Association between Beta Oscillations from Subthalamic Nucleus and Quantitative Susceptibility Mapping in Deep Gray Matter Structures in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Information
2.3. MERs Data and LFP Analysis
2.4. MRI Data Acquisition and Processing
2.5. Statistics Analysis
3. Results
3.1. Characteristics of Participants
3.2. QSM Comparison between HCs and PD patients
3.3. Correlation between PSDXb and QSM Values
3.4. Correlation between PSDXb and UPDRS Part III
3.5. Correlation between UPDRS Part III and Bilateral Average QSM Values
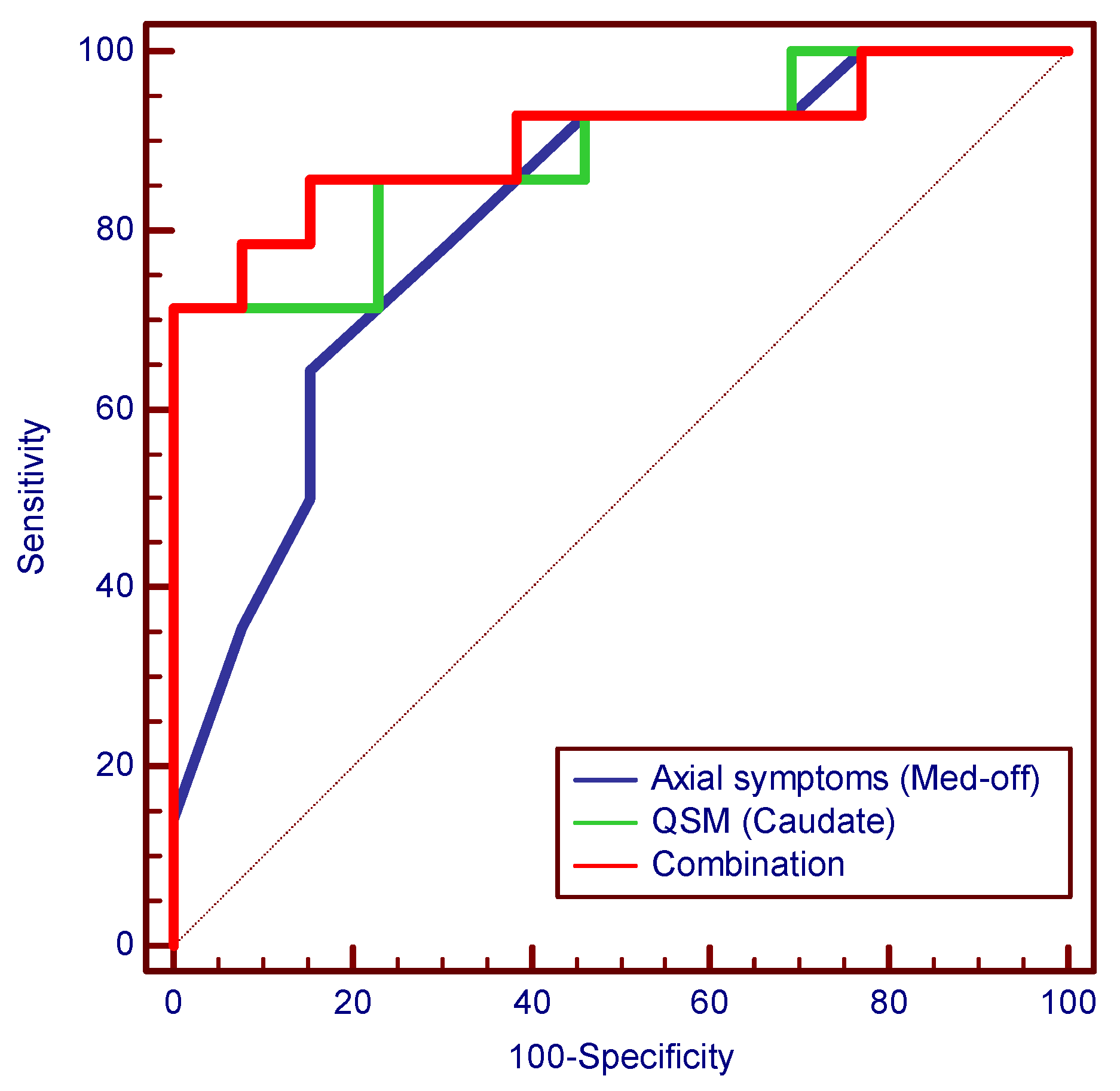
3.6. Logistic Regression and Diagnostic Performance Evaluation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou, K.; Michmizos, K.P.; Stathis, P.; Sakas, D.; Nikita, K.S.; Mitsis, G.D. Classification and Prediction of Clinical Improvement in Deep Brain Stimulation from Intraoperative Microelectrode Recordings. IEEE Trans. Biomed. Eng. 2017, 64, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Wei, J.; Zou, Z.; Li, J.; Zhang, Y. Gamma Oscillations and Coherence Are Weaker in the Dorsomedial Subregion of STN in Parkinson’s Disease. Front. Neurol. 2021, 12, 710206. [Google Scholar] [CrossRef]
- Woerd, E.S.T.; Oostenveld, R.; de Lange, F.P.; Praamstra, P. A shift from prospective to reactive modulation of beta-band oscillations in Parkinson’s disease. NeuroImage 2014, 100, 507–519. [Google Scholar] [CrossRef]
- Hammond, C.; Bergman, H.; Brown, P. Pathological synchronization in Parkinson’s disease: Networks, models and treatments. Trends Neurosci. 2007, 30, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Bouthour, W.; Mégevand, P.; Donoghue, J.; Lüscher, C.; Birbaumer, N.; Krack, P. Biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat. Rev. Neurol. 2019, 15, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Neumann, W.-J.; Staub-Bartelt, F.; Horn, A.; Schanda, J.; Schneider, G.-H.; Brown, P.; Kühn, A.A. Long term correlation of subthalamic beta band activity with motor impairment in patients with Parkinson’s disease. Clin. Neurophysiol. 2017, 128, 2286–2291. [Google Scholar] [CrossRef]
- Neumann, W.-J.; Degen, K.; Schneider, G.-H.; Brücke, C.; Huebl, J.; Brown, P.; Kühn, A.A. Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease. Mov. Disord. 2016, 31, 1748–1751. [Google Scholar] [CrossRef]
- Oswal, A.; Beudel, M.; Zrinzo, L.; Limousin, P.; Hariz, M.; Foltynie, T.; Litvak, V.; Brown, P. Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson’s disease. Brain 2016, 139, 1482–1496. [Google Scholar] [CrossRef]
- Chen, C.C.; Hsu, Y.T.; Chan, H.L.; Chiou, S.M.; Tu, P.H.; Lee, S.T.; Tsai, C.H.; Lu, C.S.; Brown, P. Complexity of subthalamic 13–35Hz oscillatory activity directly correlates with clinical impairment in patients with Parkinson’s disease. Exp. Neurol. 2010, 224, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Brown, P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann. N. Y. Acad. Sci. 2012, 1265, 9–24. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.E.C.; Leyland, L.A.; Schrag, A.-E.; Lees, A.J.; Acosta-Cabronero, J.; Weil, R.S. Brain iron deposition is linked with cognitive severity in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Melis, J.P.; van Steeg, H.; Luijten, M. Oxidative DNA Damage and Nucleotide Excision Repair. Antioxid. Redox Signal. 2013, 18, 2409–2419. [Google Scholar] [CrossRef]
- Perluigi, M.; Coccia, R.; Butterfield, D.A. 4-Hydroxy-2-Nonenal, a Reactive Product of Lipid Peroxidation, and Neurodegenerative Diseases: A Toxic Combination Illuminated by Redox Proteomics Studies. Antioxid. Redox Signal. 2012, 17, 1590–1609. [Google Scholar] [CrossRef]
- Cozzi, A.; Orellana, D.I.; Santambrogio, P.; Rubio, A.; Cancellieri, C.; Giannelli, S.G.; Ripamonti, M.; Taverna, S.; Di Lullo, G.; Rovida, E.; et al. Stem Cell Modeling of Neuroferritinopathy Reveals Iron as a Determinant of Senescence and Ferroptosis during Neuronal Aging. Stem Cell Rep. 2019, 13, 832–846. [Google Scholar] [CrossRef]
- Dexter, D.T.; Jenner, P.; Schapira, A.; Marsden, C.D. The Royal Kings and Queens Parkinson’s Disease Research Group Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia. Ann. Neurol. 1992, 32, S94–S100. [Google Scholar] [CrossRef]
- Deistung, A.; Schweser, F.; Reichenbach, J.R. Overview of quantitative susceptibility mapping. NMR Biomed. 2017, 30, e3569. [Google Scholar] [CrossRef]
- Langkammer, C.; Schweser, F.; Krebs, N.; Deistung, A.; Goessler, W.; Scheurer, E.; Sommer, K.; Reishofer, G.; Yen, K.; Fazekas, F.; et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage 2012, 62, 1593–1599. [Google Scholar] [CrossRef]
- Zang, Z.; Song, T.; Li, J.; Yan, S.; Nie, B.; Mei, S.; Ma, J.; Yang, Y.; Shan, B.; Zhang, Y.; et al. Modulation effect of substantia nigra iron deposition and functional connectivity on putamen glucose metabolism in Parkinson’s disease. Hum. Brain Mapp. 2022, 43, 3735–3744. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kakeda, S.; Watanabe, K.; Ueda, I.; Ogasawara, A.; Moriya, J.; Ide, S.; Futatsuya, K.; Sato, T.; Okada, K.; et al. Usefulness of Quantitative Susceptibility Mapping for the Diagnosis of Parkinson Disease. Am. J. Neuroradiol. 2015, 36, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Ravanfar, P.; Loi, S.M.; Syeda, W.T.; Van Rheenen, T.E.; Bush, A.I.; Desmond, P.; Cropley, V.L.; Lane, D.J.R.; Opazo, C.M.; Moffat, B.A.; et al. Systematic Review: Quantitative Susceptibility Mapping (QSM) of Brain Iron Profile in Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 618435. [Google Scholar] [CrossRef]
- Sun, H.; Walsh, A.J.; Lebel, R.M.; Blevins, G.; Catz, I.; Lu, J.-Q.; Johnson, E.S.; Emery, D.J.; Warren, K.G.; Wilman, A.H. Validation of quantitative susceptibility mapping with Perls’ iron staining for subcortical gray matter. Neuroimage 2015, 105, 486–492. [Google Scholar] [CrossRef]
- Hametner, S.; Endmayr, V.; Deistung, A.; Palmrich, P.; Prihoda, M.; Haimburger, E.; Menard, C.; Feng, X.; Haider, T.; Leisser, M.; et al. The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation—A biochemical and histological validation study. Neuroimage 2018, 179, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Baek, S.-Y.; Chun, S.Y.; Lee, J.-H.; Cho, H. Specific visualization of neuromelanin-iron complex and ferric iron in the human post-mortem substantia nigra using MR relaxometry at 7T. NeuroImage 2018, 172, 874–885. [Google Scholar] [CrossRef]
- Lewis, M.M.; Du, G.; Baccon, J.; Snyder, A.M.; Murie, B.; Cooper, F.; Stetter, C.; Kong, L.; Sica, C.; Mailman, R.B.; et al. Susceptibility MRI captures nigral pathology in patients with parkinsonian syndromes. Mov. Disord. 2018, 33, 1432–1439. [Google Scholar] [CrossRef]
- Wang, C.; Foxley, S.; Ansorge, O.; Bangerter-Christensen, S.; Chiew, M.; Leonte, A.; AL Menke, R.; Mollink, J.; Pallebage-Gamarallage, M.; Turner, M.R.; et al. Methods for quantitative susceptibility and R2* mapping in whole post-mortem brains at 7T applied to amyotrophic lateral sclerosis. Neuroimage 2020, 222, 117216. [Google Scholar] [CrossRef]
- Lee, S.H.; Lyoo, C.H.; Ahn, S.J.; Rinne, J.O.; Lee, M.S. Brain regional iron contents in progressive supranuclear palsy. Park. Relat. Disord. 2017, 45, 28–32. [Google Scholar] [CrossRef]
- Kim, R.; Kim, H.-J.; Shin, C.; Park, H.; Kim, A.; Paek, S.H.; Jeon, B. Long-term effect of subthalamic nucleus deep brain stimulation on freezing of gait in Parkinson’s disease. J. Neurosurg. 2019, 131, 1797–1804. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Hu, K.; Mo, Y.; Cao, P.; Hou, X.; He, X.; Zhang, S.; Xue, S. α and θ oscillations in the subthalamic nucleus are potential biomarkers for Parkinson’s disease with depressive symptoms. Park. Relat. Disord. 2021, 90, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Huhdanpaa, H.; Hwang, D.H.; Gasparian, G.G.; Booker, M.T.; Cen, Y.; Lerner, A.; Boyko, O.B.; Go, J.L.; Kim, P.E.; Rajamohan, A.; et al. Image Coregistration: Quantitative Processing Framework for the Assessment of Brain Lesions. J. Digit. Imaging 2014, 27, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Kutten, K.; Ceritoglu, C.; Li, Y.; Kang, N.; Hsu, J.T.; Qiao, Y.; Wei, H.; Liu, C.; et al. Multi-atlas tool for automated segmentation of brain gray matter nuclei and quantification of their magnetic susceptibility. Neuroimage 2019, 191, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, L.; Zhao, Q.; Du, X.; Bi, M.; Li, Y.; Jiao, Q.; Jiang, H. Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson’s disease. Free. Radic. Biol. Med. 2020, 152, 227–234. [Google Scholar] [CrossRef]
- Sun, Y.; He, L.; Wang, T.; Hua, W.; Qin, H.; Wang, J.; Wang, L.; Gu, W.; Li, T.; Li, N.; et al. Activation of p62-Keap1-Nrf2 Pathway Protects 6-Hydroxydopamine-Induced Ferroptosis in Dopaminergic Cells. Mol. Neurobiol. 2020, 57, 4628–4641. [Google Scholar] [CrossRef]
- Ito, K.; Eguchi, Y.; Imagawa, Y.; Akai, S.; Mochizuki, H.; Tsujimoto, Y. MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discov. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Do Van, B.; Gouel, F.; Jonneaux, A.; Timmerman, K.; Gelé, P.; Pétrault, M.; Bastide, M.; Laloux, C.; Moreau, C.; Bordet, R.; et al. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis. 2016, 94, 169–178. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Tan, E.-K. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 1–12. [Google Scholar] [CrossRef]
- Thomas, G.E.C.; Zarkali, A.; Ryten, M.; Shmueli, K.; Gil-Martinez, A.L.; Leyland, L.-A.; McColgan, P.; Acosta-Cabronero, J.; Lees, A.J.; Weil, R.S. Regional brain iron and gene expression provide insights into neurodegeneration in Parkinson’s disease. Brain 2021, 144, 1787–1798. [Google Scholar] [CrossRef]
- Li, W.; Jiang, H.; Song, N.; Xie, J. Oxidative Stress Partially Contributes to Iron-Induced Alpha-Synuclein Aggregation in SK-N-SH Cells. Neurotox. Res. 2011, 19, 435–442. [Google Scholar] [CrossRef]
- Hare, D.J.; Double, K.L. Iron and dopamine: A toxic couple. Brain 2016, 139, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-F.; Zou, T.; Tuo, Q.-Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Moreau, C.; Devedjian, J.C.; Kluza, J.; Petrault, M.; Laloux, C.; Jonneaux, A.; Ryckewaert, G.; Garcon, G.; Rouaix, N.; et al. Targeting Chelatable Iron as a Therapeutic Modality in Parkinson’s Diease. Antioxid. Redox Signal. 2014, 21, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Baaske, M.K.; Kramer, E.R.; Meka, D.P.; Engler, G.; Engel, A.K.; Moll, C.K. Parkin deficiency perturbs striatal circuit dynamics. Neurobiol. Dis. 2020, 137, 104737. [Google Scholar] [CrossRef] [PubMed]
- Vidyadhara, D.; Sasidharan, A.; Kutty, B.M.; Raju, T.; Alladi, P.A. Admixing MPTP-resistant and MPTP-vulnerable mice enhances striatal field potentials and calbindin-D28K expression to avert motor behaviour deficits. Behav. Brain Res. 2019, 360, 216–227. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, Y.; Sun, Y.; Xuan, Y.; Niu, J.; Guan, J.; Rong, Y.; Jia, Y.; Zhuang, Z.; Yan, G.; et al. Combined Application of Quantitative Susceptibility Mapping and Diffusion Kurtosis Imaging Techniques to Investigate the Effect of Iron Deposition on Microstructural Changes in the Brain in Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 792778. [Google Scholar] [CrossRef]
- Sethi, S.K.; Kisch, S.J.; Ghassaban, K.; Rajput, A.; Rajput, A.; Babyn, P.S.; Liu, S.; Szkup, P.; Haacke, E.M. Iron quantification in Parkinson’s disease using an age-based threshold on susceptibility maps: The advantage of local versus entire structure iron content measurements. Magn. Reson. Imaging 2019, 55, 145–152. [Google Scholar] [CrossRef]
- Uchida, Y.; Kan, H.; Sakurai, K.; Inui, S.; Kobayashi, S.; Ms, Y.A.; Ms, K.S.; Ueki, Y.; Matsukawa, N. Magnetic Susceptibility Associates with Dopaminergic Deficits and Cognition in Parkinson’s Disease. Mov. Disord. 2020, 35, 1396–1405. [Google Scholar] [CrossRef]
- Ghassaban, K.; He, N.; Sethi, S.K.; Huang, P.; Chen, S.; Yan, F.; Haacke, E.M. Regional High Iron in the Substantia Nigra Differentiates Parkinson’s Disease Patients from Healthy Controls. Front. Aging Neurosci. 2019, 11, 106. [Google Scholar] [CrossRef]
- De Simoni, S.; Jenkins, P.O.; Bourke, N.J.; Fleminger, J.J.; Hellyer, P.J.; Jolly, A.E.; Patel, M.C.; Cole, J.H.; Leech, R.; Sharp, D.J. Altered caudate connectivity is associated with executive dysfunction after traumatic brain injury. Brain 2018, 141, 148–164. [Google Scholar] [CrossRef]
- Morissette, M.; Morin, N.; Grégoire, L.; Rajput, A.; Rajput, A.H.; Di Paolo, T. Brain α7 nicotinic acetylcholine receptors in MPTP-lesioned monkeys and parkinsonian patients. Biochem. Pharmacol. 2016, 109, 62–69. [Google Scholar] [CrossRef]
- Cheshire, P.; Ayton, S.; Bertram, K.L.; Ling, H.; Li, A.; McLean, C.; Halliday, G.M.; O’Sullivan, S.S.; Revesz, T.; Finkelstein, D.I.; et al. Serotonergic markers in Parkinson’s disease and levodopa-induced dyskinesias. Mov. Disord. 2015, 30, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Sharott, A.; Gulberti, A.; Zittel, S.; Jones, A.A.T.; Fickel, U.; Münchau, A.; Köppen, J.A.; Gerloff, C.; Westphal, M.; Buhmann, C.; et al. Activity Parameters of Subthalamic Nucleus Neurons Selectively Predict Motor Symptom Severity in Parkinson’s Disease. J. Neurosci. 2014, 34, 6273–6285. [Google Scholar] [CrossRef] [PubMed]
- Oswal, A.; Brown, P.; Litvak, V. Synchronized neural oscillations and the pathophysiology of Parkinsonʼs disease. Curr. Opin. Neurol. 2013, 26, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Brown, P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol. 2007, 17, 656–664. [Google Scholar] [CrossRef]
- Zemel, D.; Gritton, H.; Cheung, C.; Shankar, S.; Kramer, M.; Han, X. Dopamine depletion selectively disrupts interactions between striatal neuron subtypes and LFP oscillations. Cell Rep. 2022, 38, 110265. [Google Scholar] [CrossRef]
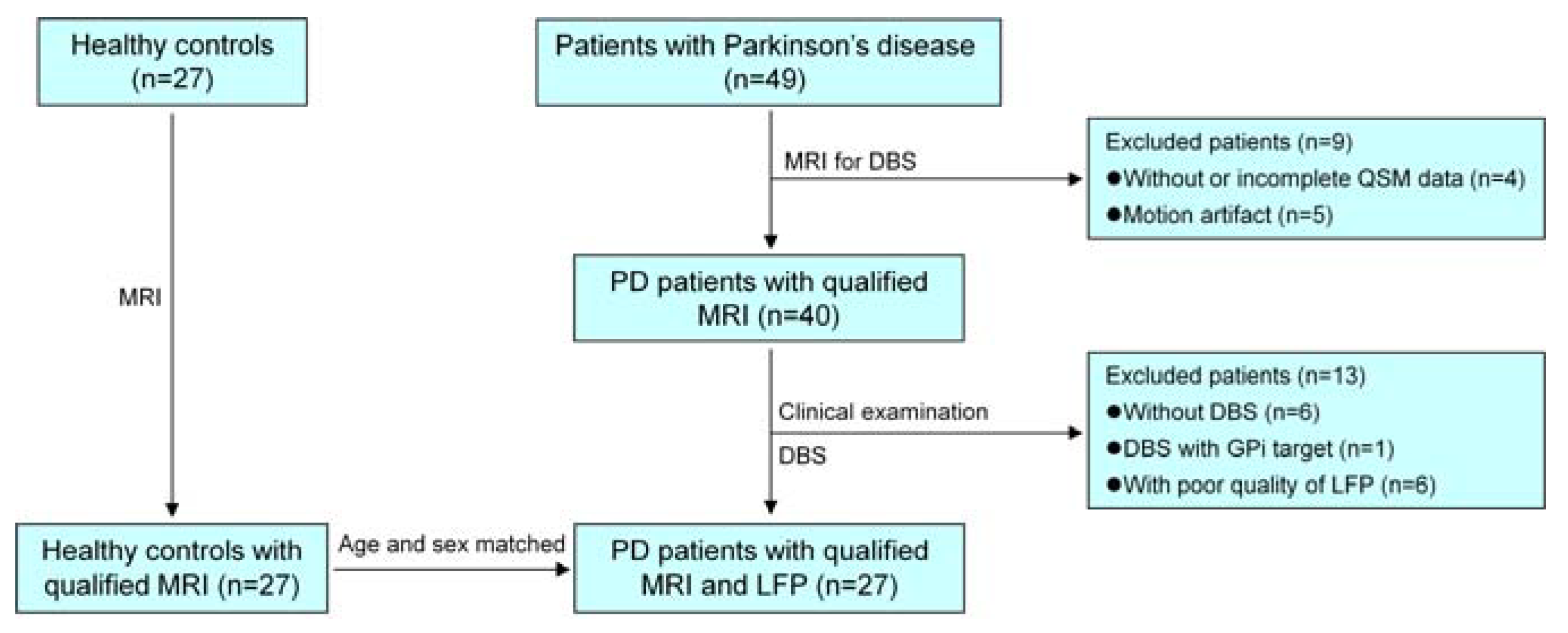
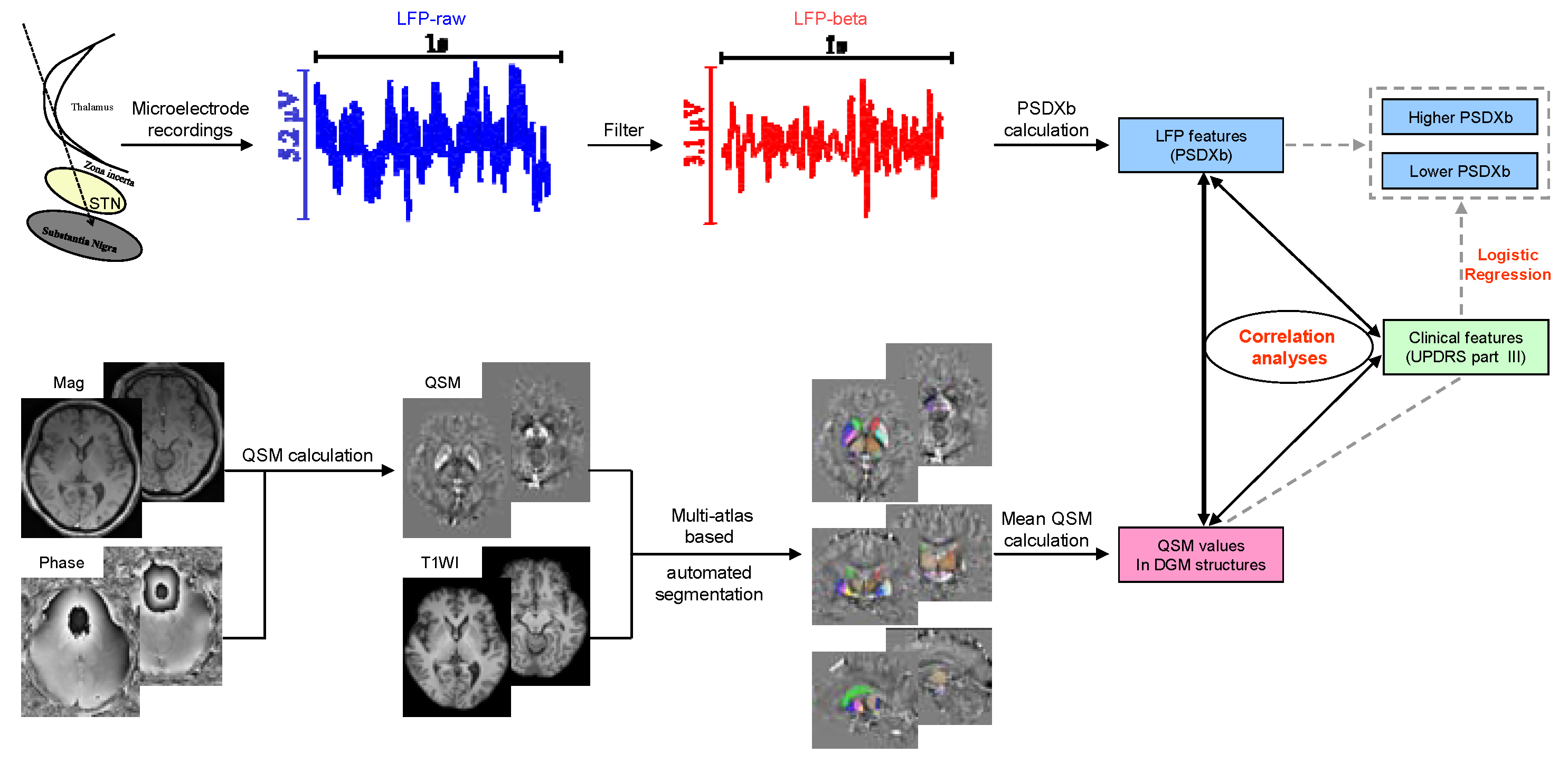
| Patient ID | Gender | Age (Years) | Disease Duration (Years) | LEDD (mg) | H-Y | UPDRS Part III | |
|---|---|---|---|---|---|---|---|
| Med-Off | Med-On | ||||||
| 1 | F | 60 | 10 | 900 | 3 | 59 | 23 |
| 2 | F | 71 | 15 | 1075 | 4 | 70 | 48 |
| 3 | F | 64 | 8 | 799 | 3 | 75 | 35 |
| 4 | M | 64 | 5 | 844 | 2.5 | 44 | 30 |
| 5 | F | 59 | 10 | 225 | 5 | 53 | 9 |
| 6 | M | 67 | 12 | 800 | 3 | 46 | 29 |
| 7 | M | 54 | 5 | 574 | 2.5 | 42 | 16 |
| 8 | F | 63 | 6 | 450 | 2 | 46 | 28 |
| 9 | M | 63 | 12 | 375 | 3 | 47 | 22 |
| 10 | M | 60 | 11 | 650 | 3 | 57 | 41 |
| 11 | F | 49 | 5 | 1450 | 2.5 | 56 | 13 |
| 12 | M | 43 | 7 | 604 | 3 | 60 | 28 |
| 13 | M | 74 | 4 | 474 | 3 | 38 | 25 |
| 14 | F | 62 | 10 | 798 | 4 | 60 | 38 |
| 15 | F | 60 | 8 | 732 | 3 | 39 | 25 |
| 16 | M | 60 | 10 | 250 | 3 | 33 | 10 |
| 17 | M | 55 | 9 | 1750 | 3 | 50 | 28 |
| 18 | M | 65 | 20 | 499 | 3 | 53 | 22 |
| 19 | F | 54 | 7 | 1148 | 3 | 55 | 26 |
| 20 | M | 67 | 10 | 997 | 2.5 | 41 | 25 |
| 21 | M | 56 | 13 | 625 | 4 | 79 | 34 |
| 22 | F | 63 | 16 | 1200 | 2.5 | 63 | 14 |
| 23 | M | 59 | 7 | 887 | 3 | 49 | 17 |
| 24 | F | 69 | 17 | 675 | 3 | 50 | 24 |
| 25 | M | 58 | 9 | 700 | 3 | 62 | 26 |
| 26 | M | 54 | 8 | 1125 | 3 | 69 | 30 |
| 27 | M | 33 | 6 | 1450 | 3 | 37 | 8 |
| Bilateral Average QSM Value | Bilateral Average PSDXb | ||
|---|---|---|---|
| rho | p | p (FDR-Corrected) | |
| Caudate | 0.582 | 0.001 * | 0.008 * |
| GPi | 0.332 | 0.091 | 0.146 |
| GPe | 0.346 | 0.077 | 0.146 |
| Putamen | 0.203 | 0.309 | 0.412 |
| STN | 0.363 | 0.063 | 0.146 |
| SN | 0.480 | 0.011 * | 0.044 * |
| RN | −0.004 | 0.983 | 0.983 |
| DN | −0.105 | 0.602 | 0.688 |
| UPDRS Part III | Bilateral Average PSDXb | ||
|---|---|---|---|
| rho | p | p (FDR-Corrected) | |
| UPDRS part III (med−off) | −0.277 | 0.162 | 0.648 |
| Tremor | −0.078 | 0.698 | 0.877 |
| Rigidity | −0.050 | 0.804 | 0.877 |
| Bradykinesia | −0.088 | 0.664 | 0.877 |
| Axial symptoms | −0.603 | 0.001 * | 0.006 * |
| Gait | −0.599 | 0.001 * | 0.006 * |
| UPDRS part III (med−on) | 0.073 | 0.718 | 0.877 |
| Tremor | −0.063 | 0.754 | 0.877 |
| Rigidity | 0.161 | 0.424 | 0.877 |
| Bradykinesia | 0.019 | 0.926 | 0.926 |
| Axial symptoms | 0.051 | 0.801 | 0.877 |
| Gait | −0.054 | 0.788 | 0.877 |
| Metric | Lower PSDXb (n = 13) vs. Higher PSDXb (n = 14) | |||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | Cut-Off | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
| Axial symptoms (med-off) | 0.813 (0.617–0.936) | 7.000 | 0.643 (0.351–0.872) | 0.846 (0.546–0.981) | 0.818 (0.482–0.977) | 0.687 (0.404–0.895) |
| Gait (med-off) | 0.788 (0.589–0.921) | 2.000 | 0.857 (0.572–0.982) | 0.692 (0.386–0.909) | 0.750 (0.476–0.927) | 0.818 (0.482–0.977) |
| QSM (caudate) | 0.885 (0.703–0.975) | 0.037 | 0.714 (0.419–0.916) | 1.000 (0.753–1.000) | 1.000 (0.692–1.000) | 0.765 (0.501–0.932) |
| QSM (SN) | 0.709 (0.503–0.866) | 0.073 | 0.857 (0.572–0.982) | 0.615 (0.316–0.861) | 0.706 (0.440–0.897) | 0.800 (0.444–0.975) |
| Combination # | 0.901 (0.724–0.982) | 0.743 | 0.714 (0.419–0.916) | 1.000 (0.753–1.000) | 1.000 (0.692–1.000) | 0.765 (0.501–0.932) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Cai, G.; Li, Y.; Sun, Y.; Song, Y.; Cai, G.; Jiang, R. Association between Beta Oscillations from Subthalamic Nucleus and Quantitative Susceptibility Mapping in Deep Gray Matter Structures in Parkinson’s Disease. Brain Sci. 2023, 13, 81. https://doi.org/10.3390/brainsci13010081
Lin M, Cai G, Li Y, Sun Y, Song Y, Cai G, Jiang R. Association between Beta Oscillations from Subthalamic Nucleus and Quantitative Susceptibility Mapping in Deep Gray Matter Structures in Parkinson’s Disease. Brain Sciences. 2023; 13(1):81. https://doi.org/10.3390/brainsci13010081
Chicago/Turabian StyleLin, Mangui, Guoen Cai, YongJie Li, Yifang Sun, Yang Song, Guofa Cai, and Rifeng Jiang. 2023. "Association between Beta Oscillations from Subthalamic Nucleus and Quantitative Susceptibility Mapping in Deep Gray Matter Structures in Parkinson’s Disease" Brain Sciences 13, no. 1: 81. https://doi.org/10.3390/brainsci13010081
APA StyleLin, M., Cai, G., Li, Y., Sun, Y., Song, Y., Cai, G., & Jiang, R. (2023). Association between Beta Oscillations from Subthalamic Nucleus and Quantitative Susceptibility Mapping in Deep Gray Matter Structures in Parkinson’s Disease. Brain Sciences, 13(1), 81. https://doi.org/10.3390/brainsci13010081





