Gross, Fine and Visual-Motor Skills in Children with Language Disorder, Speech Sound Disorder and Their Combination
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Eligibility Assessment
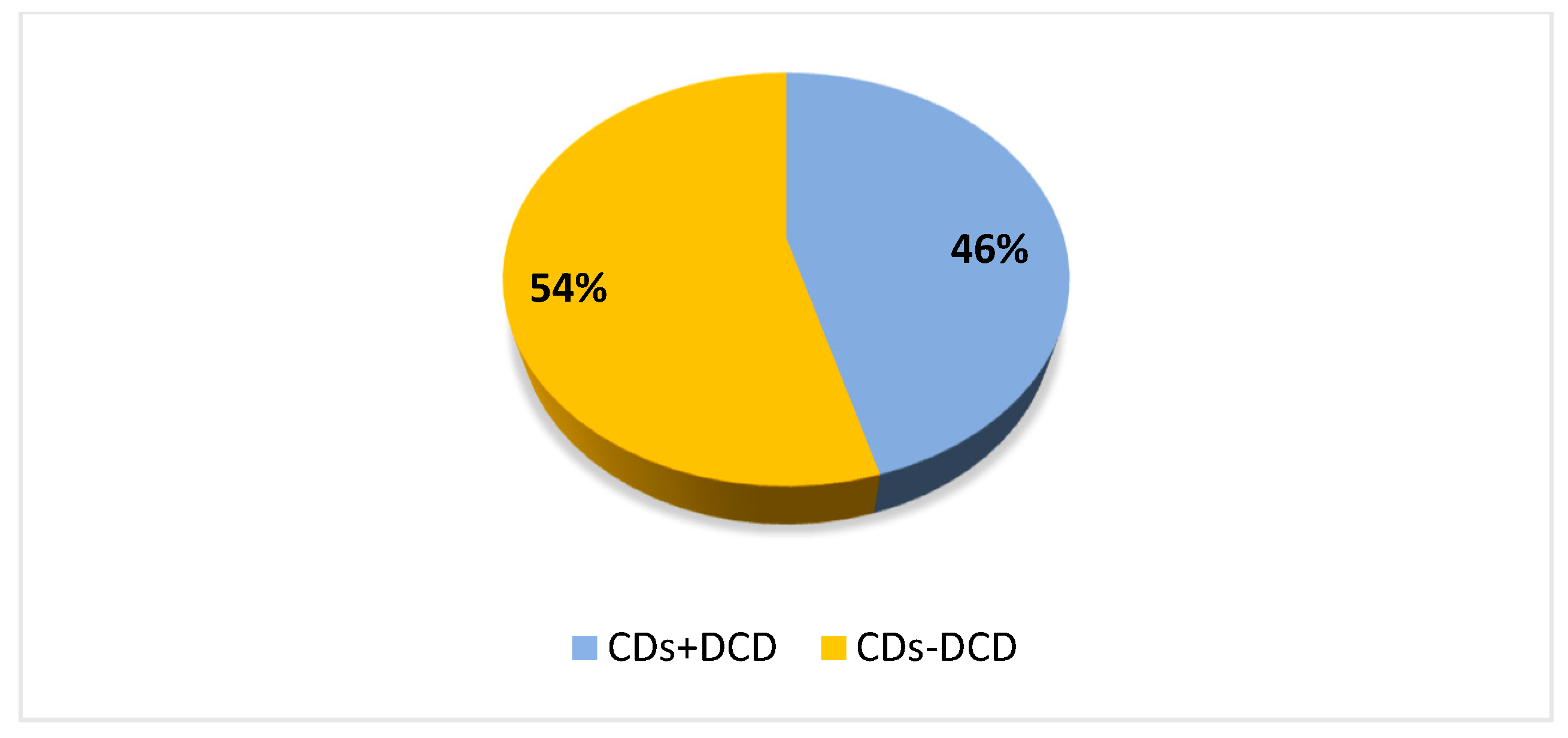
2.2. Experiment 1
2.2.1. Participants and Procedures
2.2.2. Measures
Gross and Fine Motor Skills
2.3. Experiment 2
2.3.1. Participants and Procedures
2.3.2. Measures
Visual-Motor Skills
2.4. Statistical Analysis
3. Results
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bishop, D.V.M.; North, T.; Donlan, C. Nonword Repetition as a Behavioural Marker for Inherited Language Impairment: Evidence From a Twin Study. J. Child Psychol. Psychiatry 1996, 37, 391–403. [Google Scholar] [CrossRef]
- Archibald, L.M.D.; Gathercole, S.E. Short-term and working memory in specific language impairment. Int. J. Lang. Commun. Disord. 2006, 41, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, M.; Pickles, A.; Newbury, D.F.; Addis, L.; Banfield, E.; Fisher, S.E.; Monaco, A.P.; Simkin, Z.; Conti-Ramsden, G. The SLI Consortium Genetic and phenotypic effects of phonological short-term memory and grammatical morphology in specific language impairment. Genes Brain Behav. 2008, 7, 393–402. [Google Scholar] [CrossRef]
- Bishop, D.V.M.; Bishop, S.J.; Bright, P.; James, C.; Delaney, T.; Tallal, P. Different Origin of Auditory and Phonological Processing Problems in Children with Language Impairment: Evidence from a Twin Study. J. Speech Lang. Hear. Res. 1999, 42, 155–168. [Google Scholar] [CrossRef]
- Ziegler, J.C.; Pech-Georgel, C.; George, F.; Alario, F.-X.; Lorenzi, C. Deficits in speech perception predict language learning impairment. Proc. Natl. Acad. Sci. USA 2005, 102, 14110–14115. [Google Scholar] [CrossRef]
- Spaulding, T.J.; Plante, E.; Vance, R. Sustained Selective Attention Skills of Preschool Children with Specific Language Impairment: Evidence for Separate Attentional Capacities. J. Speech Lang. Hear. Res. 2008, 51, 16–34. [Google Scholar] [CrossRef]
- Finneran, D.A.; Francis, A.L.; Leonard, L.B. Sustained Attention in Children With Specific Language Impairment (SLI). J. Speech Lang. Hear. Res. 2009, 52, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Ebert, K.D.; Kohnert, K. Sustained Attention in Children with Primary Language Impairment: A Meta-Analysis. J. Speech Lang. Hear. Res. 2011, 54, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Iverson, J.M.; Goldin-Meadow, S. Gesture Paves the Way for Language Development. Psychol. Sci. 2005, 16, 367–371. [Google Scholar] [CrossRef]
- Zambrana, I.M.; Ystrom, E.; Schjølberg, S.; Pons, F. Action Imitation at 1½ Years Is Better Than Pointing Gesture in Predicting Late Development of Language Production at 3 Years of Age. Child Dev. 2013, 84, 560–573. [Google Scholar] [CrossRef]
- Glenberg, A.M.; Kaschak, M.P. Grounding language in action. Psychon. Bull. Rev. 2002, 9, 558–565. [Google Scholar] [CrossRef]
- Fischer, M.H.; Zwaan, R.A. Embodied Language: A Review of the Role of the Motor System in Language Comprehension. Q. J. Exp. Psychol. 2008, 61, 825–850. [Google Scholar] [CrossRef] [PubMed]
- Kana, R.K.; Ammons, C.J.; Doss, C.F.; Waite, M.E.; Kana, B.; Herringshaw, A.J.; Ver Hoef, L. Language and motor cortex response to comprehending accidental and intentional action sentences. Neuropsychologia 2015, 77, 158–164. [Google Scholar] [CrossRef]
- De Vega, M.; León, I.; Hernández, J.A.; Valdés, M.; Padrón, I.; Ferstl, E.C. Action Sentences Activate Sensory Motor Regions in the Brain Independently of Their Status of Reality. J. Cogn. Neurosci. 2014, 26, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- De Grauwe, S.; Willems, R.M.; Rueschemeyer, S.-A.; Lemhöfer, K.; Schriefers, H. Embodied language in first- and second-language speakers: Neural correlates of processing motor verbs. Neuropsychologia 2014, 56, 334–349. [Google Scholar] [CrossRef]
- de Zubicaray, G.; Arciuli, J.; McMahon, K. Putting an “End” to the Motor Cortex Representations of Action Words. J. Cogn. Neurosci. 2013, 25, 1957–1974. [Google Scholar] [CrossRef] [PubMed]
- Hickok, G.; Costanzo, M.; Capasso, R.; Miceli, G. The role of Broca’s area in speech perception: Evidence from aphasia revisited. Brain Lang. 2011, 119, 214–220. [Google Scholar] [CrossRef]
- Adamaszek, M.; Kirkby, K.C. Cerebellum and Grammar Processing. In The Linguistic Cerebellum; Elsevier: Amsterdam, The Netherlands, 2016; pp. 81–105. ISBN 978-0-12-801608-4. [Google Scholar] [CrossRef]
- Lingam, R.; Golding, J.; Jongmans, M.J.; Hunt, L.P.; Ellis, M.; Emond, A. The Association between Developmental Coordination Disorder and Other Developmental Traits. Pediatrics 2010, 126, e1109–e1118. [Google Scholar] [CrossRef]
- Rechetnikov, R.P.; Maitra, K. Motor Impairments in Children Associated with Impairments of Speech or Language: A Meta-Analytic Review of Research Literature. Am. J. Occup. Ther. 2009, 63, 255–263. [Google Scholar] [CrossRef]
- Webster, R.I.; Erdos, C.; Evans, K.; Majnemer, A.; Kehayia, E.; Thordardottir, E.; Evans, A.; Shevell, M.I. The Clinical Spectrum of Developmental Language Impairment in School-Aged Children: Language, Cognitive, and Motor Findings. Pediatrics 2006, 118, e1541–e1549. [Google Scholar] [CrossRef]
- Norbury, C.F.; Gooch, D.; Wray, C.; Baird, G.; Charman, T.; Simonoff, E.; Vamvakas, G.; Pickles, A. The impact of nonverbal ability on prevalence and clinical presentation of language disorder: Evidence from a population study. J. Child Psychol. Psychiatry 2016, 57, 1247–1257. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- National Institute on Deafness and Other Communication Disorders 2016; Black, L.I.; Vahratian, A.; Hoffman, H.J. Communication Disorders and Use of Intervention Services among Children Aged 3–17 Years: United States, 2012; NCHS Data Brief, No 205; National Center for Health Statistics: Hyattsville, MD, USA, 2015.
- Reilly, S.; Wake, M.; Ukoumunne, O.C.; Bavin, E.; Prior, M.; Cini, E.; Conway, L.; Eadie, P.; Bretherton, L. Predicting Language Outcomes at 4 Years of Age: Findings From Early Language in Victoria Study. Pediatrics 2010, 126, e1530–e1537. [Google Scholar] [CrossRef] [PubMed]
- Eadie, P.; Morgan, A.; Ukoumunne, O.C.; Ttofari Eecen, K.; Wake, M.; Reilly, S. Speech sound disorder at 4 years: Prevalence, comorbidities, and predictors in a community cohort of children. Dev. Med. Child Neurol. 2015, 57, 578–584. [Google Scholar] [CrossRef]
- Bishop, D.V.M.; Price, T.S.; Dale, P.S.; Plomin, R. Outcomes of Early Language Delay: II. Etiology of Transient and Persistent Language Difficulties. J. Speech Lang. Hear. Res. 2003, 46, 561–575. [Google Scholar] [CrossRef][Green Version]
- Lewis, B.A.; Freebairn, L.; Tag, J.; Ciesla, A.A.; Iyengar, S.K.; Stein, C.M.; Taylor, H.G. Adolescent Outcomes of Children with Early Speech Sound Disorders with and without Language Impairment. Am. J. Speech Lang. Pathol. 2015, 24, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.L.; Alvarez, V.; Nelson, E.L. Do Gross and Fine Motor Skills Differentially Contribute to Language Outcomes? A Systematic Review. Front. Psychol. 2019, 10, 2670. [Google Scholar] [CrossRef] [PubMed]
- Folio, R.M.F.; Rebecca, R. Peabody Developmental Motor Scales, 2nd ed.; Pro-Ed: Austin, TX, USA, 2000. [Google Scholar]
- Newmeyer, A.J.; Grether, S.; Grasha, C.; White, J.; Akers, R.; Aylward, C.; Ishikawa, K.; deGrauw, T. Fine Motor Function and Oral-Motor Imitation Skills in Preschool-Age Children With Speech-Sound Disorders. Clin. Pediatr. 2007, 46, 604–611. [Google Scholar] [CrossRef]
- Bruininks, R.H. The Bruininks-Oseretsky Test of Motor Proficiency; American Guidance Service: Circle Pines, MN, USA, 1978. [Google Scholar]
- Zelaznik, H.N.; Goffman, L. Generalized Motor Abilities and Timing Behavior in Children With Specific Language Impairment. J. Speech Lang. Hear. Res. 2010, 53, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Beery, K.; Buktenica, N. Beery VMI: Development Test of Visual-Motor Integration; Pearson: London, UK, 2004. [Google Scholar]
- Ercan, Z.G.; Yilmaz, Ş.; Taş, M.; Aral, N. Investigation of Visual Motor Integration Skills in Children with Speech Sound Problems. Percept. Mot. Skills 2016, 123, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Nicola, K.; Watter, P. Visual-motor integration performance in children with severe specific language impairment: Visual-motor integration in children with specific language impairment. Child Care Health Dev. 2016, 42, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.E.; Sugden, D.; Barnett, A.L. Movement assessment battery for children-2. Res. Dev. Disabil. 2007. [Google Scholar] [CrossRef]
- Visscher, C.; Houwen, S.; Scherder, E.J.A.; Moolenaar, B.; Hartman, E. Motor Profile of Children with Developmental Speech and Language Disorders. Pediatrics 2007, 120, e158–e163. [Google Scholar] [CrossRef] [PubMed]
- Gaines, R.; Missiuna, C. Early identification: Are speech/language-impaired toddlers at increased risk for Developmental Coordination Disorder? Child Care Health Dev. 2007, 33, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Roid, G.H.; Koch, C. Leiter-3: Nonverbal cognitive and neuropsychological assessment. In Handbook of Nonverbal Assessment; Springer: Cham, Switzerland, 2017; pp. 127–150. [Google Scholar]
- Marini, A.; Marotta, L.; Bulgheroni, S.; Fabbro, F. Batteria per la Valutazione del Linguaggio in Bambini dai 4 ai 12 Anni; Giunti OS: Firenze, Italy, 2015. [Google Scholar]
- Beery, K.E.; Buktenica, N.A.; Beery, N.A. The Beery-Buktenica Developmental Test of Visual-Motor Integration: Administration, Scoring, and Teaching Manual, 6th ed.; NSC Pearson: Minneapolis, MN, USA, 2010. [Google Scholar]
- Bishop, D.V.M.; McArthur, G.M. Individual Differences in Auditory Processing in Specific Language Impairment: A Follow-Up Study Using Event-Related Potentials and Behavioural Thresholds. Cortex 2005, 41, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Snowling, M.J.; Nash, H.M.; Gooch, D.C.; Hayiou-Thomas, M.E.; Hulme, C. Wellcome Language and Reading Project Team Developmental Outcomes for Children at High Risk of Dyslexia and Children with Developmental Language Disorder. Child Dev. 2019, 90, e548–e564. [Google Scholar] [CrossRef]
- Bravenboer, M.; de Groot, R.; Visser, E. MetaBorg in Action: Examples of Domain-Specific Language Embedding and Assimilation Using Stratego/XT. In Generative and Transformational Techniques in Software Engineering; Lämmel, R., Saraiva, J., Visser, J., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2006; Volume 4143, pp. 297–311. ISBN 978-3-540-45778-7. [Google Scholar] [CrossRef]
- Leisman, G.; Braun-Benjamin, O.; Melillo, R. Cognitive-motor interactions of the basal ganglia in development. Front. Syst. Neurosci. 2014, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Close Interrelation of Motor Development and Cognitive Development and of the Cerebellum and Prefrontal Cortex. Child Dev. 2000, 71, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Hedenius, M. Procedural and Declarative Memory in Children with Developmental Disorders of Language and Literacy; Acta Universitatis Upsaliensis: Uppsala, Sweden, 2013; ISBN 978-91-554-8707-2. [Google Scholar]
- Lum, J.A.G.; Conti-Ramsden, G. Long-Term Memory: A Review and Meta-Analysis of Studies of Declarative and Procedural Memory in Specific Language Impairment. Top. Lang. Disord. 2013, 33, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, R.I.; Fawcett, A.J. Procedural learning difficulties: Reuniting the developmental disorders? Trends Neurosci. 2007, 30, 135–141. [Google Scholar] [CrossRef]
- Ullman, M.T. A cognitive neuroscience perspective on second language acquisition: The declarative/procedural model. Mind Context Adult Second. Lang. Acquis. 2005, 2005, 141–178. [Google Scholar]
- Bombonato, C.; Casalini, C.; Pecini, C.; Angelucci, G.; Vicari, S.; Podda, I.; Cipriani, P.; Chilosi, A.M.; Menghini, D. Implicit learning in children with Childhood Apraxia of Speech. Res. Dev. Disabil. 2022, 122, 104170. [Google Scholar] [CrossRef] [PubMed]
- Sices, L.; Taylor, H.G.; Freebairn, L.; Hansen, A.; Lewis, B. Relationship Between Speech-Sound Disorders and Early Literacy Skills in Preschool-Age Children: Impact of Comorbid Language Impairment. J. Dev. Behav. Pediatr. 2007, 28, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Baah, C.; Schoenhaut, A.M.; Vassall, S.G.; Tovar, D.A.; Ramachandran, R.; Wallace, M.T. Visual Influences on Auditory Behavioral, Neural, and Perceptual Processes: A Review. J. Assoc. Res. Otolaryngol. 2021, 22, 365–386. [Google Scholar] [CrossRef]
- Huyse, A.; Berthommier, F.; Leybaert, J. Degradation of Labial Information Modifies Audiovisual Speech Perception in Cochlear-Implanted Children. Ear Hear. 2013, 34, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Paris, T.; Kim, J.; Davis, C. Visual speech form influences the speed of auditory speech processing. Brain Lang. 2013, 126, 350–356. [Google Scholar] [CrossRef]
- Chen, F.; Wang, L.; Peng, G.; Yan, N.; Pan, X. Development and evaluation of a 3-D virtual pronunciation tutor for children with autism spectrum disorders. PLoS ONE 2019, 14, e0210858. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yabushita, T.; Sakamoto, S.; Katori, Y.; Kawase, T. In-home auditory training using audiovisual stimuli on a tablet computer: Feasibility and preliminary results. Auris. Nasus. Larynx 2020, 47, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Ghassabian, A.; Sundaram, R.; Bell, E.; Bello, S.C.; Kus, C.; Yeung, E. Gross Motor Milestones and Subsequent Development. Pediatrics 2016, 138, e20154372. [Google Scholar] [CrossRef]
- Mancini, V.O.; Rigoli, D.; Heritage, B.; Roberts, L.D.; Piek, J.P. The Relationship between Motor Skills, Perceived Social Support, and Internalizing Problems in a Community Adolescent Sample. Front. Psychol. 2016, 7, 543. [Google Scholar] [CrossRef] [PubMed]
- Walle, E.A.; Campos, J.J. Infant language development is related to the acquisition of walking. Dev. Psychol. 2014, 50, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Iverson, J.M.; Shic, F.; Wall, C.A.; Chawarska, K.; Curtin, S.; Estes, A.; Gardner, J.M.; Hutman, T.; Landa, R.J.; Levin, A.R.; et al. Early motor abilities in infants at heightened versus low risk for ASD: A Baby Siblings Research Consortium (BSRC) study. J. Abnorm. Psychol. 2019, 128, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.S.; Seitz, A.R.; Shams, L. Benefits of Stimulus Congruency for Multisensory Facilitation of Visual Learning. PLoS ONE 2008, 3, e1532. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.; Jenni, O.G.; Molinari, L.; Caflisch, J.; Largo, R.H.; Latal Hajnal, B. Correlations between Motor Performance and Cognitive Functions in Children Born < 1250 g at School Age. Neuropediatrics 2006, 37, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Shams, L.; Seitz, A.R. Benefits of multisensory learning. Trends Cogn. Sci. 2008, 12, 411–417. [Google Scholar] [CrossRef] [PubMed]
- von Kriegstein, K.; Giraud, A.-L. Implicit Multisensory Associations Influence Voice Recognition. PLoS Biol. 2006, 4, e326. [Google Scholar] [CrossRef] [PubMed]
- Council on Children with Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children with Special Needs Project Advisory Committee. Identifying Infants and Young Children with Developmental Disorders in the Medical Home: An Algorithm for Developmental Surveillance and Screening. Pediatrics 2006, 118, 405–420. [Google Scholar] [CrossRef]

| Lexical Processing | Grammatical Processing | Speech Processing | |||
|---|---|---|---|---|---|
| Groups | Naming % 1 | Comprehension % 1 | Sentence Repetition % 1 | Comprehension % 1 | Articulation % 1 |
| LD | 14.3 | 38.1 | 85.7 | 47.6 | - |
| SSD | - | - | - | - | 100 |
| LD + SSD | 40 | 57.8 | 76.7 | 61.1 | 100 |
| Lexical Processing | Grammatical Processing | Speech Processing | |||
|---|---|---|---|---|---|
| Groups | Naming % 1 | Comprehension % 1 | Sentence Repetition % 1 | Comprehension % 1 | Articulation % 1 |
| LD | 29.4 | 44.1 | 64.7 | 38.2 | - |
| SSD | - | - | - | - | 100 |
| LD + SSD | 48 | 61.5 | 82.4 | 39.8 | 100 |
| MABC-2 | LD Mean (SD) | SSD Mean (SD) | LD + SSD Mean (SD) | H(2, N = 147) | p-Value |
|---|---|---|---|---|---|
| Total | 5.57 ^ (2.56) | 6.27 ^ (2.09) | 5.34 ^ (2.49) | 3.88 | 0.14 |
| Manual Dexterity | 6.47 ^ (2.94) | 7.72 (3.93) | 6.43 ^ (3.20) | 2.52 | 0.28 |
| Aiming and Catching | 7.33 (3.91) | 7.44 (2.82) | 6.91 ^ (2.71) | 1.67 | 0.43 |
| Balance | 6.04 ^ (2.33) | 6.55 ^ (1.25) | 5.81 ^ (2.46) | 3.8 | 0.14 |
| VMI | LD Mean (SD) | SSD Mean (SD) | LD + SSD Mean (SD) | H (2, N = 237) | p-Value |
|---|---|---|---|---|---|
| Visual Motor Integration | 95.64 (12.31) | 100.19 (14.7) | 96.16 (14.10) | 4.16 | 0.12 |
| Visual Perception | 99.22 (20.41) | 100.45 (15.89) | 90.79 ** (19.72) | 13.03 | 0.0015 |
| Fine Motor Coordination | 90.71 (19.09) | 94.80 (15.24) | 89.63 * (14.69) | 6.36 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varuzza, C.; D’Aiello, B.; Lazzaro, G.; Quarin, F.; De Rose, P.; Bergonzini, P.; Menghini, D.; Marini, A.; Vicari, S. Gross, Fine and Visual-Motor Skills in Children with Language Disorder, Speech Sound Disorder and Their Combination. Brain Sci. 2023, 13, 59. https://doi.org/10.3390/brainsci13010059
Varuzza C, D’Aiello B, Lazzaro G, Quarin F, De Rose P, Bergonzini P, Menghini D, Marini A, Vicari S. Gross, Fine and Visual-Motor Skills in Children with Language Disorder, Speech Sound Disorder and Their Combination. Brain Sciences. 2023; 13(1):59. https://doi.org/10.3390/brainsci13010059
Chicago/Turabian StyleVaruzza, Cristiana, Barbara D’Aiello, Giulia Lazzaro, Fabio Quarin, Paola De Rose, Paola Bergonzini, Deny Menghini, Andrea Marini, and Stefano Vicari. 2023. "Gross, Fine and Visual-Motor Skills in Children with Language Disorder, Speech Sound Disorder and Their Combination" Brain Sciences 13, no. 1: 59. https://doi.org/10.3390/brainsci13010059
APA StyleVaruzza, C., D’Aiello, B., Lazzaro, G., Quarin, F., De Rose, P., Bergonzini, P., Menghini, D., Marini, A., & Vicari, S. (2023). Gross, Fine and Visual-Motor Skills in Children with Language Disorder, Speech Sound Disorder and Their Combination. Brain Sciences, 13(1), 59. https://doi.org/10.3390/brainsci13010059








