The Mediation Role of Sleep Disturbances between Vitamin D and Depressive Symptoms: A Cross-Sectional Study
Abstract
:1. Introduction
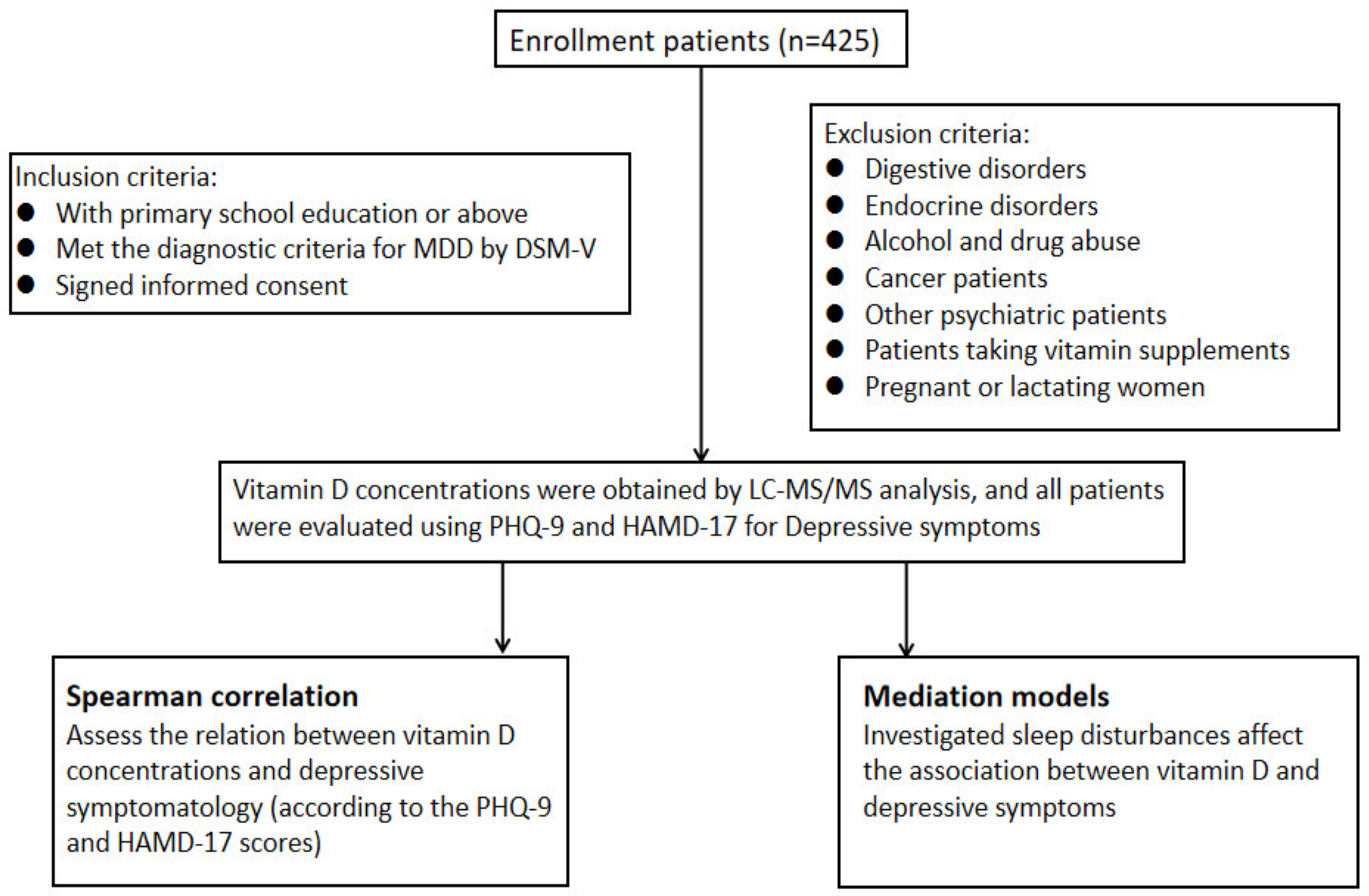
2. Materials and Methods
2.1. Patients
2.2. Measures
2.3. Statistical Analyses
3. Results
3.1. Participant Characteristics and Scores of Scales
3.2. The Correlation Test of Scale Scores with Vitamin D Concentration
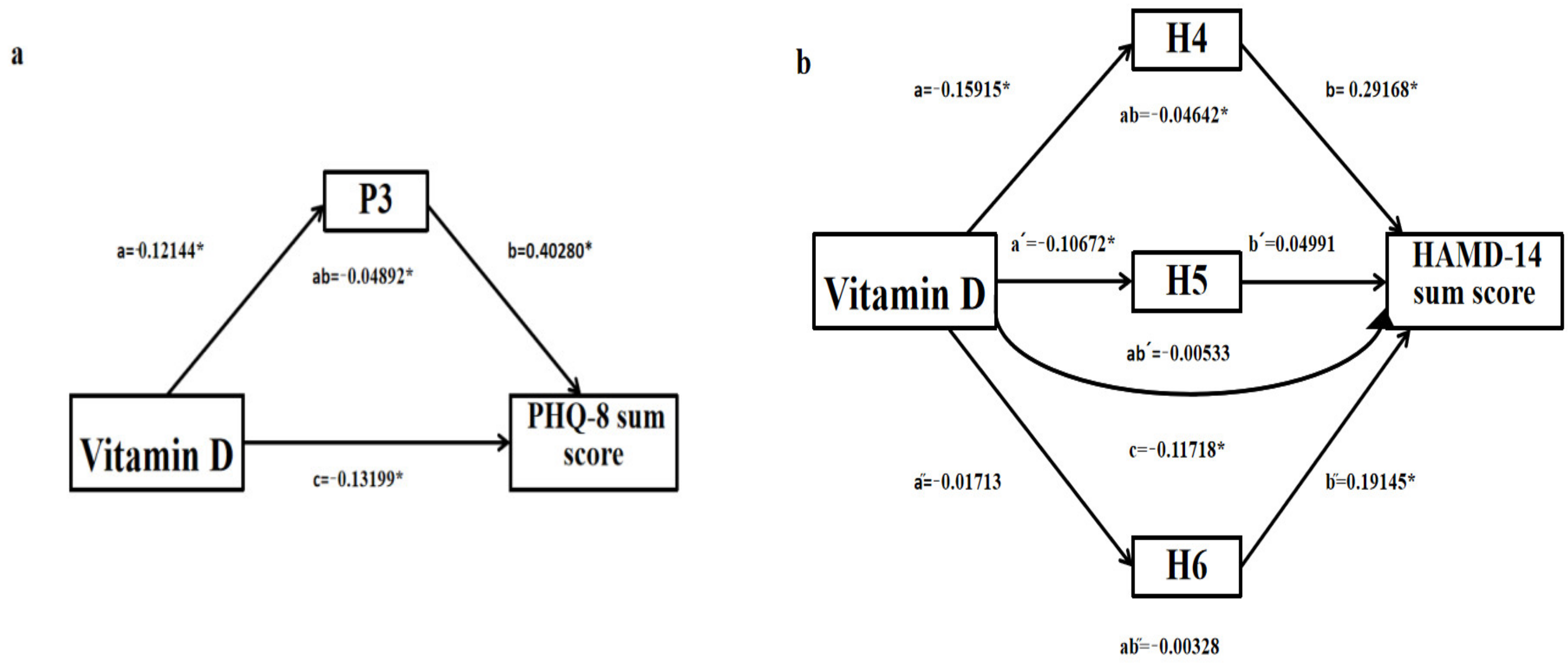
3.3. The Mediation Models for Sleep
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.K.; Karlin, B.E.; Trockel, M.; Gordienko, M.; Yesavage, J.; Taylor, C.B. Effectiveness of Cognitive Behavioral Therapy for Veterans with Depression and Suicidal Ideation. Arch. Suicide Res. 2016, 20, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Pandarakalam, J.P. Challenges of Treatment-resistant Depression. Psychiatr. Danub. 2018, 30, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, X.; Huang, Y.; Li, T.; Ma, C.; Xu, G.; Yin, H.; Xu, X.; Ma, Y.; Wang, L.; et al. Prevalence of depressive disorders and treatment in China: A cross-sectional epidemiological study. Lancet Psychiatr. 2021, 8, 981–990. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—A review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Eyles, D.W.; Burne, T.H.; McGrath, J.J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrin. 2013, 34, 47–64. [Google Scholar] [CrossRef]
- Al-Wardat, M.; Alwardat, N.; Lou, D.S.G.; Zomparelli, S.; Gualtieri, P.; Bigioni, G.; Romano, L.; Di Renzo, L. The association between serum vitamin D and mood disorders in a cohort of lipedema patients. Horm. Mol. Biol. Clin. I 2021, 42, 351–355. [Google Scholar] [CrossRef]
- Bicikova, M.; Duskova, M.; Vitku, J.; Kalvachova, B.; Ripova, D.; Mohr, P.; Stárka, L. Vitamin D in anxiety and affective disorders. Physiol. Res. 2015, 64, S101–S103. [Google Scholar] [CrossRef]
- Parker, G.B.; Brotchie, H.; Graham, R.K. Vitamin D and depression. J. Affect. Disorders. 2017, 208, 56–61. [Google Scholar] [CrossRef]
- Anglin, R.E.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiat. 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Bersani, F.S.; Ghezzi, F.; Maraone, A.; Vicinanza, R.; Cavaggioni, G.; Biondi, M.; Pasquini, M. The relationship between Vitamin D and depressive disorders. Riv. Psichiatr. 2019, 54, 229–234. [Google Scholar] [PubMed]
- Murphy, P.K.; Wagner, C.L. Vitamin D and mood disorders among women: An integrative review. J. Midwifery Women Health 2008, 53, 440–446. [Google Scholar] [CrossRef]
- Vellekkatt, F.; Menon, V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2019, 65, 74–80. [Google Scholar] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Schibler, U. Circadian rhythms: Mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 593–628. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.P.; Ames, B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef]
- Huiberts, L.M.; Smolders, K. Effects of vitamin D on mood and sleep in the healthy population: Interpretations from the serotonergic pathway. Sleep. Med. Rev. 2021, 55, 101379. [Google Scholar] [CrossRef]
- de Oliveira, D.L.; Hirotsu, C.; Tufik, S.; Andersen, M.L. The interfaces between vitamin D, sleep and pain. J. Endocrinol. 2017, 234, R23–R36. [Google Scholar] [CrossRef]
- Romano, F.; Muscogiuri, G.; Di Benedetto, E.; Zhukouskaya, V.V.; Barrea, L.; Savastano, S.; Colao, A.; Di Somma, C. Vitamin D and Sleep Regulation: Is there a Role for Vitamin D? Curr. Pharm. Des. 2020, 26, 2492–2496. [Google Scholar] [CrossRef]
- Gao, Q.; Kou, T.; Zhuang, B.; Ren, Y.; Dong, X.; Wang, Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1395. [Google Scholar] [CrossRef]
- Al-Shawwa, B.; Ehsan, Z.; Ingram, D.G. Vitamin D and sleep in children. J. Clin. Sleep Med. 2020, 16, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.L.; Mancuso, R.A.; Hobel, C.J.; Schetter, C.D.; Coussons-Read, M. Poor sleep quality increases symptoms of depression and anxiety in postpartum women. J. Behav. Med. 2018, 41, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E.; Duong, H.T. The prospective association between sleep deprivation and depression among adolescents. Sleep 2014, 37, 239–244. [Google Scholar] [CrossRef]
- Le, J.; Yuan, T.; Zhang, Y.; Wang, S.; Li, Y. New LC-MS/MS method with single-step pretreatment analyzes fat-soluble vitamins in plasma and amniotic fluid. J. Lipid Res. 2018, 59, 1783–1790. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 2nd ed.; Guilford Publications: New York, NY, USA, 2018. [Google Scholar]
- Lu, Y.; Li, J.; Liu, Y. Depression as a mediator of quality of life in patients with neuropathic pain: A cross-sectional study. J. Adv. Nurs. 2019, 75, 2719–2726. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Jones, K.S.; Redmond, J.; Fulford, A.J.; Jarjou, L.; Zhou, B.; Prentice, A.; Schoenmakers, I. Diurnal rhythms of vitamin D binding protein and total and free vitamin D metabolites. J. Steroid Biochem. 2017, 172, 130–135. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef]
- Patel, P.D.; Pontrello, C.; Burke, S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol. Psychiat. 2004, 55, 428–433. [Google Scholar] [CrossRef]
- Okereke, O.I.; Reynolds, C.R.; Mischoulon, D.; Chang, G.; Vyas, C.M.; Cook, N.R.; Weinberg, A.; Bubes, V.; Copeland, T.; Friedenberg, G.; et al. Effect of Long-term Vitamin D3 Supplementation vs Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial. JAMA—J. Am. Med. Assoc. 2020, 324, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, Y.; Wang, T.; Lin, Y.; Yu, J.; Xia, Q.; Zhu, P.; Zhu, D.-M. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020, 10, e1760. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M.; Majde, J.A.; Rector, D.M. Cytokines in immune function and sleep regulation. Handb. Clin. Neurol. 2011, 98, 229–240. [Google Scholar] [PubMed]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the gut microbiome: A systematic review of in vivo studies. Eur. J. Nutr. 2019, 58, 2895–2910. [Google Scholar] [CrossRef]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef]
- Torki, M.; Gholamrezaei, A.; Mirbagher, L.; Danesh, M.; Kheiri, S.; Emami, M.H. Vitamin D Deficiency Associated with Disease Activity in Patients with Inflammatory Bowel Diseases. Dig. Dis. Sci. 2015, 60, 3085–3091. [Google Scholar] [CrossRef]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Nena, E.; Papanas, N.; Steiropoulos, P. The role of vitamin D in obstructive sleep apnoea syndrome. Breathe 2018, 14, 206–215. [Google Scholar] [CrossRef]
- Lucock, M.; Jones, P.; Martin, C.; Beckett, E.; Yates, Z.; Furst, J.; Veysey, M. Vitamin D: Beyond Metabolism. J. Evid. Based Complement. Altern. Med. 2015, 20, 310–322. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Vranic, L.; Mikolasevic, I.; Milic, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Han, L.; Liu, Q.; Zhao, Y.; Wang, L.; Wang, Y. Effects of Vitamin D Supplementation on General and Central Obesity: Results from 20 Randomized Controlled Trials Involving Apparently Healthy Populations. Ann. Nutr. Metab. 2020, 76, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Abiri, B.; Vafa, M. Effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammatory biomarkers, and SIRT1 in obese women: A study protocol for a double-blind, randomized, placebo-controlled trial. Trials 2020, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Kar, S.K.; Suthar, N.; Nebhinani, N. Vitamin D and Depression: A Critical Appraisal of the Evidence and Future Directions. Indian J. Psychol. Med. 2020, 42, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Cardoso, C.K.d.S.; Moura, L.d.A.N.e.; Rodrigues, A.P.d.S.; de Oliveira, C. Serum and Dietary Vitamin D in Individuals with Class II and III Obesity: Prevalence and Association with Metabolic Syndrome. Nutrients 2021, 13, 2138. [Google Scholar] [CrossRef] [PubMed]
- Wirz-Justice, A.; Skene, D.J.; Munch, M. The relevance of daylight for humans. Biochem. Pharmacol. 2021, 191, 114304. [Google Scholar] [CrossRef]
- Mergl, R.; Dogan-Sander, E.; Willenberg, A.; Wirkner, K.; Kratzsch, J.; Riedel-Heller, S.; Allgaier, A.-K.; Hegerl, U.; Sander, C. The effect of depressive symptomatology on the association of vitamin D and sleep. BMC Psychiatry 2021, 21, 178. [Google Scholar] [CrossRef]


| Variables | Frequency/Mean | Percentage (%)/SD |
|---|---|---|
| Gender | ||
| Male | 105 | 24.7 |
| Female | 320 | 75.3 |
| Relationship status | ||
| Single a | 287 | 67.5 |
| Has a partner b | 138 | 32.5 |
| Occupation | ||
| Student | 287 | 67.5 |
| Unemployed | 38 | 8.9 |
| Employed | 100 | 23.5 |
| Education level | ||
| High school or less | 15 | 3.5 |
| Undergraduate | 351 | 82.6 |
| Postgraduate or higher | 59 | 13.9 |
| First episode | ||
| Yes | 263 | 61.9 |
| No | 162 | 38.1 |
| Race | ||
| Ethnic Han | 393 | 92.5 |
| Minority | 32 | 7.5 |
| Habitation | ||
| Urban | 355 | 83.5 |
| Rural | 70 | 16.5 |
| Age | 23 | 5.3 |
| BMI | 20.7 | 3.0 |
| Vitamin D concentration (ng/mL) | 14.5 | 6.9 |
| P3 score | 2.1 | 0.9 |
| H4 score | 1.1 | 0.8 |
| H5 score | 1.0 | 0.7 |
| H6 score | 0.9 | 0.8 |
| PHQ-8 sum score | 14.7 | 4.9 |
| HAMD-14 sum score | 16.8 | 5.8 |
| PHQ-9 sum score | 16.9 | 5.4 |
| HAMD-17 sum score | 19.7 | 6.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Chen, M.; Zhang, N.; Ma, S.; Xie, X.; Xu, S.; Nie, Z.; Wang, W.; Zhou, E.; Xu, S.; et al. The Mediation Role of Sleep Disturbances between Vitamin D and Depressive Symptoms: A Cross-Sectional Study. Brain Sci. 2023, 13, 1501. https://doi.org/10.3390/brainsci13111501
Yao L, Chen M, Zhang N, Ma S, Xie X, Xu S, Nie Z, Wang W, Zhou E, Xu S, et al. The Mediation Role of Sleep Disturbances between Vitamin D and Depressive Symptoms: A Cross-Sectional Study. Brain Sciences. 2023; 13(11):1501. https://doi.org/10.3390/brainsci13111501
Chicago/Turabian StyleYao, Lihua, Mianmian Chen, Nan Zhang, Simeng Ma, Xinhui Xie, Shuxian Xu, Zhaowen Nie, Wei Wang, Enqi Zhou, Shunsheng Xu, and et al. 2023. "The Mediation Role of Sleep Disturbances between Vitamin D and Depressive Symptoms: A Cross-Sectional Study" Brain Sciences 13, no. 11: 1501. https://doi.org/10.3390/brainsci13111501
APA StyleYao, L., Chen, M., Zhang, N., Ma, S., Xie, X., Xu, S., Nie, Z., Wang, W., Zhou, E., Xu, S., Weng, S., Chen, H., Xiang, D., & Liu, Z. (2023). The Mediation Role of Sleep Disturbances between Vitamin D and Depressive Symptoms: A Cross-Sectional Study. Brain Sciences, 13(11), 1501. https://doi.org/10.3390/brainsci13111501







