Investigating Sex-Based Neural Differences in Autism and Their Extended Reality Intervention Implications
Abstract
:1. Introduction
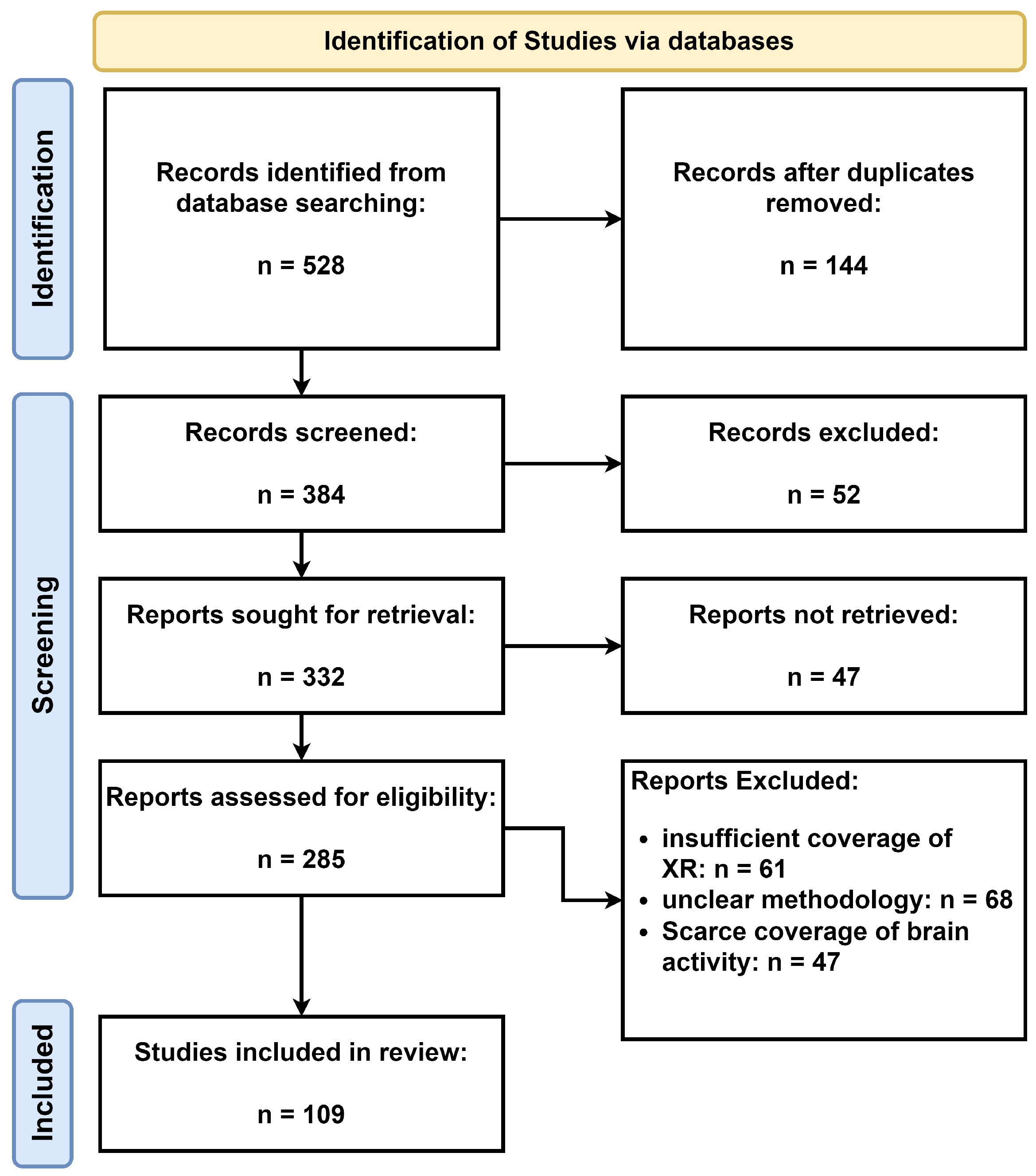
1.1. Methodology
- PubMed: PubMed: Specializing in medical sciences, this database was instrumental for acquiring the literature on the diagnostic and neurological aspects of ASD.
- IEEEXplore: Selected for its extensive collection of technology-related publications, particularly those focused on the application of VR, AR, and MR in ASD.
- Wiley Online: Contributed to a well-rounded review with its comprehensive range of subjects, including health, physical sciences, social sciences, and the humanities.
- MDPI: Particularly useful for its focus on extended reality (XR) technologies and as a supplementary source for articles not available in other databases.
- Frontiers: An open-access platform that publishes peer-reviewed articles across multiple disciplines, including science and technology, medicine, and the humanities.
- Elsevier, Springer, Semantic Scholar: These multi-disciplinary databases provided a broad scientific backdrop for our review.
- The research must focus on at least one of the following key terms: ASD, brain structure, brain function, sex differences, or XR technology interventions.
- The studies must present empirical data relating to XR-based ASD interventions or brain activity. For the purpose of this review, “empirical data” refers to quantitative or qualitative information collected through observation or experimentation. This includes, but is not limited to, randomized controlled trials, observational studies, and validated surveys or questionnaires.
- The studies should provide some detailed participant characteristics, including, but not limited to, age, sex, and diagnosis criteria met. This also extends down to their methodology in terms of procedure, type of XR technology used, and equipment.
- The studies should offer significant findings—whether positive or negative—pertaining to the interventions or brain activities under investigation. The studies that had inconclusive findings were excluded.
- Autism Spectrum Disorder (ASD)
- ASD brain activity
- XR and ASD
- Virtual reality and autism
- Augmented reality and autism
- ASD in women
1.2. Paper Organization
2. The Related Literature
2.1. Feasibility of XR-Based Interventions for ASD
- social interaction
- communication and speech
- emotion recognition and control
- daily living skills
- problem behavior reduction
- anxiety symptom reduction
- insomnia control
2.2. Differences in Brain Structures and Function Based on Sex for ASD
2.3. Remarks
- A critical evaluation of the existing research, complete with the identification of gaps and suggestions for future work.
- A comprehensive overview focusing on the specific brain regions most impacted by ASD.
- Novel insights into the sex-specific differences in the neurological manifestations of ASD.
3. Divergent Neurological Correlations in Males and Females with ASD
4. XR Use Cases for ASD
4.1. Social Skills
4.2. Life Skills and Daily Activities
4.3. Concentration
4.4. Neurofeedback
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Diagnostic Criteria for Autism Spectrum Disorder (ASD). Available online: https://www.cdc.gov/ncbddd/autism/hcp-dsm.html (accessed on 11 April 2023).
- Centers for Disease Control and Prevention. Data & Statistics on Autism Spectrum Disorder. Available online: https://www.cdc.gov/ncbddd/autism/data.html (accessed on 28 March 2023).
- Tsang, L.P.M.; How, C.H.; Yeleswarapu, S.P.; Wong, C.M. Autism Spectrum Disorder: Early identification and management in primary care. Singap. Med. J. 2019, 60, 324. [Google Scholar] [CrossRef]
- Boccaccio, F.M.; Platania, G.A.; Guerrera, C.S.; Varrasi, S.; Privitera, C.R.; Caponnetto, P.; Pirrone, C.; Castellano, S. Autism Spectrum Disorder: Recommended psychodiagnostic tools for early diagnosis. Health Psychol. Res. 2023, 11, 77357. [Google Scholar] [CrossRef]
- Napolitano, A.; Schiavi, S.; Rosa, P.L.; Rossi-Espagnet, M.C.; Petrillo, S.; Bottino, F.; Tagliente, E.; Longo, D.; Lupi, E.; Casula, L.; et al. Sex Differences in Autism Spectrum Disorder: Diagnostic, Neurobiological, and Behavioral Features. Front. Psych. 2022, 13, 889636. [Google Scholar] [CrossRef]
- McCrossin, R. Finding the True Number of Females with Autistic Spectrum Disorder by Estimating the Biases in Initial Recognition and Clinical Diagnosis. Children 2022, 9, 272. [Google Scholar] [CrossRef]
- Gesi, C.; Migliarese, G.; Torriero, S.; Capellazzi, M.; Omboni, A.C.; Cerveri, G.; Mencacci, C. Gender Differences in Misdiagnosis and Delayed Diagnosis among Adults with Autism Spectrum Disorder with No Language or Intellectual Disability. Brain Sci. 2021, 11, 912. [Google Scholar] [CrossRef]
- White, S.W.; Elias, R.; Capriola-Hall, N.N.; Smith, I.C.; Conner, C.M.; Asselin, S.B.; Howlin, P.; Getzel, E.E.; Mazefsky, C.A. Development of a College Transition and Support Program for Students with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 3072–3078. [Google Scholar] [CrossRef]
- Richey, J.A.; Damiano, C.R.; Sabatino, A.; Rittenberg, A.; Petty, C.; Bizzell, J.; Voyvodic, J.; Heller, A.S.; Coffman, M.C.; Smoski, M.; et al. Neural Mechanisms of Emotion Regulation in Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 3409–3423. [Google Scholar] [CrossRef]
- Horwitz, E.; Vos, M.; Bildt, A.D.; Greaves-Lord, K.; Rommelse, N.; Schoevers, R.; Hartman, C. Sex differences in the course of autistic and co-occurring psychopathological symptoms in adolescents with and without autism spectrum disorder. Autism 2023, 27, 1716–1729. [Google Scholar] [CrossRef]
- Cerritelli, F.; Chiera, M.; Abbro, M.; Megale, V.; Esteves, J.; Gallace, A.; Manzotti, A. The Challenges and Perspectives of the Integration between Virtual and Augmented Reality and Manual Therapies. Front. Neurol. 2021, 12, 700211. [Google Scholar] [CrossRef]
- Ke, F.; Moon, J.; Sokolikj, Z. Virtual Reality–Based Social Skills Training for Children with Autism Spectrum Disorder. J. Spec. Educ. Technol. 2020, 37, 49–62. [Google Scholar] [CrossRef]
- Berenguer, C.; Baixauli, I.; Gómez, S.; de El Puig Andrés, M.; Stasio, S.D. Exploring the Impact of Augmented Reality in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6143. [Google Scholar] [CrossRef]
- Mosher, M.A.; Carreon, A.C. Teaching social skills to students with Autism Spectrum Disorder through augmented, virtual and mixed reality. Res. Learn. Technol. 2021, 29, 1–22. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.S.; Lee, S.; Jang, J.; Yoo, S.; Choi, Y.; Park, Y.R. Development and Application of a Metaverse-Based Social Skills Training Program for Children with Autism Spectrum Disorder to Improve Social Interaction: Protocol for a Randomized Controlled Trial. JMIR Res. Prot. 2022, 11, e35960. [Google Scholar] [CrossRef]
- Seaman, R.L.; Cannella-Malone, H.I. Vocational Skills Interventions for Adults with Autism Spectrum Disorder: A Review of the Literature. J. Dev. Phys. Disabil. 2016, 28, 479–494. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Suckling, J.; Ruigrok, A.N.V.; Chakrabarti, B.; Ecker, C.; Deoni, S.C.L.; Craig, M.C.; Murphy, D.G.M.; Bullmore, E.T.; et al. Biological sex affects the neurobiology of autism. Brain 2013, 136, 2799–2815. [Google Scholar] [CrossRef]
- Weng, S.J.; Wiggins, J.L.; Peltier, S.J.; Carrasco, M.; Risi, S.; Lord, C.; Monk, C.S. Alterations of resting state functional connectivity in the default network in adolescents with Autism Spectrum Disorders. Brain Res. 2010, 1313, 202–214. [Google Scholar] [CrossRef]
- Ecker, C. Brain Anatomy and Its Relationship to Behavior in Adults with Autism Spectrum Disorder. Arch. Gen. Psychiatry 2012, 69, 195. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Cao, M.; Liu, M.; Lin, Z.; Yang, W.; Yang, X.; Dhaidhai, D.; Xiong, P. Extended Reality (XR) and telehealth interventions for children or adolescents with Autism Spectrum Disorder: Systematic review of qualitative and quantitative studies. Neurosci. Biobehav. Rev. 2022, 138, 104683. [Google Scholar] [CrossRef]
- Ploog, B.O.; Scharf, A.; Nelson, D.; Brooks, P.J. Use of computer-assisted technologies (CAT) to enhance social, communicative, and language development in children with autism spectrum disorders. J. Autism Dev. Dis. 2013, 43, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Hussain, I.; Mirza, H.T.; Tayyab, M. The role of information and innovative technology for rehabilitation of children with Autism: A Systematic Literature Review. In Proceedings of the 2017 17th International Conference on Computational Science and Its Applications (ICCSA), Trieste, Italy, 3–6 July 2017; pp. 1–10. [Google Scholar] [CrossRef]
- Mubin, S.A.; Thiruchelvam, V.; Andrew, Y.W. Extended Reality: How They Incorporated for ASD Intervention. In Proceedings of the 2020 8th International Conference on Information Technology and Multimedia (ICIMU), Selangor, Malaysia, 24–26 August 2020. [Google Scholar] [CrossRef]
- Roberts, R.; Stacey, J.; Jenner, S.; Maguire, E. Are Extended Reality Interventions Effective in Helping Autistic Children to Enhance Their Social Skills? A Systematic Review. Rev. J. Autism Dev. Disord. 2022, 1–20. [Google Scholar] [CrossRef]
- Maran, P.L.; Daniëls, R.; Slegers, K. The use of extended reality (XR) for people with moderate to severe intellectual disabilities (ID): A scoping review. Technol. Disabil. 2022, 34, 53–67. [Google Scholar] [CrossRef]
- Gu, P.; Xu, X.; Qian, X.; Weng, T.H. Leveraging Extended Reality for Autistic Individuals: A Scoping Review of Technical Features and Technology Affordances. IEEE Trans. Learn. Technol. 2023, 16, 133–149. [Google Scholar] [CrossRef]
- Karamanoli, P.; Tsinakos, A.; Karagiannidis, C. The Application of Augmented Reality for Intervention to People with Autism Spectrum Disorders. IOSR J. Mob. Comput. Appl. 2017, 4, 42–51. [Google Scholar] [CrossRef]
- Wedyan, M.; Al-Jumaily, A.; Dorgham, O. The use of augmented reality in the diagnosis and treatment of autistic children: A review and a new system. Multimed. Tools Appl. 2020, 79, 18245–18291. [Google Scholar] [CrossRef]
- Lian, X.; Sunar, M.S. Mobile Augmented Reality Technologies for Autism Spectrum Disorder Interventions: A Systematic Literature Review. Appl. Sci. 2021, 11, 4550. [Google Scholar] [CrossRef]
- Cavus, N.; Al-Dosakee, K.; Abdi, A.; Sadiq, S. The Utilization of Augmented Reality Technology for Sustainable Skill Development for People with Special Needs: A Systematic Literature Review. Sustainability 2021, 13, 10532. [Google Scholar] [CrossRef]
- Huamanchahua, D.; Valenzuela-Lino, Y.; Ortiz-Zacarias, J.; Manco-Fernandez, F. AR and VR Training System for Children with ASD: A Detailed and Innovative Review. In Proceedings of the 2022 IEEE ANDESCON, Barranquilla, Colombia, 16–19 November 2022. [Google Scholar] [CrossRef]
- Shahmoradi, L.; Rezayi, S. Cognitive rehabilitation in people with Autism Spectrum Disorder: A systematic review of emerging virtual reality-based approaches. J. Neuroeng. Rehab. 2022, 19, 91. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, H.; Naumceska, M.; Zhang, Y. Virtual Reality Technology as an Educational and Intervention Tool for Children with Autism Spectrum Disorder: Current Perspectives and Future Directions. Behav. Sci. 2022, 12, 138. [Google Scholar] [CrossRef]
- Glaser, N.; Schmidt, M. Systematic Literature Review of Virtual Reality Intervention Design Patterns for Individuals with Autism Spectrum Disorders. Int. J. Hum. Comput. Interact. 2022, 38, 753–788. [Google Scholar] [CrossRef]
- Bozgeyikli, L.; Raij, A.; Katkoori, S.; Alqasemi, R. A Survey on Virtual Reality for Individuals with Autism Spectrum Disorder: Design Considerations. IEEE Trans. Learn. Technol. 2018, 11, 133–151. [Google Scholar] [CrossRef]
- Mesa-Gresa, P.; Gil-Gómez, H.; Lozano-Quilis, J.A.; Gil-Gómez, J.A. Effectiveness of Virtual Reality for Children and Adolescents with Autism Spectrum Disorder: An Evidence-Based Systematic Review. Sensors 2018, 18, 2486. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schmidt, C.; Glaser, N.; Beck, D.; Lim, M.; Palmer, H. Evaluation of a spherical video-based virtual reality intervention designed to teach adaptive skills for adults with autism: A preliminary report. Interact. Learn. Environ. 2019, 29, 345–364. [Google Scholar] [CrossRef]
- Supekar, K. Brain Hyperconnectivity in Children with Autism and its Links to Social Deficits. Cell Rep. 2013, 5, 738–747. [Google Scholar] [CrossRef]
- Ecker, C.; Bookheimer, S.Y.; Murphy, D.G.M. Neuroimaging in Autism Spectrum Disorder: Brain structure and function across the lifespan. Lancet Neurol. 2015, 14, 1121–1134. [Google Scholar] [CrossRef]
- Hull, J.V.; Dokovna, L.B.; Jacokes, Z.J.; Torgerson, C.M.; Irimia, A.; Van Horn, J.D. Resting-State Functional Connectivity in Autism Spectrum Disorders: A Review. Front. Psychiatry 2017, 7, 205. [Google Scholar] [CrossRef]
- Subbaraju, V.; Suresh, M.; Sundaram, S.; Narasimhan, S. Identifying differences in brain activities and an accurate detection of Autism Spectrum Disorder using resting state functional-magnetic resonance imaging: A spatial filtering approach. Med. Image Anal. 2017, 35, 375–389. [Google Scholar] [CrossRef]
- Paakki, J.J. Alterations in regional homogeneity of resting-state brain activity in Autism Spectrum Disorder. Brain Res. 2010, 1321, 169–179. [Google Scholar] [CrossRef]
- Alaerts, K.; Swinnen, S.; Wenderoth, N. Sex differences in autism: A resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cogn. Affect. Neurosci. 2016, 11, 1002–1016. [Google Scholar] [CrossRef]
- Schumann, C.M.; Bloss, C.S.; Barnes, C.C.; Wideman, G.M.; Carper, R.A.; Akshoomoff, N.; Pierce, K.; Hagler, D.; Schork, N.; Lord, C.; et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 2010, 30, 4419–4427. [Google Scholar] [CrossRef] [PubMed]
- Ecker, C. The neuroanatomy of Autism Spectrum Disorder: An overview of structural neuroimaging findings and their translatability to the clinical setting. Autism 2016, 21, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Lombardo, M.V.; Ruigrok, A.N.V.; Chakrabarti, B.; Wheelwright, S.J.; Auyeung, B.; Allison, C.; MRC AIMS Consortium; Baron-Cohen, S. Cognition in Males and Females with Autism: Similarities and Differences. PLoS ONE 2012, 7, e47198. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Olivieri, C.; Buscema, M. Diagnosis of autism through EEG processed by advanced computational algorithms: A pilot study. Comput. Methods Programs Biomed. 2017, 142, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.M.; Vértes, P.E.; Seidlitz, J.; Kim, S.H.; Grosenick, L.; Liston, C. Molecular and network-level mechanisms explaining individual differences in Autism Spectrum Disorder. Nat. Neurosci. 2023, 26, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.; Georgiades, S.; Bishop, S.; Hardan, A. Behavioral and Cognitive Characteristics of Females and Males with Autism in the Simons Simplex Collection. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 329–340. [Google Scholar] [CrossRef]
- Hull, L.; Mandy, W.; Petrides, K. Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism 2017, 21, 706–727. [Google Scholar] [CrossRef]
- Greenberg, D.; Warrier, V.; Allison, C.; Baron-Cohen, S. Testing the Empathizing–Systemizing theory of sex differences and the Extreme Male Brain theory of autism in half a million people. Proc. Natl. Acad. Sci. USA 2018, 115, 12152–12157. [Google Scholar] [CrossRef]
- Floris, D.L.; Lai, M.C.; Giavasis, S.; Oldehinkel, M.; Mennes, M.; Charman, T.; Tillmann, J.; Dumas, G.; Ecker, C.; Dell’Acqua, F.; et al. Towards robust and replicable sex differences in the intrinsic brain function of autism. Mol. Autism 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Coburn, K.; Williams, D. Development of Neural Structure and Function in Autism Spectrum Disorder: Potential Implications for Learning Language. Am. J. Speech-Lang. Pathol. 2020, 29, 1783–1797. [Google Scholar] [CrossRef]
- Di, X.; Biswal, B.B. Similarly Expanded Bilateral Temporal Lobe Volumes in Female and Male Children with Autism Spectrum Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.M.; Geschwind, D.H. Sex differences in Autism Spectrum Disorders. Curr. Opin. Neurol. 2013, 26, 146. [Google Scholar] [CrossRef] [PubMed]
- de Giambattista, C.; Ventura, P.; Trerotoli, P.; Margari, F.; Margari, L. Sex Differences in Autism Spectrum Disorder: Focus on High Functioning Children and Adolescents. Front. Psychiatry 2021, 12, 539835. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; de los Angeles, C.; Ryali, S.; Cao, K.; Ma, T.; Menon, V. Deep learning identifies robust gender differences in functional brain organization and their dissociable links to clinical symptoms in autism. Br. J. Psychiatry 2022, 220, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Wallace, G.; Gallegos, S.; Braden, B. Brain-based sex differences in Autism Spectrum Disorder across the lifespan: A systematic review of structural MRI, fMRI, and DTI findings. Neuroimage Clin. 2021, 31, 102719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dougherty, C.C.; Baum, S.A.; White, T.; Michael, A.M. Functional connectivity predicts gender: Evidence for gender differences in resting brain connectivity. Hum. Brain Mapp. 2018, 39, 1765–1776. [Google Scholar] [CrossRef]
- Lawrence, K. Neural responsivity to social rewards in autistic female youth. Transl. Psych. 2020, 10, 178. [Google Scholar] [CrossRef]
- Lange, N.; Travers, B.G.; Bigler, E.D.; Prigge, M.B.D.; Froehlich, A.L.; Nielsen, J.A.; Cariello, A.N.; Zielinski, B.A.; Anderson, J.S.; Fletcher, P.T. Longitudinal Volumetric Brain Changes in Autism Spectrum Disorder Ages 6–35 Years. Autism Res. 2014, 8, 82–93. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Chakrabarti, B.; Ruigrok, A.N.; Bullmore, E.T.; Suckling, J.; Auyeung, B.; Happé, F.; Szatmari, P.; Baron-Cohen, S.; et al. Neural self-representation in autistic women and association with ‘compensatory camouflaging’. Autism 2018, 23, 1210–1223. [Google Scholar] [CrossRef]
- Yang, Y.; Allen, T.; Abdullahi, S.; Pelphrey, K.; Volkmar, F.; Chapman, S. Neural mechanisms of behavioral change in young adults with high-functioning autism receiving virtual reality social cognition training: A pilot study. Autism Res. 2018, 11, 713–725. [Google Scholar] [CrossRef]
- Cauvet, É.; Westeinde, A.v.; Toro, R.; Kuja-Halkola, R.; Neufeld, J.; Mevel, K.; Bölte, S. The social brain in female autism: A structural imaging study of twins. Soc. Cogn. Affect. Neurosci. 2020, 15, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Jack, A. A neurogenetic analysis of female autism. Brain 2021, 144, 1911–1926. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Lawrence, K.E.; Padgaonkar, N.T.; Inada, M.; Hoekstra, J.N.; Lowe, J.K.; Eilbott, J.; Jack, A.; Aylward, E.; Gaab, N.; et al. Imaging-genetics of sex differences in ASD: Distinct effects of OXTR variants on brain connectivity. Transl. Psych. 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; Menon, V. Sex differences in structural organization of motor systems and their dissociable links with repetitive/restricted behaviors in children with autism. Mol. Autism 2015, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D.R.; Blatt, G.J. Autism Spectrum Disorders and neuropathology of the cerebellum. Front. Neurosci. 2015, 9, 420. [Google Scholar] [CrossRef]
- Scott, J.A.; Schumann, C.M.; Goodlin-Jones, B.L.; Amaral, D.G. A comprehensive volumetric analysis of the cerebellum in children and adolescents with Autism Spectrum Disorder. Autism Res. 2009, 2, 246–257. [Google Scholar] [CrossRef]
- D’Mello, A.M.; Stoodley, C.J. Cerebro-cerebellar circuits in Autism Spectrum Disorder. Front. Neurosci. 2015, 9, 408. [Google Scholar] [CrossRef]
- Becker, E.B.; Stoodley, C.J. Autism Spectrum Disorder and the cerebellum. Int. Rev. Neurobiol. 2013, 113, 1–34. [Google Scholar] [CrossRef]
- van der Heijden, M.E.; Gill, J.S.; Sillitoe, R.V. Abnormal cerebellar development in Autism Spectrum Disorder. Dev. Neurosci. 2021, 43, 181–190. [Google Scholar] [CrossRef]
- Mo, K.; Sadoway, T.; Bonato, S.; Ameis, S.H.; Anagnostou, E.; Lerch, J.P.; Taylor, M.J.; Lai, M.C. Sex/gender differences in the human autistic brains: A systematic review of 20 years of neuroimaging research. Neuroimage Clin. 2021, 32, 102811. [Google Scholar] [CrossRef]
- Valenti, M.; Pino, M.C.; Mazza, M.; Panzarino, G.; Di Paolantonio, C.; Verrotti, A. Abnormal Structural and Functional Connectivity of the Corpus Callosum in Autism Spectrum Disorders: A Review. Rev. J. Autism Dev. Disord. 2019, 7, 46–62. [Google Scholar] [CrossRef]
- Nordahl, C.W.; Iosif, A.M.; Young, G.S.; Perry, L.M.; Dougherty, R.; Lee, A.; Li, D.; Buonocore, M.H.; Simon, T.; Rogers, S.; et al. Sex differences in the corpus callosum in preschool-aged children with Autism Spectrum Disorder. Mol. Autism 2015, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.; Saviozzi, I.; Brambilla, P.; Muratori, F.; Retico, A.; Calderoni, S. The effect of age, sex and clinical features on the volume of Corpus Callosum in pre-schoolers with Autism Spectrum Disorder: A case-control study. Eur. J. Neurosci. 2017, 47, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, B.; Wang, L.; Zhang, K.; Song, C.; Chen, J.; Cai, J.; Li, T. Corpus callosum volumes in children with Autism Spectrum Disorders: Sex-Associated differences. J. Autism Dev. Disord. 2023, 53, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- QEEG-Guided Neurotherapy for Autism Spectrum Disorder, Melbourne. Available online: https://www.adhd.com.au/autism/neurotherapy-autism-spectrum-disorder (accessed on 27 March 2023).
- Hurt, E.; Arnold, L.E.; Lofthouse, N. Quantitative EEG neurofeedback for the treatment of pediatric attention-deficit/hyperactivity disorder, Autism Spectrum Disorders, learning disorders, and epilepsy. Child Adolesc. Psychiatr. Clin. 2014, 23, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Billeci, L.; Sicca, F.; Maharatna, K.; Apicella, F.; Narzisi, A.; Campatelli, G.; Calderoni, S.; Pioggia, G.; Muratori, F. On the application of quantitative EEG for characterizing autistic brain: A systematic review. Front. Hum. Neurosci. 2013, 7, 442. [Google Scholar] [CrossRef]
- Coben, R.; Mohammad-Rezazadeh, I.; Cannon, R.L. Using quantitative and analytic EEG methods in the understanding of connectivity in Autism Spectrum Disorders: A theory of mixed over-and under-connectivity. Front. Hum. Neurosci. 2014, 8, 45. [Google Scholar] [CrossRef]
- Chabot, R.; Coben, R.; Hirshberg, L.; Cantor, D. QEEG and VARETA based neurophysiological indices of brain dysfunction in attention deficit and autistic spectrum disorder. J. Autism Relat. Disabil. 2015, 1, 1007. [Google Scholar]
- Ouattara, C. QEEG Characteristic and Spectral Analysis in Adults Diagnosed with Autism Spectrum Disorder. 2021. Available online: http://arno.uvt.nl/show.cgi?fid=157278 (accessed on 14 March 2023).
- Dimitrov, P.D.; Petrov, P.; Aleksandrov, I.; Dimitrov, I.; Mihailova, M.; Radkova, G.; Dimitrova, R. Quantitative EEG Comparative Analysis between Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD). J. IMAB Ann. Proc. Sci. Pap. 2017, 23, 1441–1443. [Google Scholar] [CrossRef]
- Pop-Jordanova, N.; Zorcec, T.; Demerdzieva, A.; Gucev, Z. QEEG characteristics and spectrum weighted frequency for children diagnosed as autistic spectrum disorder. Nonlin. Biomed. Phys. 2010, 4, 1–7. [Google Scholar] [CrossRef]
- McVoy, M.; Lytle, S.; Fulchiero, E.; Aebi, M.E.; Adeleye, O.; Sajatovic, M. A systematic review of quantitative EEG as a possible biomarker in child psychiatric disorders. Psych. Res. 2019, 279, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Al-Salihy, A.A.R.S. Resting-state QEEG Neuro-Biomarkers for Diagnosis and Treatment Planning of Autism Spectrum Disorders. Kufa Med. J. 2022, 18, 1–18. [Google Scholar] [CrossRef]
- Alabood, L.; Krul, E.; Shahidi, A.; Jaswal, V.K.; Krishnamurthy, D.; Wang, M. HoloType-CR: Cross Reality Communication Training for Minimally Verbal Autistic Persons. In Proceedings of the 2022 IEEE International Symposium on Mixed and Augmented Reality Adjunct (ISMAR-Adjunct), Singapore, 17–21 October 2022; pp. 187–190. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, I.J.; Lin, L.Y. Augmented reality-based video-modeling storybook of nonverbal facial cues for children with Autism Spectrum Disorder to improve their perceptions and judgments of facial expressions and emotions. Comput. Hum. Behav. 2016, 55, 477–485. [Google Scholar] [CrossRef]
- Lee, I.J. Kinect-for-windows with augmented reality in an interactive roleplay system for children with an Autism Spectrum Disorder. Interact. Learn. Environ. 2020, 29, 688–704. [Google Scholar] [CrossRef]
- Wedyan, M.; Falah, J.; Alturki, R.; Giannopulu, I.; Alfalah, S.F.M.; Elshaweesh, O.; Al-Jumaily, A. Augmented Reality for Autistic Children to Enhance Their Understanding of Facial Expressions. Multimodal Technol. Interact. 2021, 5, 48. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Z.; Chai, Y.; Li, X.; Wei, X. FaceMe: An agent-based social game using augmented reality for the emotional development of children with Autism Spectrum Disorder. Int. J. Hum.-Comput. Stud. 2023, 175, 103032. [Google Scholar] [CrossRef]
- Mora-Guiard, J.; Crowell, C.; Pares, N.; Heaton, P. Lands of fog: Helping children with autism in social interaction through a full-body interactive experience. In Proceedings of the 15th International Conference on Interaction Design and Children, Manchester, UK, 21–24 June 2016; pp. 262–274. [Google Scholar] [CrossRef]
- Rosenfield, N.; Lamkin, K.; Re, J.; Day, K.; Boyd, L.; Linstead, E. A Virtual Reality System for Practicing Conversation Skills for Children with Autism. Multimodal Technol. Interact. 2019, 3, 28. [Google Scholar] [CrossRef]
- Amaral, C.; Mouga, S.; Simões, M.; Pereira, H.C.; Bernardino, I.; Quental, H.; Playle, R.; McNamara, R.; Oliveira, G.; Castelo-Branco, M. A feasibility clinical trial to improve social attention in autistic spectrum disorder (ASD) using a brain computer interface. Front. Neurosci. 2018, 12, 477. [Google Scholar] [CrossRef]
- Madsen, M.; Kaliouby, R.; Goodwin, M.; Picard, R. Technology for just-in-time in-situ learning of facial affect for persons diagnosed with an Autism Spectrum Disorder. In Proceedings of the 10th International ACM SIGACCESS Conference on Computers and Accessibility, Halifax, NS, Canada, 13–15 October 2008; pp. 19–26. [Google Scholar] [CrossRef]
- Lyu, Y.; An, P.; Xiao, Y.; Zhang, Z.; Zhang, H.; Katsuragawa, K.; Zhao, J. Eggly: Designing Mobile Augmented Reality Neurofeedback Training Games for Children with Autism Spectrum Disorder. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2023, 7, 1–29. [Google Scholar] [CrossRef]
- Bauer, V.; Bouchara, T.; Bourdot, P. Designing an extended reality application to expand clinic-based sensory strategies for autistic children requiring substantial support: Participation of practitioners. In Proceedings of the 2021 IEEE International Symposium on Mixed and Augmented Reality Adjunct (ISMAR-Adjunct), Bari, Italy, 4–8 October 2021; pp. 254–259. [Google Scholar] [CrossRef]
- Strickland, D.; Marcus, L.M.; Mesibov, G.B.; Hogan, K. Brief report: Two case studies using virtual reality as a learning tool for autistic children. J. Autism Dev. Disord. 1996, 26, 651–659. [Google Scholar] [CrossRef]
- Dixon, D.; Miyake, C.; Nohelty, K.; Novack, M.; Granpeesheh, D. Evaluation of an Immersive Virtual Reality Safety Training Used to Teach Pedestrian Skills to Children with Autism Spectrum Disorder. Behav. Anal. Pract. 2020, 13, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, S.; Nickel, A.; Barnes, T.; Suma, E. Astrojumper: Designing a virtual reality exergame to motivate children with autism to exercise. In Proceedings of the 2010 IEEE Virtual Reality Conference (VR), Boston, MA, USA, 20–24 March 2010; pp. 267–268. [Google Scholar] [CrossRef]
- Zheng, Z.K.; Sarkar, N.; Swanson, A.; Weitlauf, A.; Warren, Z.; Sarkar, N. CheerBrush: A novel interactive augmented reality coaching system for toothbrushing skills in children with Autism Spectrum Disorder. ACM Trans. Access. Comput. 2021, 14, 1–20. [Google Scholar] [CrossRef]
- Ahmed, S.; Deneke, W.; Mai, V.; Veneruso, A.; Stepita, M.; Dawson, A.; Hoefel, B.; Claeys, G.; Lam, N.; Sharmin, M. InterViewR: A mixed-reality based interview training simulation platform for individuals with autism. In Proceedings of the 2020 IEEE 44th Annual Computers, Software, and Applications Conference (COMPSAC), Madrid, Spain, 13–17 July 2020; pp. 439–448. [Google Scholar] [CrossRef]
- Wade, J.; Zhang, L.; Bian, D.; Fan, J.; Swanson, A.; Weitlauf, A.; Sarkar, M.; Warren, Z.; Sarkar, N. A gaze-contingent adaptive virtual reality driving environment for intervention in individuals with Autism Spectrum Disorders. ACM Trans. Interact. Intell. Syst. 2016, 6, 1–23. [Google Scholar] [CrossRef]
- Escobedo, L.; Tentori, M.; Quintana, E.; Favela, J.; Garcia-Rosas, D. Using augmented reality to help children with autism stay focused. IEEE Pervasive Comput. 2014, 13, 38–46. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, B.; Cho, Y. Using Mobile Augmented Reality to Improve Attention in Adults with Autism Spectrum Disorder. In Proceedings of the Extended Abstracts of the 2020 CHI Conference on Human Factors in Computing Systems, Honolulu, HI, USA, 25–30 April 2020; pp. 1–9. [Google Scholar] [CrossRef]
- Lu, K.; Yueh, K.; Hu, H.; Guo, M.; Liu, Y. A Novel Neurofeedback Attentional Enhancement Approach Based on Virtual Reality. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 5140–5143. [Google Scholar] [CrossRef]
- Fraser, D.; Marder, T.; deBettencourt, L.; Myers, L.; Kalymon, K.; Harrell, R. Using a Mixed-Reality Environment to Train Special Educators Working with Students with Autism Spectrum Disorder to Implement Discrete Trial Teaching. Focus Autism Other Dev. Disabil. 2019, 35, 3–14. [Google Scholar] [CrossRef]
- Hashim, H.; Yunus, M.; Norman, H. ‘AReal-Vocab’: An Augmented Reality English Vocabulary Mobile Application to Cater to Mild Autism Children in Response towards Sustainable Education for Children with Disabilities. Sustainability 2022, 14, 4831. [Google Scholar] [CrossRef]
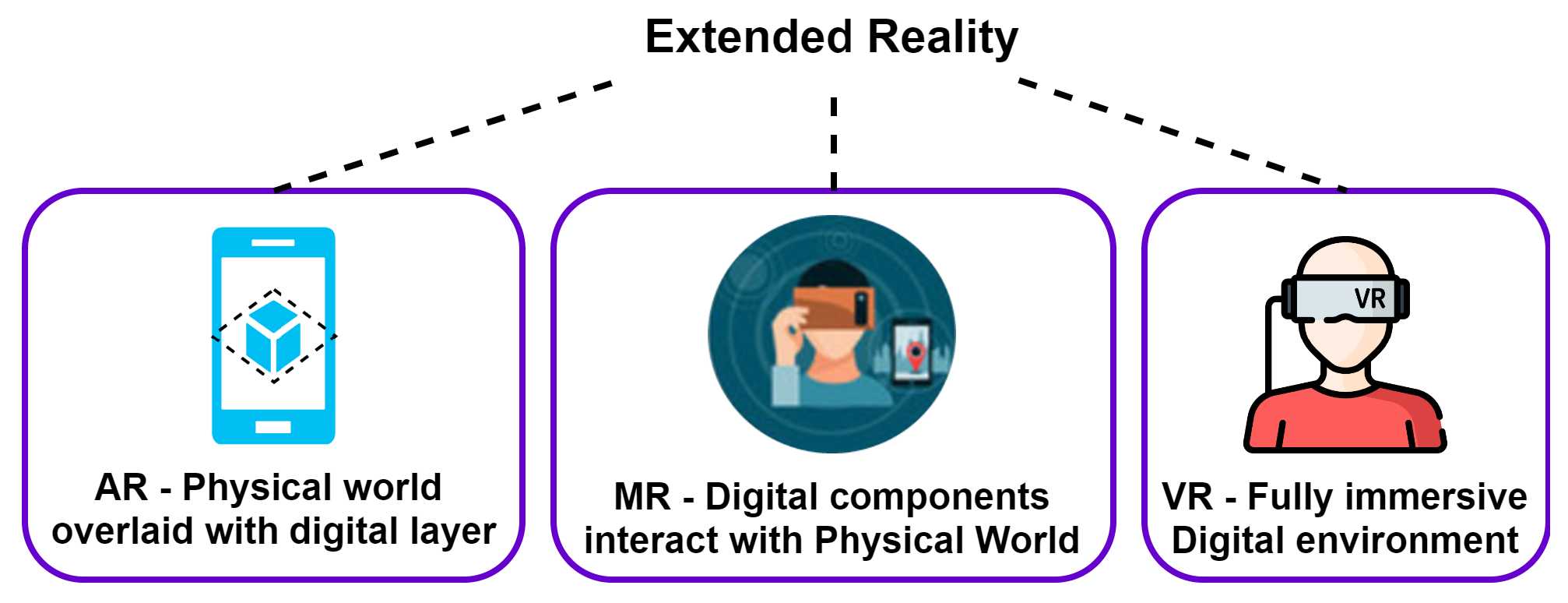
| Levels of ASD | Characteristics |
|---|---|
| Level 1: Requiring Support |
|
| Level 2: Requiring Substantial Support |
|
| Level 3: Requiring Very Substantial Support |
|
| Author(s) | Topic | General Findings | Pros | Cons |
|---|---|---|---|---|
| Bertram et al. [24] | XR | CAT shows promise, lacks rigorous assessment | Covers multiple areas, provides VR use cases | No consideration for alternatives |
| Shoaib et al. [25] | XR | Benefits those with ASD, improves skills | Provides VR use cases | Limited tech coverage, insufficient research |
| Mubin et al. [26] | XR | XR improves behavior, attention, reduces stress in ASD | Covers XR use cases and weaknesses | No participant info, limited analysis |
| Chen et al. [23] | XR | XR improves ASD outcomes across numerous areas | Categorizes literature by XR tech type | Lack of info on hybrid interventions |
| Roberts et al. [27] | XR | Limited evidence on XR’s effectiveness for ASD children | Quality of XR-based studies, mentions of brain anatomy | Unclear effectiveness on older teens |
| Maran et al. [28] | XR | XR improves navigation and daily living skills for ID individuals | Wide age range of participants, varying degrees of ID | Small sample sizes, no control group |
| Gu et al. [29] | XR | XR platform usage tied to technical features and study traits | Review of XR technologies, high number of studies | No consistent quality check |
| Karagiannidis et al. [30] | AR | AR can help people with ASD learn new skills | Coverage of use cases | Very low number of studies |
| Wedyan et al. [31] | AR | Limited long-term research on AR’s effectiveness with autistic children | Proposes new AR system, strong evidence for AR usage | Small sample sizes, short study durations |
| Lian et al. [32] | AR | Mobile AR aids autism intervention to some degree | Discusses mobile applications of AR | Studies involved mostly children, small sample size |
| Cavus et al. [33] | AR | AR applicable for multiple fields and disabilities | Calls for more AR-based research | Limited search criteria |
| Huamanchahua et al. [34] | AR | AR/VR can enhance education, but usage is limited | Discusses AR and VR platforms used | Participants’ characteristics not discussed |
| Shahmoradi et al. [35] | VR | VR technologies had beneficial effects on reducing cognitive problems | Strong assessment of study quality | Very low number of studies, low numbers of women participants |
| Zhang et al. [36] | VR | VR improves social functioning, emotion recognition, speech, and language | Overview of popular VR products | Does not discuss applicability for adults with ASD |
| Glaser et al. [37] | VR | Inconsistencies in how VR is conceptualized | Need for clear VR definition | Does not address other types of XR technologies |
| Bozgeyikli et al. [38] | VR | VR design guidelines for ASD individuals rely on observation and lack generalizability | Covers VR system types, design considerations | Low number of participants |
| Mesa-Gresa et al. [39] | VR | VR-based treatment improves at least one common ASD deficit | Reviews clinical and technical databases | Limited insight into variations between males and females. Focus on ‘high performance autism’ |
| Author | Technique(s) Used | Pros | Cons |
|---|---|---|---|
| Supekar et al. [41] | fMRI (functional magnetic resonance imaging) |
|
|
| Alaerts et al. [46] |
|
| |
| Schumann et al. [47] | MRI |
|
|
| Ecker et al. [21,42,48] |
|
| |
| Lai et al. [49] |
|
| |
| Hull et al. [43] | rs-FMRI |
|
|
| Subbaraju et al. [44] |
|
| |
| Grossi et al. [50] | EEG |
|
|
| Buch et al. [51] | Machine Learning |
|
|
| Frazier et al. [52] | Data analysis from the Simons Simplex Collection. t-tests were also used for determining clinical characteristics among women. |
|
|
| Paaki et al. [45] | Regional Homogeneity (ReHo) Analysis |
|
|
| Brain Region(s) | Related References | Associated Organs |
|---|---|---|
| Temporal Lobe | [45,47,57,58,59,60,61,62,63,64] | Middle Temporal Gyrus (MTG) |
| Amygdala | ||
| Fusiform gyrus | ||
| Hippocampus * | ||
| Insula * | ||
| Frontal Lobe | [7,47,58,62,64,65,66,67,68] | Anterior cingulate cortex (ACC) |
| Cingulate gyrus | ||
| Dorsal anterior cingulate cortex (dACC) * | ||
| Dorsolateral prefrontal cortex (DLPFC) | ||
| Inferior frontal gyrus (IFG) | ||
| Medial prefrontal cortex (MPFC) | ||
| Prefrontal cortex | ||
| Superior frontal gyrus (SFG) | ||
| Subcortical Structures | [69,70] | Basal ganglia |
| Cerebellum | [71,72,73,74,75] | Cerebellar vermis |
| Occipital Lobe | [64,76] | Visual cortex |
| Lateral occipital cortex | ||
| Parietal Lobe | [7,62,64,66] | Superior parietal lobule |
| Posterior cingulate cortex (PCC) * | ||
| Corpus Callosum | [76,77,78,79,80] | N/A |
| Areas of Improvement | Sub-Category | Citation | Method | Specific Use Case(s) |
|---|---|---|---|---|
| Social Skills | Nonverbal Communication | [91] | MR | Nonverbal communication |
| Body Language and Facial Expressions | [92,93,94,95] | AR | Body language and facial expressions | |
| Social Interaction | [96] | MR | Collaboration | |
| [97,98] | VR | Social interaction and social cognition | ||
| Emotions | [99,100,101] | - AR MR | Understanding Emotions Empathy Relaxation | |
| Life Skills and Daily Activities | Visual Perception 1 | [102,103] | VR | Street crossing |
| Physical Well-being 2 | [104,105] | VR AR | Exercise Improving fine motor skills | |
| Daily Living 3 | [106,107] | MR VR | Preparing for job interviews Driving | |
| Concentration | Task Engagement | [108] | AR | Therapy sessions |
| Attention Management 4 | [109] | AR | Attention management in terms of distraction | |
| Attention Enhancement 5 | [110] | VR | Attention enhancement | |
| Education | Special Education 6 | [111,112] | MR AR | Helping special educators teach children with ASD Improving English Vocabulary |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzak, R.; Li, J.; He, S.; Sokhadze, E. Investigating Sex-Based Neural Differences in Autism and Their Extended Reality Intervention Implications. Brain Sci. 2023, 13, 1571. https://doi.org/10.3390/brainsci13111571
Razzak R, Li J, He S, Sokhadze E. Investigating Sex-Based Neural Differences in Autism and Their Extended Reality Intervention Implications. Brain Sciences. 2023; 13(11):1571. https://doi.org/10.3390/brainsci13111571
Chicago/Turabian StyleRazzak, Rehma, Joy Li, Selena He, and Estate Sokhadze. 2023. "Investigating Sex-Based Neural Differences in Autism and Their Extended Reality Intervention Implications" Brain Sciences 13, no. 11: 1571. https://doi.org/10.3390/brainsci13111571
APA StyleRazzak, R., Li, J., He, S., & Sokhadze, E. (2023). Investigating Sex-Based Neural Differences in Autism and Their Extended Reality Intervention Implications. Brain Sciences, 13(11), 1571. https://doi.org/10.3390/brainsci13111571






