Hippocampal Resting State Functional Connectivity Associated with Physical Activity in Periadolescent Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Physical Activity
2.3. MRI
2.3.1. Procedures
2.3.2. Scanning Parameters
2.3.3. Data Processing and Analysis
2.3.4. Statistical Analysis
3. Results
3.1. Behavioral Data
3.2. Neuroimaging Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fair, D.A.; Dosenbach, N.U.F.; Church, J.A.; Cohen, A.L.; Brahmbhatt, S.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. USA 2007, 104, 13507–13512. [Google Scholar] [CrossRef] [PubMed]
- Fair, D.A.; Cohen, A.L.; Dosenbach, N.U.F.; Church, J.A.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. The maturing architecture of the brain’s default network. Proc. Natl. Acad. Sci. USA 2008, 105, 4028–4032. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Hoff, G.E.A.-J.; Van den Heuvel, M.P.; Benders, M.J.N.L.; Kersbergen, K.J.; De Vries, L.S. On development of functional brain connectivity in the young brain. Front. Hum. Neurosci. 2013, 7, 650. [Google Scholar] [CrossRef] [PubMed]
- Lenroot, R.K.; Giedd, J.N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006, 30, 718–729. [Google Scholar] [CrossRef]
- Menon, V. Developmental pathways to functional brain networks: Emerging principles. Trends Cogn. Sci. 2013, 17, 627–640. [Google Scholar] [CrossRef]
- Stevens, M.C.; Pearlson, G.D.; Calhoun, V.D. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum. Brain Mapp. 2009, 30, 2356–2366. [Google Scholar] [CrossRef]
- Stevens, W.D.; Spreng, R.N. Resting-state functional connectivity MRI reveals active processes central to cognition: Cognition and resting-state functional connectivity. WIREs Cogn. Sci. 2014, 5, 233–245. [Google Scholar] [CrossRef]
- Blankenship, S.L.; Redcay, E.; Dougherty, L.R.; Riggins, T. Development of hippocampal functional connectivity during childhood. Hum. Brain Mapp. 2017, 38, 182–201. [Google Scholar] [CrossRef]
- Warren, D.E.; Rangel, A.J.; Christopher-Hayes, N.J.; Eastman, J.A.; Frenzel, M.R.; Stephen, J.M.; Calhoun, V.D.; Wang, Y.; Wilson, T.W. Resting-state functional connectivity of the human hippocampus in periadolescent children: Associations with age and memory performance. Hum. Brain Mapp. 2021, 42, 3620–3642. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Wendel, C.; Voss, M.W. Physical Activity and Cognitive Training: Impact on Hippocampal Structure and Function. In The Hippocampus from Cells to Systems; Hannula, D.E., Duff, M.C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 209–243. ISBN 978-3-319-50405-6. [Google Scholar]
- Meijer, A.; Königs, M.; Vermeulen, G.T.; Visscher, C.; Bosker, R.J.; Hartman, E.; Oosterlaan, J. The effects of physical activity on brain structure and neurophysiological functioning in children: A systematic review and meta-analysis. Dev. Cogn. Neurosci. 2020, 45, 100828. [Google Scholar] [CrossRef] [PubMed]
- Valkenborghs, S.R.; Noetel, M.; Hillman, C.H.; Nilsson, M.; Smith, J.J.; Ortega, F.B.; Lubans, D.R. The Impact of Physical Activity on Brain Structure and Function in Youth: A Systematic Review. Pediatrics 2019, 144, e20184032. [Google Scholar] [CrossRef] [PubMed]
- Voelcker-Rehage, C.; Niemann, C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci. Biobehav. Rev. 2013, 37, 2268–2295. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Soto, C.; Yoo, S.; Sodoma, M.; Vivar, C.; Van Praag, H. Exercise and Hippocampal Memory Systems. Trends Cogn. Sci. 2019, 23, 318–333. [Google Scholar] [CrossRef]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Kim, J.S.; Voss, M.W.; Vanpatter, M.; Pontifex, M.B.; Raine, L.B.; Konkel, A.; Hillman, C.H.; et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010, 1358, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage 2018, 166, 230–238. [Google Scholar] [CrossRef]
- Herting, M.M.; Nagel, B.J. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav. Brain Res. 2012, 233, 517–525. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, W.; Jiang, F.; Wu, F.; Li, G.; Hu, Y.; Zhang, W.; Wang, J.; Fan, X.; Wei, X.; et al. Associations among body mass index, working memory performance, gray matter volume, and brain activation in healthy children. Cereb. Cortex 2023, 33, 6335–6344. [Google Scholar] [CrossRef]
- Esteban-Cornejo, I.; Stillman, C.M.; Rodriguez-Ayllon, M.; Kramer, A.F.; Hillman, C.H.; Catena, A.; Erickson, K.I.; Ortega, F.B. Physical fitness, hippocampal functional connectivity and academic performance in children with overweight/obesity: The ActiveBrains project. Brain Behav. Immun. 2021, 91, 284–295. [Google Scholar] [CrossRef]
- Voss, M.W.; Chaddock, L.; Kim, J.S.; VanPatter, M.; Pontifex, M.B.; Raine, L.B.; Cohen, N.J.; Hillman, C.H.; Kramer, A.F. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience 2011, 199, 166–176. [Google Scholar] [CrossRef]
- Brooks, S.J.; Parks, S.M.; Stamoulis, C. Widespread Positive Direct and Indirect Effects of Regular Physical Activity on the Developing Functional Connectome in Early Adolescence. Cereb. Cortex 2021, 31, 4840–4852. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.E.; Pierce, J.E.; Schwarz, N.F.; Chi, L.; Weinberger, A.L.; Schaeffer, D.J.; Rodrigue, A.L.; Camchong, J.; Allison, J.D.; Yanasak, N.E.; et al. An eight month randomized controlled exercise intervention alters resting state synchrony in overweight children. Neuroscience 2014, 256, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Ruotsalainen, I.; Glerean, E.; Karvanen, J.; Gorbach, T.; Renvall, V.; Syväoja, H.J.; Tammelin, T.H.; Parviainen, T. Physical activity and aerobic fitness in relation to local and interhemispheric functional connectivity in adolescents’ brains. Brain Behav. 2021, 11, e01941. [Google Scholar] [CrossRef] [PubMed]
- Fung, H.; Yeo, B.T.T.; Chen, C.; Lo, J.C.; Chee, M.W.L.; Ong, J.L. Adherence to 24-Hour Movement Recommendations and Health Indicators in Early Adolescence: Cross-Sectional and Longitudinal Associations in the Adolescent Brain Cognitive Development Study. J. Adolesc. Health 2023, 72, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, K.; Pontifex, M.B.; O’Leary, K.C.; Scudder, M.R.; Wu, C.-T.; Castelli, D.M.; Hillman, C.H. The effects of an afterschool physical activity program on working memory in preadolescent children: Fitness and working memory in children. Dev. Sci. 2011, 14, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Pontifex, M.B.; Castelli, D.M.; Khan, N.A.; Raine, L.B.; Scudder, M.R.; Drollette, E.S.; Moore, R.D.; Wu, C.-T.; Kamijo, K. Effects of the FITKids Randomized Controlled Trial on Executive Control and Brain Function. Pediatrics 2014, 134, e1063–e1071. [Google Scholar] [CrossRef]
- Chaddock-Heyman, L.; Erickson, K.I.; Chappell, M.A.; Johnson, C.L.; Kienzler, C.; Knecht, A.; Drollette, E.S.; Raine, L.B.; Scudder, M.R.; Kao, S.-C.; et al. Aerobic fitness is associated with greater hippocampal cerebral blood flow in children. Dev. Cogn. Neurosci. 2016, 20, 52–58. [Google Scholar] [CrossRef]
- Fair, D.A.; Miranda-Dominguez, O.; Snyder, A.Z.; Perrone, A.; Earl, E.A.; Van, A.N.; Koller, J.M.; Feczko, E.; Tisdall, M.D.; van der Kouwe, A.; et al. Correction of respiratory artifacts in MRI head motion estimates. NeuroImage 2020, 208, 116400. [Google Scholar] [CrossRef]
- Barch, D.M.; Albaugh, M.D.; Avenevoli, S.; Chang, L.; Clark, D.B.; Glantz, M.D.; Hudziak, J.J.; Jernigan, T.L.; Tapert, S.F.; Yurgelun-Todd, D.; et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev. Cogn. Neurosci. 2018, 32, 55–66. [Google Scholar] [CrossRef]
- Casey, B.J.; Cannonier, T.; Conley, M.I.; Cohen, A.O.; Barch, D.M.; Heitzeg, M.M.; Soules, M.E.; Teslovich, T.; Dellarco, D.V.; Garavan, H.; et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018, 32, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.P.; Somerville, L.H.; Ances, B.M.; Andersson, J.; Barch, D.M.; Bastiani, M.; Bookheimer, S.Y.; Brown, T.B.; Buckner, R.L.; Burgess, G.C.; et al. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. NeuroImage 2018, 183, 972–984. [Google Scholar] [CrossRef]
- Glasser, M.F.; Sotiropoulos, S.N.; Wilson, J.A.; Coalson, T.S.; Fischl, B.; Andersson, J.L.; Xu, J.; Jbabdi, S.; Webster, M.; Polimeni, J.R.; et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 2013, 80, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.W.; Chen, G.; Glen, D.R.; Reynolds, R.C.; Taylor, P.A. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 2017, 7, 152–171. [Google Scholar] [CrossRef] [PubMed]
- Eklund, A.; Knutsson, H.; Nichols, T.E. Cluster Failure Revisited: Impact of First Level Design and Data Quality on Cluster False Positive Rates. Hum Brain Mapp. 2019, 40, 2017–2032. [Google Scholar] [CrossRef] [PubMed]
- Brodoehl, S.; Gaser, C.; Dahnke, R.; Witte, O.W.; Klingner, C.M. Surface-based analysis increases the specificity of cortical activation patterns and connectivity results. Sci. Rep. 2020, 10, 5737. [Google Scholar] [CrossRef]
- Gordon, E.M.; Laumann, T.O.; Adeyemo, B.; Huckins, J.F.; Kelley, W.M.; Petersen, S.E. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb. Cortex 2016, 26, 288–303. [Google Scholar] [CrossRef]
- Marcus, D.S.; Harwell, J.; Olsen, T.; Hodge, M.; Glasser, M.F.; Prior, F.; Jenkinson, M.; Laumann, T.; Curtiss, S.W.; Van Essen, D.C. Informatics and Data Mining Tools and Strategies for the Human Connectome Project. Front. Neuroinform. 2011, 5, 4. [Google Scholar] [CrossRef]
- Ikuta, T.; Loprinzi, P.D. Association of cardiorespiratory fitness on interhemispheric hippocampal and parahippocampal functional connectivity. Eur. J. Neurosci. 2019, 50, 1871–1877. [Google Scholar] [CrossRef]
- Kronman, C.A.; Kern, K.L.; Nauer, R.K.; Dunne, M.F.; Storer, T.W.; Schon, K. Cardiorespiratory fitness predicts effective connectivity between the hippocampus and default mode network nodes in young adults. Hippocampus 2020, 30, 526–541. [Google Scholar] [CrossRef]
- Li, M.; Huang, M.; Li, S.; Tao, J.; Zheng, G.; Chen, L. The effects of aerobic exercise on the structure and function of DMN-related brain regions: A systematic review. Int. J. Neurosci. 2017, 127, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Uyar, F.; Huang, H.; Grove, G.A.; Watt, J.C.; Wollam, M.E.; Erickson, K.I. Cardiorespiratory fitness is associated with enhanced hippocampal functional connectivity in healthy young adults. Hippocampus 2018, 28, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Malkowski, E.; Alves, H.; Kim, J.S.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; et al. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia 2010, 48, 1394–1406. [Google Scholar] [CrossRef]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and Ventral Attention Systems: Distinct Neural Circuits but Collaborative Roles. Neuroscientist 2014, 20, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Kincade, J.M.; Shulman, G.L. Neural Systems for Visual Orienting and Their Relationships to Spatial Working Memory. J. Cogn. Neurosci. 2002, 14, 508–523. [Google Scholar] [CrossRef]
- Konrad, K.; Neufang, S.; Thiel, C.M.; Specht, K.; Hanisch, C.; Fan, J.; Herpertz-Dahlmann, B.; Fink, G.R. Development of attentional networks: An fMRI study with children and adults. NeuroImage 2005, 28, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Rohr, C.S.; Vinette, S.A.; Parsons, K.A.L.; Cho, I.Y.K.; Dimond, D.; Benischek, A.; Lebel, C.; Dewey, D.; Bray, S. Functional Connectivity of the Dorsal Attention Network Predicts Selective Attention in 4–7 year-old Girls. Cereb. Cortex 2017, 27, 4350–4360. [Google Scholar] [CrossRef]
- Rohr, C.S.; Arora, A.; Cho, I.Y.K.; Katlariwala, P.; Dimond, D.; Dewey, D.; Bray, S. Functional network integration and attention skills in young children. Dev. Cogn. Neurosci. 2018, 30, 200–211. [Google Scholar] [CrossRef]
- Szczepanski, S.M.; Pinsk, M.A.; Douglas, M.M.; Kastner, S.; Saalmann, Y.B. Functional and structural architecture of the human dorsal frontoparietal attention network. Proc. Natl. Acad. Sci. USA 2013, 110, 15806–15811. [Google Scholar] [CrossRef] [PubMed]
- Fair, D.A.; Cohen, A.L.; Power, J.D.; Dosenbach, N.U.F.; Church, J.A.; Miezin, F.M.; Schlaggar, B.L.; Petersen, S.E. Functional Brain Networks Develop from a “Local to Distributed” Organization. PLoS Comput. Biol. 2009, 5, e1000381. [Google Scholar] [CrossRef]
- Supekar, K.; Musen, M.; Menon, V. Development of Large-Scale Functional Brain Networks in Children. PLoS Biol. 2009, 7, e1000157. [Google Scholar] [CrossRef] [PubMed]
- Faghiri, A.; Stephen, J.M.; Wang, Y.; Wilson, T.W.; Calhoun, V.D. Changing brain connectivity dynamics: From early childhood to adulthood. Hum. Brain Mapp. 2018, 39, 1108–1117. [Google Scholar] [CrossRef]
- Johnson, M.H. Functional brain development in humans. Nat. Rev. Neurosci. 2001, 2, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Baym, C.L.; Monti, J.M.; Raine, L.B.; Drollette, E.S.; Scudder, M.R.; Moore, R.D.; Kramer, A.F.; Hillman, C.H.; Cohen, N.J. Central Adiposity Is Negatively Associated with Hippocampal-Dependent Relational Memory among Overweight and Obese Children. J. Pediatr. 2015, 166, 302–308.e1. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; McGlade, E.C.; Huber, R.S.; Lyoo, I.K.; Renshaw, P.F.; Yurgelun-Todd, D.A. Overweight/Obesity-related microstructural alterations of the fimbria-fornix in the ABCD study: The role of aerobic physical activity. PLoS ONE 2023, 18, e0287682. [Google Scholar] [CrossRef]
- Petrican, R.; Fornito, A.; Boyland, E. Lifestyle Factors Counteract the Neurodevelopmental Impact of Genetic Risk for Accelerated Brain Aging in Adolescence. Biol. Psychiatry, 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Power, J.; Mitra, A.; Laumann, T.; Snyder, A.; Schlaggar, B.; Petersen, S. Methods to detect, charac-terize, and remove motion artifact in resting state Fmri. NeuroImage 2014, 84, 320–341. [Google Scholar] [CrossRef]
- Murphy, K.; Birn, R.; Handwerker, D.; Jones, T.; Bandettini, P. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage 2009, 44, 893–905. [Google Scholar] [CrossRef]
- Enguix, V.; Easson, K.; Gilbert, G.; Saint-Martin, C.; Rohlicek, C.; Luck, D.; Lodygensky, G.A.; Brossard-Racine, M. Altered resting state functional connectivity in youth with congenital heart disease operated during infancy. PLoS ONE 2022, 17, e0264781. [Google Scholar] [CrossRef]
- Haneef, Z.; Lenartowicz, A.; Yeh, H.J.; Levin, H.S.; Engel, J.; Stern, J.M. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 2014, 55, 137–145. [Google Scholar] [CrossRef]
- Schwab, S.; Afyouni, S.; Chen, Y.; Han, Z.; Guo, Q.; Dierks, T.; Wahlund, L.-O.; Grieder, M. Functional Connectivity Alterations of the Temporal Lobe and Hippocampus in Semantic Dementia and Alzheimer’s Disease. JAD 2020, 76, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zang, Y.; He, Y.; Liang, M.; Zhang, X.; Tian, L.; Wu, T.; Jiang, T.; Li, K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. NeuroImage 2006, 31, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Jiang, Y.; Wang, Z.; Zhang, H.; Li, H.; Biswal, B.B. Hippocampus-Based Dynamic Functional Connectivity Mapping in the Early Stages of Alzheimer’s Disease. JAD 2022, 85, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
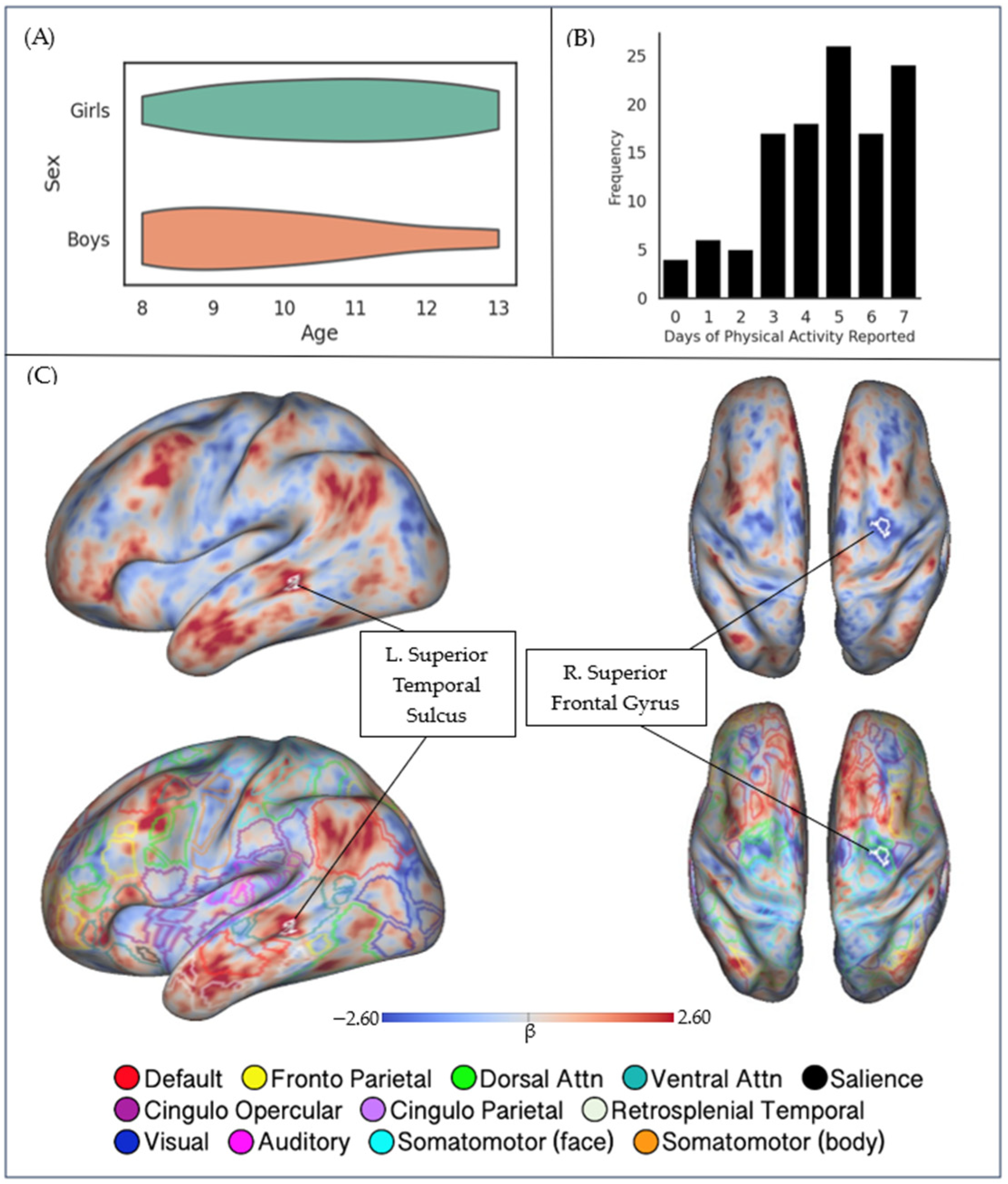
| Characteristic | M (SD) | Range |
|---|---|---|
| Age (at time of MRI scan, years) | 10.92 (1.61) | 8.24–14.0 |
| Pubertal stage | 1.48 (0.69) | 0.0–3.4 |
| Average number of days reporting PA | 4.61(1.91) | 0–7 |
| BMI (kg/m2) | 19.09 (4.25) | 12.75–32.81 |
| Household income | 8.74 (1.13) | 5.00–10.00 |
| Seed | Nature of Correlation | Cluster Location | Peak MNI Coordinate (X, Y, Z) | Cluster Area (mm2) | t Value |
|---|---|---|---|---|---|
| Bilateral Hippocampus | Negative | R. Superior Frontal Gyrus | +30.7, −3.8, +50.1 | 48.79 | 4.724 |
| Bilateral Hippocampus | Positive | L. Superior Temporal Sulcus | −57.3, −35.4, −3.2 | 52.04 | 4.231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heller-Wight, A.; Phipps, C.; Sexton, J.; Ramirez, M.; Warren, D.E. Hippocampal Resting State Functional Connectivity Associated with Physical Activity in Periadolescent Children. Brain Sci. 2023, 13, 1558. https://doi.org/10.3390/brainsci13111558
Heller-Wight A, Phipps C, Sexton J, Ramirez M, Warren DE. Hippocampal Resting State Functional Connectivity Associated with Physical Activity in Periadolescent Children. Brain Sciences. 2023; 13(11):1558. https://doi.org/10.3390/brainsci13111558
Chicago/Turabian StyleHeller-Wight, Abi, Connor Phipps, Jennifer Sexton, Meghan Ramirez, and David E. Warren. 2023. "Hippocampal Resting State Functional Connectivity Associated with Physical Activity in Periadolescent Children" Brain Sciences 13, no. 11: 1558. https://doi.org/10.3390/brainsci13111558
APA StyleHeller-Wight, A., Phipps, C., Sexton, J., Ramirez, M., & Warren, D. E. (2023). Hippocampal Resting State Functional Connectivity Associated with Physical Activity in Periadolescent Children. Brain Sciences, 13(11), 1558. https://doi.org/10.3390/brainsci13111558





