsch, M.E.; Engelke, J.; Simmet, T.; Corbacioglu, S.; Debatin, K.M. RIST: A potent new combination therapy for glioblastoma. Int. J. Cancer 2015, 136, E173–E187. [Google Scholar] [CrossRef] [PubMed]
- Pall, A.E.; Juratli, L.; Guntur, D.; Bandyopadhyay, K.; Kondapalli, K.C. A gain of function paradox: Targeted therapy for glioblastoma associated with abnormal NHE9 expression. J. Cell. Mol. Med. 2019, 23, 7859–7872. [Google Scholar] [CrossRef] [PubMed]
- Paternot, S.; Roger, P.P. Combined inhibition of MEK and mammalian target of rapamycin abolishes phosphorylation of cyclin-dependent kinase 4 in glioblastoma cell lines and prevents their proliferation. Cancer Res. 2009, 69, 4577–4581. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Yang, C.; Liu, Y.; Shen, C. miR-25-3p promotes glioma cell proliferation and migration by targeting FBXW7 and DKK3. Exp. Ther. Med. 2019, 18, 769–778. [Google Scholar] [CrossRef]
- Peng, R.; Jiang, B.; Ma, J.; Ma, Z.; Wan, X.; Liu, H.; Chen, Z.; Cheng, Q.; Chen, R. Forced downregulation of RACK1 inhibits glioma development by suppressing Src/Akt signaling activity. Oncol. Rep. 2013, 30, 2195–2202. [Google Scholar] [CrossRef]
- Pezuk, J.A.; Brassesco, M.S.; Morales, A.G.; de Oliveira, J.C.; de Paula Queiroz, R.G.; Machado, H.R.; Carlotti, C.G.; Neder, L.; Scrideli, C.A.; Tone, L.G. Polo-like kinase 1 inhibition causes decreased proliferation by cell cycle arrest, leading to cell death in glioblastoma. Cancer Gene Ther. 2013, 20, 499–506. [Google Scholar] [CrossRef]
- Premkumar, D.R.; Jane, E.P.; Pollack, I.F. Co-administration of NVP-AEW541 and dasatinib induces mitochondrial-mediated apoptosis through Bax activation in malignant human glioma cell lines. Int. J. Oncol. 2010, 37, 633–643. [Google Scholar]
- Preukschas, M.; Hagel, C.; Schulte, A.; Weber, K.; Lamszus, K.; Sievert, H.; Pällmann, N.; Bokemeyer, C.; Hauber, J.; Braig, M.; et al. Expression of eukaryotic initiation factor 5A and hypusine forming enzymes in glioblastoma patient samples: Implications for new targeted therapies. PLoS ONE 2012, 7, e43468. [Google Scholar] [CrossRef]
- Punganuru, S.R.; Arutla, V.; Zhao, W.; Rajaei, M.; Deokar, H.; Zhang, R.; Buolamwini, J.K.; Srivenugopal, K.S.; Wang, W. Targeted brain tumor therapy by inhibiting the MDM2 oncogene: In vitro and in vivo antitumor activity and mechanism of action. Cells 2020, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Raub, T.J.; Wishart, G.N.; Kulanthaivel, P.; Staton, B.A.; Ajamie, R.T.; Sawada, G.A.; Gelbert, L.M.; Shannon, H.E.; Sanchez-Martinez, C.; De Dios, A. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab. Dispos. 2015, 43, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Renfrow, J.J.; Soike, M.H.; West, J.L.; Ramkissoon, S.H.; Metheny-Barlow, L.; Mott, R.T.; Kittel, C.A.; D’Agostino, R.B., Jr.; Tatter, S.B.; Laxton, A.W. Attenuating hypoxia driven malignant behavior in glioblastoma with a novel hypoxia-inducible factor 2 alpha inhibitor. Sci. Rep. 2020, 10, 15195. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Iizuka, Y.; Ohta, S.; Takahashi, S.; Nakamura, K.; Saya, H.; Yoshida, K.; Kawakami, Y.; Toda, M. Functional analysis of a novel glioma antigen, EFTUD1. Neuro-Oncology 2014, 16, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Bringas, J.R.; Panner, A.; Tamas, M.; Pieper, R.O.; Berger, M.S.; Bankiewicz, K.S. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004, 64, 6858–6862. [Google Scholar] [CrossRef]
- Salphati, L.; Heffron, T.P.; Alicke, B.; Nishimura, M.; Barck, K.; Carano, R.A.; Cheong, J.; Edgar, K.A.; Greve, J.; Kharbanda, S. Targeting the PI3K pathway in the brain—Efficacy of a PI3K inhibitor optimized to cross the blood–brain barrier. Clin. Cancer Res. 2012, 18, 6239–6248. [Google Scholar] [CrossRef]
- Sanai, N.; Li, J.; Boerner, J.; Stark, K.; Wu, J.; Kim, S.; Derogatis, A.; Mehta, S.; Dhruv, H.D.; Heilbrun, L.K. Phase 0 trial of AZD1775 in first-recurrence glioblastoma patients. Clin. Cancer Res. 2018, 24, 3820–3828. [Google Scholar] [CrossRef]
- Sanzey, M.; Abdul Rahim, S.A.; Oudin, A.; Dirkse, A.; Kaoma, T.; Vallar, L.; Herold-Mende, C.; Bjerkvig, R.; Golebiewska, A.; Niclou, S.P. Comprehensive analysis of glycolytic enzymes as therapeutic targets in the treatment of glioblastoma. PLoS ONE 2015, 10, e0123544. [Google Scholar] [CrossRef]
- Sathornsumetee, S.; Hjelmeland, A.B.; Keir, S.T.; McLendon, R.E.; Batt, D.; Ramsey, T.; Yusuff, N.; Rasheed, B.A.; Kieran, M.W.; Laforme, A. AAL881, a novel small molecule inhibitor of RAF and vascular endothelial growth factor receptor activities, blocks the growth of malignant glioma. Cancer Res. 2006, 66, 8722–8730. [Google Scholar] [CrossRef]
- Saunders, J.T.; Holmes, B.; Benavides-Serrato, A.; Kumar, S.; Nishimura, R.N.; Gera, J. Targeting the YAP-TEAD interaction interface for therapeutic intervention in glioblastoma. J. Neuro-Oncol. 2021, 152, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Sautter, L.; Hofheinz, R.; Tuettenberg, J.; Grimm, M.; Vajkoczy, P.; Groden, C.; Schmieder, K.; Hochhaus, A.; Wenz, F.; Giordano, F.A. Open-label phase II evaluation of imatinib in primary inoperable or incompletely resected and recurrent glioblastoma. Oncology 2020, 98, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, S.M.; Thotala, D.K.; Linkous, A.G.; Hu, R.; Leahy, K.M.; Yazlovitskaya, E.M.; Hallahan, D.E. Autotaxin and LPA receptors represent potential molecular targets for the radiosensitization of murine glioma through effects on tumor vasculature. PLoS ONE 2011, 6, e22182. [Google Scholar] [CrossRef] [PubMed]
- See, W.L.; Tan, I.-L.; Mukherjee, J.; Nicolaides, T.; Pieper, R.O. Sensitivity of Glioblastomas to Clinically Available MEK Inhibitors Is Defined by Neurofibromin 1 Deficiency. Cancer Res. 2012, 72, 3350–3359. [Google Scholar] [CrossRef] [PubMed]
- Selvasaravanan, K.D.; Wiederspohn, N.; Hadzalic, A.; Strobel, H.; Payer, C.; Schuster, A.; Karpel-Massler, G.; Siegelin, M.D.; Halatsch, M.-E.; Debatin, K.-M. The limitations of targeting MEK signalling in Glioblastoma therapy. Sci. Rep. 2020, 10, 7401. [Google Scholar] [CrossRef] [PubMed]
- Shingu, T.; Holmes, L.; Henry, V.; Wang, Q.; Latha, K.; Gururaj, A.E.; Gibson, L.A.; Doucette, T.; Lang, F.F.; Rao, G. Suppression of RAF/MEK or PI3K synergizes cytotoxicity of receptor tyrosine kinase inhibitors in glioma tumor-initiating cells. J. Transl. Med. 2016, 14, 46. [Google Scholar] [CrossRef]
- Shulepko, M.; Bychkov, M.; Lyukmanova, E.; Kirpichnikov, M. Recombinant analogue of the human protein SLURP-1 inhibits the growth of U251 MG and A172 glioma cells. Dokl. Biochem. Biophys. 2020, 493, 211–214. [Google Scholar] [CrossRef]
- Signore, M.; Pelacchi, F.; Di Martino, S.; Runci, D.; Biffoni, M.; Giannetti, S.; Morgante, L.; De Majo, M.; Petricoin, E.; Stancato, L.; et al. Combined PDK1 and CHK1 inhibition is required to kill glioblastoma stem-like cells in vitro and in vivo. Cell Death Dis. 2014, 5, e1223. [Google Scholar] [CrossRef]
- Takano, S.; Tsuboi, K.; Matsumura, A.; Nose, T. Anti-vascular endothelial growth factor antibody and nimustine as combined therapy: Effects on tumor growth and angiogenesis in human glioblastoma xenografts. Neuro-Oncology 2003, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tchoghandjian, A.; Soubéran, A.; Tabouret, E.; Colin, C.; Denicolaï, E.; Jiguet-Jiglaire, C.; El-Battari, A.; Villard, C.; Baeza-Kallee, N.; Figarella-Branger, D. Inhibitor of apoptosis protein expression in glioblastomas and their in vitro and in vivo targeting by SMAC mimetic GDC-0152. Cell Death Dis. 2016, 7, e2325. [Google Scholar] [CrossRef]
- Thanasupawat, T.; Glogowska, A.; Burg, M.; Krcek, J.; Beiko, J.; Pitz, M.; Zhang, G.J.; Hombach-Klonisch, S.; Klonisch, T. C1q/TNF-related peptide 8 (CTRP 8) promotes temozolomide resistance in human glioblastoma. Mol. Oncol. 2018, 12, 1464–1479. [Google Scholar] [CrossRef] [PubMed]
- Tsigelny, I.F.; Mukthavaram, R.; Kouznetsova, V.L.; Chao, Y.; Babic, I.; Nurmemmedov, E.; Pastorino, S.; Jiang, P.; Calligaris, D.; Agar, N. Multiple spatially related pharmacophores define small molecule inhibitors of OLIG2 in glioblastoma. Oncotarget 2017, 8, 22370. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Niu, M.; Xie, P.; Yue, C.; Liu, N.; Qi, Z.; Gao, S.; Liu, H.; Shi, Q.; Yu, R. Smoothened is a poor prognosis factor and a potential therapeutic target in glioma. Sci. Rep. 2017, 7, 42630. [Google Scholar] [CrossRef]
- Venere, M.; Horbinski, C.; Crish, J.F.; Jin, X.; Vasanji, A.; Major, J.; Burrows, A.C.; Chang, C.; Prokop, J.; Wu, Q. The mitotic kinesin KIF11 is a driver of invasion, proliferation, and self-renewal in glioblastoma. Sci. Transl. Med. 2015, 7, 304ra143. [Google Scholar] [CrossRef]
- Vengoji, R.; Macha, M.A.; Nimmakayala, R.K.; Rachagani, S.; Siddiqui, J.A.; Mallya, K.; Gorantla, S.; Jain, M.; Ponnusamy, M.P.; Batra, S.K.; et al. Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 266. [Google Scholar] [CrossRef]
- von Spreckelsen, N.; Fadzen, C.M.; Hartrampf, N.; Ghotmi, Y.; Wolfe, J.M.; Dubey, S.; Yang, B.Y.; Kijewski, M.F.; Wang, S.; Farquhar, C. Targeting glioblastoma using a novel peptide specific to a deglycosylated isoform of brevican. Adv. Ther. 2021, 4, 2000244. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Kirkpatrick, J.P.; Reardon, D.A.; Peters, K.B.; Herndon, J.E., II; Marcello, J.; Bailey, L.; Threatt, S.; Sampson, J.; et al. Addition of Bevacizumab to Standard Radiation Therapy and Daily Temozolomide Is Associated With Minimal Toxicity in Newly Diagnosed Glioblastoma Multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 58–66. [Google Scholar] [CrossRef]
- Wang, F.; Bai, H.; Wang, J.; Bai, Y.; Dou, C. Glioma growth inhibition in vitro and in vivo by single chain variable fragments of the transferrin receptor conjugated to survivin small interfering RNA. J. Int. Med. Res. 2011, 39, 1701–1712. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Q.; Zhou, L.-Y. Analysis of the treatment of gliomas with SEC therapy combined with radiochemotherapy. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2400–2405. [Google Scholar]
- Wang, X.; Hua, Y.; Xu, G.; Deng, S.; Yang, D.; Gao, X. Targeting eZh2 for glioma therapy with a novel nanoparticle–sirNa complex. Int. J. Nanomed. 2019, 14, 2637–2653. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Wu, Q.; Kim, L.J.; Morton, A.R.; Gimple, R.C.; Prager, B.C.; Shi, Y.; Zhou, W.; Bhargava, S. Targeting pyrimidine synthesis accentuates molecular therapy response in glioblastoma stem cells. Sci. Transl. Med. 2019, 11, eaau4972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, L.; Sheng, X.F.; Chen, S.; Dai, J.Z. Nimotuzumab, a humanized monoclonal antibody specific for the EGFR, in combination with temozolomide and radiation therapy for newly diagnosed glioblastoma multiforme: First results in Chinese patients. Asia Pac. J. Clin. Oncol. 2016, 12, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Nishijima, N.; Hirai, K.; Shibata, K.; Hase, A.; Yamanaka, T.; Inazu, M. Anticancer activity of Amb4269951, a choline transporter-like protein 1 inhibitor, in human glioma cells. Pharmaceuticals 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, H.; Güttler, A.; Bache, M.; Taubert, H.; Rot, S.; Kessler, J.; Eckert, A.W.; Kappler, M.; Vordermark, D. Targeting of EGFR and HER2 with therapeutic antibodies and siRNA. Strahlenther. Onkol. 2015, 191, 180. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Dettmer, S.; Berberich, A.; Kessler, T.; Karapanagiotou-Schenkel, I.; Wick, A.; Winkler, F.; Pfaff, E.; Brors, B.; Debus, J. N2M2 (NOA-20) phase I/II trial of molecularly matched targeted therapies plus radiotherapy in patients with newly diagnosed non-MGMT hypermethylated glioblastoma. Neuro-Oncology 2019, 21, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Gong, M.; Zou, Y.; Wang, Z.; Wu, B.; Zhang, S.; Li, L.; Jin, K.; Sun, C. Apatinib induces ferroptosis of glioma cells through modulation of the VEGFR2/Nrf2 pathway. Oxidative Med. Cell. Longev. 2022, 2022, 9925919. [Google Scholar] [CrossRef]
- Xiong, D.D.; Xu, W.Q.; He, R.Q.; Dang, Y.W.; Chen, G.; Luo, D.Z. In silico analysis identified miRNA-based therapeutic agents against glioblastoma multiforme. Oncol. Rep. 2019, 41, 2194–2208. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, C.; Han, H.; Liu, H.; Tang, M.; Liu, L.; Ji, W.; Lu, X.; Yang, X.; Zhang, Y. Icaritin inhibits the invasion and epithelial-to-mesenchymal transition of glioblastoma cells by targeting EMMPRIN via PTEN/AKt/HIF-1α signalling. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1296–1307. [Google Scholar] [CrossRef]
- Xu, T.-J.; Qiu, P.; Zhang, Y.-B.; Yu, S.-Y.; Xu, G.-M.; Yang, W. MiR-148a inhibits the proliferation and migration of glioblastoma by targeting ITGA9. Hum. Cell 2019, 32, 548–556. [Google Scholar] [CrossRef]
- Yan, D.; Kowal, J.; Akkari, L.; Schuhmacher, A.; Huse, J.; West, B.; Joyce, J. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene 2017, 36, 6049–6058. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Barth, R.F.; Wu, G.; Kawabata, S.; Sferra, T.J.; Bandyopadhyaya, A.K.; Tjarks, W.; Ferketich, A.K.; Moeschberger, M.L.; Binns, P.J. Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin. Cancer Res. 2006, 12, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, G.; Barth, R.F.; Swindall, M.R.; Bandyopadhyaya, A.K.; Tjarks, W.; Tordoff, K.; Moeschberger, M.; Sferra, T.J.; Binns, P.J.; et al. Molecular targeting and treatment of composite EGFR and EGFRvIII-positive gliomas using boronated monoclonal antibodies. Clin. Cancer Res. 2008, 14, 883–891. [Google Scholar] [CrossRef]
- Yao, T.-W.; Zhang, J.; Prados, M.; Weiss, W.A.; James, C.D.; Nicolaides, T. EGFR blockade prevents glioma escape from BRAFV600E targeted therapy. Oncotarget 2015, 6, 21993. [Google Scholar] [CrossRef]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genßler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J. Natl. Cancer Inst. 2016, 108, djv375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, X.-R.; Li, H.-Y.; Zhao, Z.-J.; Liao, Y.-W.; Wang, X.-Y.; Su, J.; Sang, S.-S.; Liu, Q. Anti-cancer effect of metabotropic glutamate receptor 1 inhibition in human glioma U87 cells: Involvement of PI3K/Akt/mTOR pathway. Cell. Physiol. Biochem. 2015, 35, 419–432. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Liu, X.Y.; Qin, Z.H.; Yang, J.M. Expression of elongation factor-2 kinase contributes to anoikis resistance and invasion of human glioma cells. Acta Pharmacol. Sin. 2011, 32, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; He, H.; Wang, C.; Tao, B.; Zhou, H.; Dong, Y.; Xiang, J.; Wang, L.; Luo, C.; Lu, Y. Downregulation of Id2 increases chemosensitivity of glioma. Tumor Biol. 2015, 36, 4189–4196. [Google Scholar] [CrossRef]
- Zustovich, F.; Landi, L.; Lombardi, G.; Porta, C.; Galli, L.; Fontana, A.; Amoroso, D.; Galli, C.; Andreuccetti, M.; Falcone, A. Sorafenib plus daily low-dose temozolomide for relapsed glioblastoma: A phase II study. Anticancer. Res. 2013, 33, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, X.; Gao, C.; Jiang, S.; Wu, H.; Liu, Z.; Dou, T. Burden and trends of brain and central nervous system cancer from 1990 to 2019 at the global, regional, and country levels. Arch. Public Health 2022, 80, 209. [Google Scholar] [CrossRef]
- Raja, N.; Hayes, L.; Basta, N.; McNally, R.J.Q. International trends in the incidence of brain tumours in children and young-adults and their association with indicators of economic development. Cancer Epidemiol. 2021, 74, 102006. [Google Scholar] [CrossRef] [PubMed]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R. Rising incidence of glioblastoma and meningioma in the United States: Projections through 2050. J. Clin. Oncol. 2012, 30, 2065. [Google Scholar] [CrossRef]
- Tebha, S.S.; Ali Memon, S.; Mehmood, Q.; Mukherjee, D.; Abdi, H.; Negida, A. Glioblastoma management in low and middle-income countries; existing challenges and policy recommendations. Brain Spine 2023, 3, 101775. [Google Scholar] [CrossRef]
- Cagney, D.N.; Alexander, B.M. The cost and value of glioblastoma therapy. Expert Rev. Anticancer. Ther. 2017, 17, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.S.; Koffie, R.M.; Rattani, A.; Dewan, M.C.; Baticulon, R.E.; Qureshi, M.M.; Wahjoepramono, E.J.; Rosseau, G.; Park, K.; Nahed, B.V. Global incidence of brain and spinal tumors by geographic region and income level based on cancer registry data. J. Clin. Neurosci. 2019, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Shagufta, B.I.; Abbas, S.Q.; Patel, S.; Khan, I.; Shah, S.A.A.; Akhter, N.; ul Hassan, S.S. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed. Pharmacother. 2017, 92, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Hashem, S.A.; Al-Rashdan, A.; Ezam, N.; Nour, A.; Alsharbaji, A.; Sughayer, M.; Mohamad, I.; Elyan, M.; Addas, A.; et al. The challenges of managing glioblastoma multiforme in developing countries: A trade-off between cost and quality of care. Hematol. Oncol. Stem Cell Ther. 2011, 4, 116–120. [Google Scholar] [CrossRef]
- Wang, T.; Pham, A.; Yoo, S.; Attenello, F.J.; Jennelle, R.; Wagle, N.; Chang, E.L.; Zada, G. Identifying Disparities in Care in Treating Glioblastoma: A Retrospective Cohort Study of Patients Treated at a Safety-net Versus Private Hospital Setting. World Neurosurg. 2020, 137, e213–e220. [Google Scholar] [CrossRef]
- Ooi, S.Z.Y.; de Koning, R.; Egiz, A.; Dalle, D.U.; Denou, M.; Tsopmene, M.R.D.; Khan, M.; Takoukam, R.; Kotecha, J.; Sichimba, D.; et al. Management and outcomes of low-grade gliomas in Africa: A scoping review. Ann. Med. Surg. 2022, 74, 103246. [Google Scholar] [CrossRef]
- Balogun, J.A.; Adekanmbi, A.A. Management of glioblastoma: A perspective from Nigeria. Chin. Clin. Oncol. 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Kanmounye, U.S.; Karekezi, C.; Nyalundja, A.D.; Awad, A.K.; Laeke, T.; Balogun, J.A. Adult brain tumors in Sub-Saharan Africa: A scoping review. Neuro-Oncology 2022, 24, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Steinbach, J.P.; Wick, W. Temozolomide: A milestone in the pharmacotherapy of brain tumors. Future Oncol. 2005, 1, 747–754. [Google Scholar] [CrossRef]
- Trinh, V.A.; Patel, S.P.; Hwu, W.J. The safety of temozolomide in the treatment of malignancies. Expert Opin. Drug Saf. 2009, 8, 493–499. [Google Scholar] [CrossRef]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- Ghosh, D.; Nandi, S.; Bhattacharjee, S. Combination therapy to checkmate Glioblastoma: Clinical challenges and advances. Clin. Transl. Med. 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qiu, W.; Sun, T.; Wang, L.; Du, C.; Hu, Y.; Liu, W.; Feng, F.; Chen, Y.; Sun, H. Therapeutic strategies of glioblastoma (GBM): The current advances in the molecular targets and bioactive small molecule compounds. Acta Pharm. Sin. B 2022, 12, 1781–1804. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Zhu, P.; Du, X.L.; Lu, G.; Zhu, J.J. Survival benefit of glioblastoma patients after FDA approval of temozolomide concomitant with radiation and bevacizumab: A population-based study. Oncotarget 2017, 8, 44015–44031. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, Z.; Dai, S.; Qian, L.; Sun, L.; Gong, Z. Targeting autophagy to sensitive glioma to temozolomide treatment. J. Exp. Clin. Cancer Res. 2016, 35, 23. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, L.; Li, Y.; Fang, B.; Li, G.; Chen, L.; Xu, L. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. BioMed Res. Int. 2019, 2019, 2820853. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Teraiya, M.; Perreault, H.; Chen, V.C. An overview of glioblastoma multiforme and temozolomide resistance: Can LC-MS-based proteomics reveal the fundamental mechanism of temozolomide resistance? Front. Oncol. 2023, 13, 1166207. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-H.; Hsueh, W.-T.; Chuang, J.-Y.; Chang, K.-Y. Dissecting the mechanism of temozolomide resistance and its association with the regulatory roles of intracellular reactive oxygen species in glioblastoma. J. Biomed. Sci. 2021, 28, 18. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.Y.; Cho, B.J.; Choi, E.J.; Kim, J.H.; Chie, E.K.; Wu, H.G.; Kim, I.H.; Paek, S.H.; Kim, J.S.; Kim, I.A. The Effect of Chemoradiotherapy with SRC Tyrosine Kinase Inhibitor, PP2 and Temozolomide on Malignant Glioma Cells In vitro and In vivo. Cancer Res. Treat. 2016, 48, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Gainer, J.L.; Sheehan, J.P.; Larner, J.M.; Jones, D.R. Trans sodium crocetinate with temozolomide and radiation therapy for glioblastoma multiforme. J. Neurosurg. 2017, 126, 460–466. [Google Scholar] [CrossRef]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Vu Han, T.-L.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Ling, S.; Song, L.; Fan, N.; Feng, T.; Liu, L.; Yang, X.; Wang, M.; Li, Y.; Tian, Y.; Zhao, F.; et al. Combination of metformin and sorafenib suppresses proliferation and induces autophagy of hepatocellular carcinoma via targeting the mTOR pathway. Int. J. Oncol. 2017, 50, 297–309. [Google Scholar] [CrossRef]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef]
- Minniti, G.; Muni, R.; Lanzetta, G.; Marchetti, P.; Enrici, R.M. Chemotherapy for Glioblastoma: Current Treatment and Future Perspectives for Cytotoxic and Targeted Agents. Anticancer. Res. 2009, 29, 5171. [Google Scholar]
- Veliz, I.; Loo, Y.; Castillo, O.; Karachaliou, N.; Nigro, O.; Rosell, R. Advances and challenges in the molecular biology and treatment of glioblastoma-is there any hope for the future? Ann. Transl. Med. 2015, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.K.; Brothers, S.P.; Wahlestedt, C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.; Bankhead, A., 3rd; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef]
- Tangsrivimol, J.A.; Schonfeld, E.; Zhang, M.; Veeravagu, A.; Smith, T.R.; Härtl, R.; Lawton, M.T.; El-Sherbini, A.H.; Prevedello, D.M.; Glicksberg, B.S.; et al. Artificial Intelligence in Neurosurgery: A State-of-the-Art Review from Past to Future. Diagnostics 2023, 13, 2429. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).