Dynamic Changes of Brain Activity in Different Responsive Groups of Patients with Prolonged Disorders of Consciousness
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Coma Recovery Scale
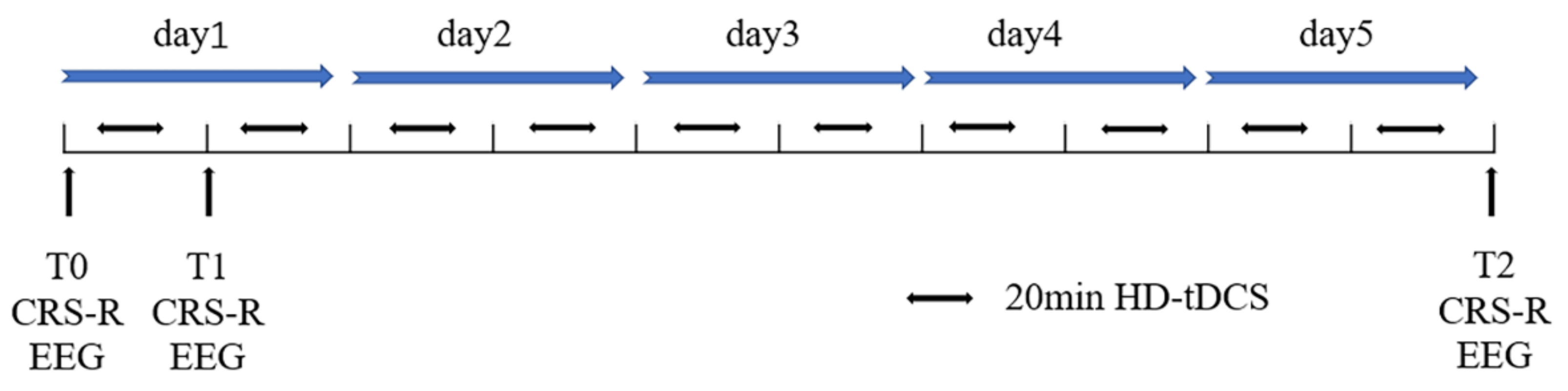
2.3. Stimulation Protocol
2.4. EEG Data Acquisition and Pre-Processing
2.5. Microstate Analysis
2.6. Microstate Segmentation
2.7. Microstate Parameters
2.8. Statistical Analysis
3. Results
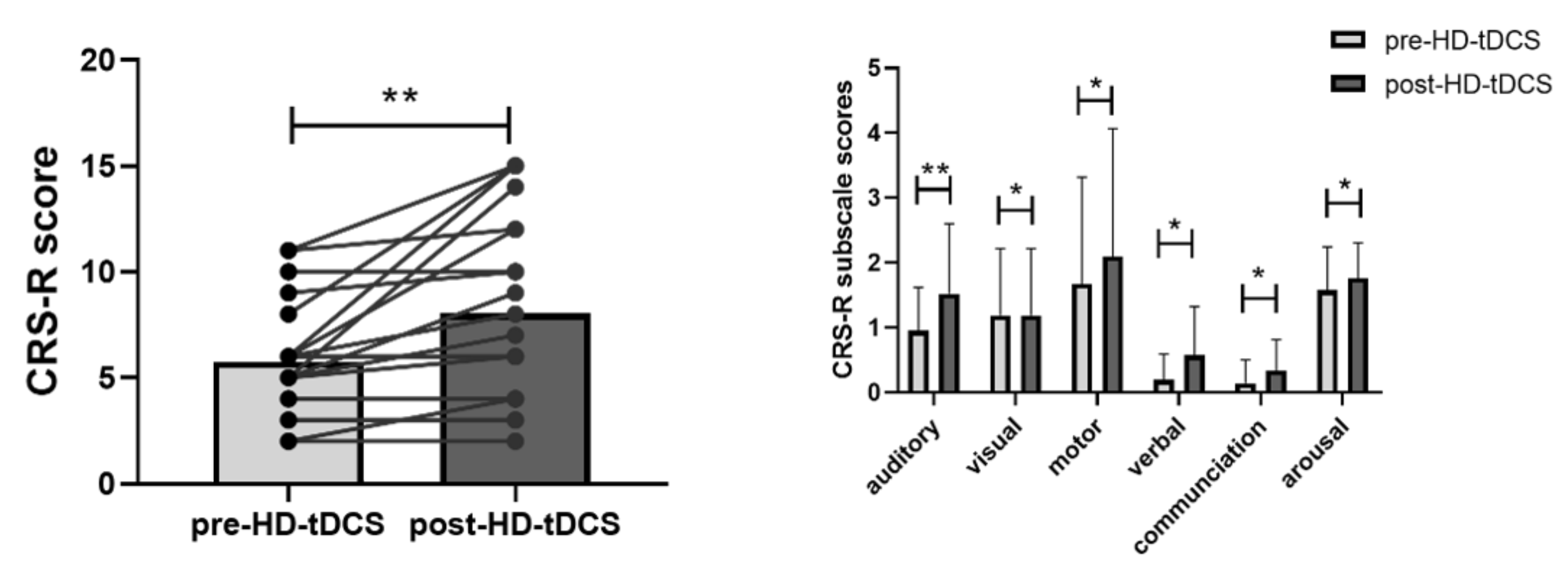
3.1. Demographic and Clinical Behavioral Results
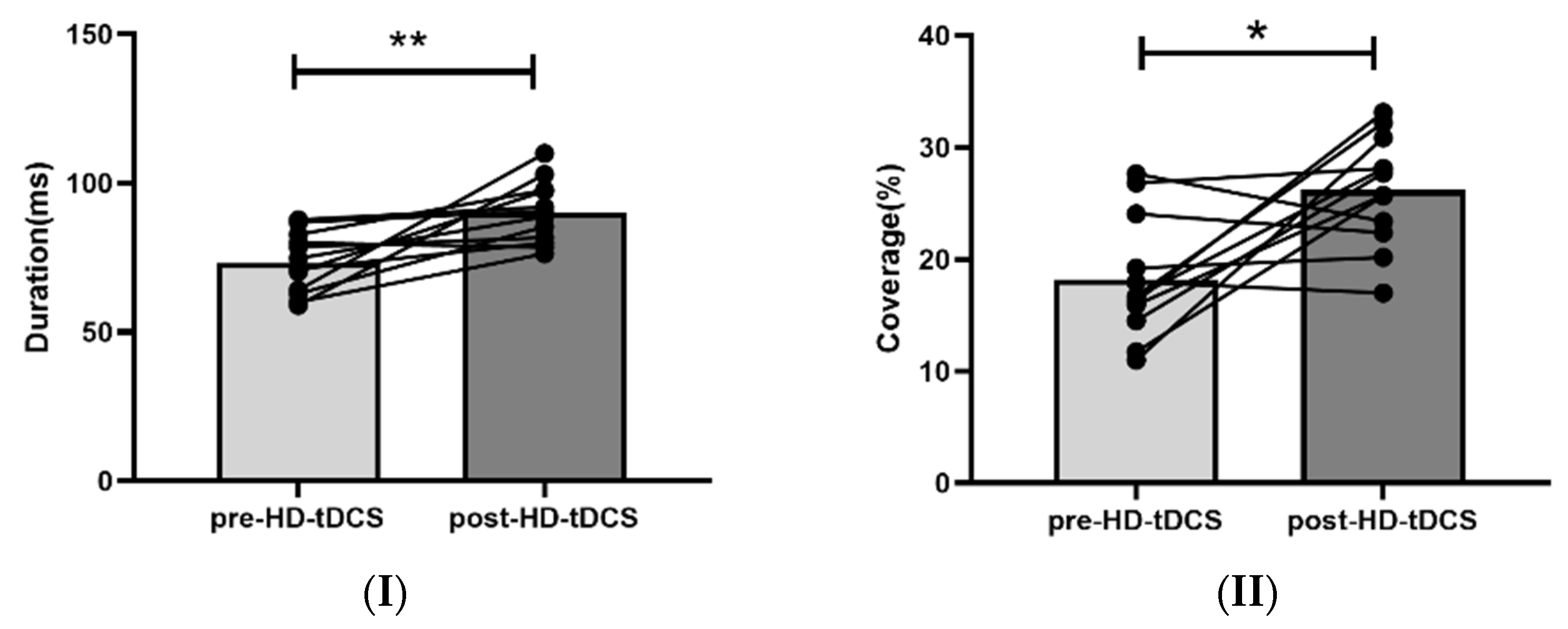
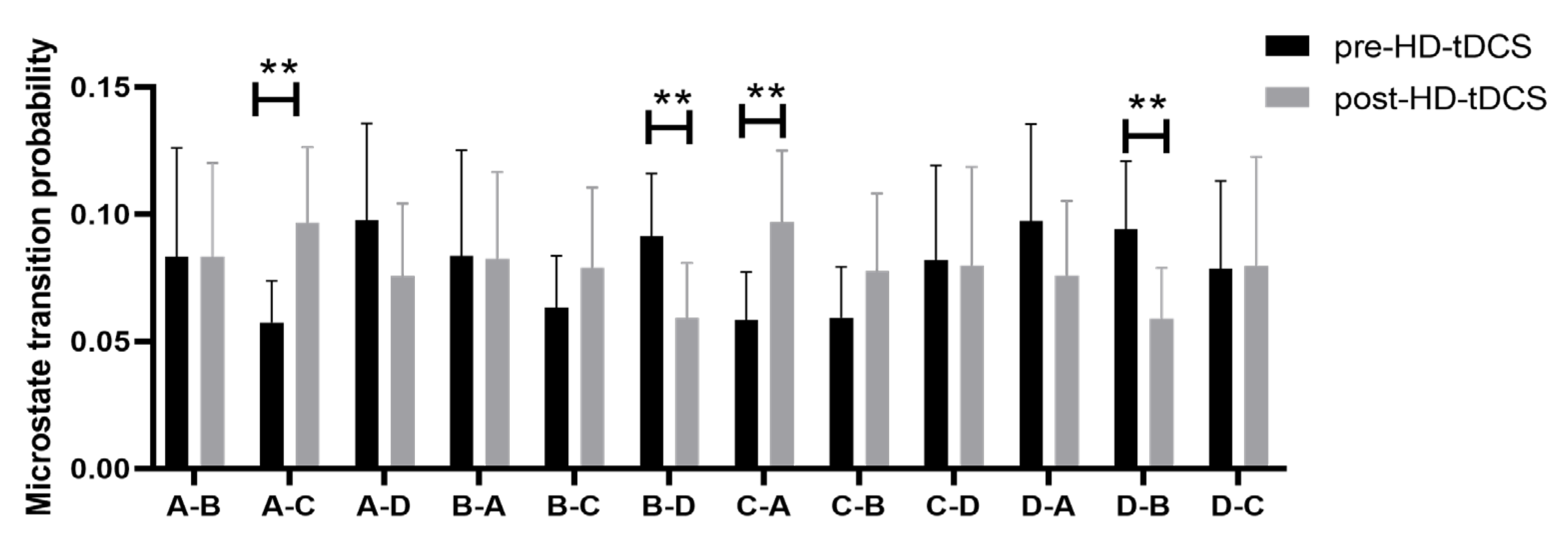
3.2. Changes in EEG Microstate Parameters following HD-tDCS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comanducci, A.; Boly, M.; Claassen, J.; De Lucia, M.; Gibson, R.M.; Juan, E.; Laureys, S.; Naccache, L.; Owen, A.M.; Rosanova, M.; et al. Clinical and advanced neurophysiology in the prognostic and diagnostic evaluation of disorders of consciousness: Review of an IFCN-endorsed expert group. Clin. Neurophysiol. 2020, 131, 2736–2765. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Comprehensive Systematic Review Update Summary: Disorders of Consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch. Phys. Med. Rehabil. 2018, 99, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D. Cognitive Motor Dissociation Following Severe Brain Injuries. JAMA Neurol. 2015, 72, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Wannez, S.; Gosseries, O.; Azzolini, D.; Martial, C.; Cassol, H.; Aubinet, C.; Annen, J.; Martens, G.; Bodart, O.; Heine, L.; et al. Prevalence of coma-recovery scale-revised signs of consciousness in patients in minimally conscious state. Neuropsychol. Rehabil. 2018, 28, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.A.; Vanhaudenhuyse, A.; Thibaut, A.; Moonen, G.; Laureys, S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: Recent advances in our understanding of disorders of consciousness. J. Neurol. 2011, 258, 1373–1384. [Google Scholar] [CrossRef]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009, 2, 201–207.e201. [Google Scholar] [CrossRef]
- Dmochowski, J.P.; Datta, A.; Bikson, M.; Su, Y.; Parra, L.C. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural. Eng. 2011, 8, 046011. [Google Scholar] [CrossRef]
- Thibaut, A.; Bruno, M.A.; Ledoux, D.; Demertzi, A.; Laureys, S. tDCS in patients with disorders of consciousness: Sham-controlled randomized double-blind study. Neurology 2014, 82, 1112–1118. [Google Scholar] [CrossRef]
- Estraneo, A.; Pascarella, A.; Moretta, P.; Masotta, O.; Fiorenza, S.; Chirico, G.; Crispino, E.; Loreto, V.; Trojano, L. Repeated transcranial direct current stimulation in prolonged disorders of consciousness: A double-blind cross-over study. J. Neurol. Sci. 2017, 375, 464–470. [Google Scholar] [CrossRef]
- Seitzman, B.A.; Abell, M.; Bartley, S.C.; Erickson, M.A.; Bolbecker, A.R.; Hetrick, W.P. Cognitive manipulation of brain electric microstates. Neuroimage 2017, 146, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Koenig, T.; Prichep, L.; Lehmann, D.; Sosa, P.V.; Braeker, E.; Kleinlogel, H.; Isenhart, R.; John, E.R. Millisecond by millisecond, year by year: Normative EEG microstates and developmental stages. Neuroimage 2002, 16, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Pascual-Leone, A.; Michel, C.M.; Farzan, F. Microstates in resting-state EEG: Current status and future directions. Neurosci. Biobehav. Rev. 2015, 49, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Britz, J.; Van De Ville, D.; Michel, C.M. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage 2010, 52, 1162–1170. [Google Scholar] [CrossRef]
- Michel, C.M.; Koenig, T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. Neuroimage 2018, 180, 577–593. [Google Scholar] [CrossRef]
- Irisawa, S.; Isotani, T.; Yagyu, T.; Morita, S.; Nishida, K.; Yamada, K.; Yoshimura, M.; Okugawa, G.; Nobuhara, K.; Kinoshita, T. Increased omega complexity and decreased microstate duration in nonmedicated schizophrenic patients. Neuropsychobiology 2006, 54, 134–139. [Google Scholar] [CrossRef]
- Nishida, K.; Morishima, Y.; Yoshimura, M.; Isotani, T.; Irisawa, S.; Jann, K.; Dierks, T.; Strik, W.; Kinoshita, T.; Koenig, T. EEG microstates associated with salience and frontoparietal networks in frontotemporal dementia, schizophrenia and Alzheimer’s disease. Clin. Neurophysiol. 2013, 124, 1106–1114. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, M.; Hu, Y.; Wang, K. Altered Resting-State Electroencephalography Microstates in Idiopathic Generalized Epilepsy: A Prospective Case-Control Study. Front. Neurol. 2021, 12, 710952. [Google Scholar] [CrossRef]
- Drissi, N.M.; Szakacs, A.; Witt, S.T.; Wretman, A.; Ulander, M.; Stahlbrandt, H.; Darin, N.; Hallbook, T.; Landtblom, A.M.; Engstrom, M. Altered Brain Microstate Dynamics in Adolescents with Narcolepsy. Front. Hum. Neurosci. 2016, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, V.; Kuhn, A.; von Wegner, F.; Morzelewski, A.; Tagliazucchi, E.; Borisov, S.; Michel, C.M.; Laufs, H. EEG microstates of wakefulness and NREM sleep. Neuroimage 2012, 62, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Gianotti, L.R.; Isotani, T.; Faber, P.L.; Sasada, K.; Kinoshita, T.; Lehmann, D. Classes of multichannel EEG microstates in light and deep hypnotic conditions. Brain Topogr. 2007, 20, 7–14. [Google Scholar] [CrossRef]
- Panda, R.; Bharath, R.D.; Upadhyay, N.; Mangalore, S.; Chennu, S.; Rao, S.L. Temporal Dynamics of the Default Mode Network Characterize Meditation-Induced Alterations in Consciousness. Front. Hum. Neurosci. 2016, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Wutzl, B.; Golaszewski, S.M.; Leibnitz, K.; Langthaler, P.B.; Kunz, A.B.; Leis, S.; Schwenker, K.; Thomschewski, A.; Bergmann, J.; Trinka, E. Narrative Review: Quantitative EEG in Disorders of Consciousness. Brain Sci. 2021, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Bagnato, S.; Boccagni, C.; Galardi, G. EEG oscillatory states as neuro-phenomenology of consciousness as revealed from patients in vegetative and minimally conscious states. Conscious Cogn. 2012, 21, 149–169. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Bagnato, S.; Boccagni, C.; Galardi, G. The value of spontaneous EEG oscillations in distinguishing patients in vegetative and minimally conscious states. Suppl. Clin. Neurophysiol. 2013, 62, 81–99. [Google Scholar] [CrossRef]
- Stefan, S.; Schorr, B.; Lopez-Rolon, A.; Kolassa, I.T.; Shock, J.P.; Rosenfelder, M.; Heck, S.; Bender, A. Consciousness Indexing and Outcome Prediction with Resting-State EEG in Severe Disorders of Consciousness. Brain Topogr. 2018, 31, 848–862. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Han, S.; Xu, L.; Chen, X.; Geng, X.; Bie, L.; He, J. The temporal dynamics of Large-Scale brain network changes in disorders of consciousness: A Microstate-Based study. CNS Neurosci. Ther. 2022, 1–10. [Google Scholar] [CrossRef]
- Panda, R.; Thibaut, A.; Lopez-Gonzalez, A.; Escrichs, A.; Bahri, M.A.; Hillebrand, A.; Deco, G.; Laureys, S.; Gosseries, O.; Annen, J.; et al. Disruption in structural-functional network repertoire and time-resolved subcortical fronto-temporoparietal connectivity in disorders of consciousness. Elife 2022, 11, e77462. [Google Scholar] [CrossRef]
- Giacino, J.T.; Fins, J.J.; Laureys, S.; Schiff, N.D. Disorders of consciousness after acquired brain injury: The state of the science. Nat. Rev. Neurol. 2014, 10, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bai, Y.; Xia, X.; Li, J.; Wang, X.; Dai, Y.; Dang, Y.; He, J.; Liu, C.; Zhang, H. Effects of Long-Lasting High-Definition Transcranial Direct Current Stimulation in Chronic Disorders of Consciousness: A Pilot Study. Front. Neurosci. 2019, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, R.; Zhang, R.; Liu, C.; Zhang, L.; Zhao, D.; Shan, Q.; Wang, X.; Hu, Y. Dynamic Changes of Brain Activity in Patients With Disorders of Consciousness During Recovery of Consciousness. Front. Neurosci. 2022, 16, 878203. [Google Scholar] [CrossRef] [PubMed]
- Gui, P.; Jiang, Y.; Zang, D.; Qi, Z.; Tan, J.; Tanigawa, H.; Jiang, J.; Wen, Y.; Xu, L.; Zhao, J.; et al. Assessing the depth of language processing in patients with disorders of consciousness. Nat. Neurosci. 2020, 23, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.-P.; Makeig, S.; Westerfield, M.; Townsend, J.; Courchesne, E.; Sejnowski, T.J. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. 2000, 111, 1745–1758. [Google Scholar] [CrossRef]
- He, R.; Fan, J.; Wang, H.; Zhong, Y.; Ma, J. Differentiating Responders and Non-responders to rTMS Treatment for Disorder of Consciousness Using EEG After-Effects. Front. Neurol. 2020, 11, 583268. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Segmentation of brain electrical activity into microstates: Model estimation and validation. IEEE Trans. Biomed. Eng. 1995, 42, 658–665. [Google Scholar] [CrossRef]
- Poulsen, A.T.; Pedroni, A.; Langer, N.; Hansen, L.K. Microstate EEGlab toolbox: An introductory guide. bioRxiv 2018, 289850. [Google Scholar] [CrossRef]
- Lehmann, D.; Faber, P.L.; Galderisi, S.; Herrmann, W.M.; Kinoshita, T.; Koukkou, M.; Mucci, A.; Pascual-Marqui, R.D.; Saito, N.; Wackermann, J.; et al. EEG microstate duration and syntax in acute, medication-naive, first-episode schizophrenia: A multi-center study. Psych. Res. 2005, 138, 141–156. [Google Scholar] [CrossRef]
- Tomescu, M.I.; Rihs, T.A.; Roinishvili, M.; Karahanoglu, F.I.; Schneider, M.; Menghetti, S.; Van De Ville, D.; Brand, A.; Chkonia, E.; Eliez, S.; et al. Schizophrenia patients and 22q11.2 deletion syndrome adolescents at risk express the same deviant patterns of resting state EEG microstates: A candidate endophenotype of schizophrenia. Schizophr. Res. Cogn. 2015, 2, 159–165. [Google Scholar] [CrossRef]
- Koenig, T.; Lehmann, D.; Merlo, M.C.; Kochi, K.; Hell, D.; Koukkou, M. A deviant EEG brain microstate in acute, neuroleptic-naive schizophrenics at rest. Eur. Arch. Psych. Clin. Neurosci. 1999, 249, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Li, R. Study on the Process of Consciousness Recovery in DOC Patients Based on Microstate. Int. J. Psychophysiol. 2021, 168, S204. [Google Scholar] [CrossRef]
- Vanhaudenhuyse, A.; Noirhomme, Q.; Tshibanda, L.J.; Bruno, M.A.; Boveroux, P.; Schnakers, C.; Soddu, A.; Perlbarg, V.; Ledoux, D.; Brichant, J.F.; et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2010, 133, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Vanhaudenhuyse, A.; Demertzi, A.; Schabus, M.; Noirhomme, Q.; Bredart, S.; Boly, M.; Phillips, C.; Soddu, A.; Luxen, A.; Moonen, G.; et al. Two distinct neuronal networks mediate the awareness of environment and of self. J. Cogn. Neurosci. 2011, 23, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Yeshurun, Y.; Nguyen, M.; Hasson, U. The default mode network: Where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 2021, 22, 181–192. [Google Scholar] [CrossRef]
- Tommasino, C.; Grana, C.; Lucignani, G.; Torri, G.; Fazio, F. Regional cerebral metabolism of glucose in comatose and vegetative state patients. J. Neurosurg. Anesthesiol. 1995, 7, 109–116. [Google Scholar] [CrossRef]
- Qin, P.; Wu, X.; Huang, Z.; Duncan, N.W.; Tang, W.; Wolff, A.; Hu, J.; Gao, L.; Jin, Y.; Wu, X.; et al. How are different neural networks related to consciousness? Ann. Neurol. 2015, 78, 594–605. [Google Scholar] [CrossRef]
- Qin, P.; Wu, X.; Wu, C.; Wu, H.; Zhang, J.; Huang, Z.; Weng, X.; Zang, D.; Qi, Z.; Tang, W.; et al. Higher-order sensorimotor circuit of the brain’s global network supports human consciousness. Neuroimage 2021, 231, 117850. [Google Scholar] [CrossRef]

| ID | Sex | Age | Etiology | Course (Days) | T0 (CRS-R) | T0-Diagnosis | T1 (CRS-R) | T1-Diagnosis | T2 (CRS-R) | T2-Diagnosis | Follow-Up at 3 Months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RE1 | M | 52 | Trauma | 84 | 11 | MCS+ | 11 | MCS+ | 12 | MCS+ | EMCS |
| RE2 | F | 49 | HIE | 30 | 6 | MCS- | 7 | MCS- | 15 | MCS+ | EMCS |
| RE3 | M | 53 | Trauma | 34 | 5 | MCS- | 7 | MCS- | 14 | MCS+ | EMCS |
| RE4 | F | 74 | Hemorrhage | 101 | 11 | MCS+ | 11 | MCS+ | 15 | MCS+ | Dead |
| RE5 | M | 49 | Hemorrhage | 50 | 5 | VS | 6 | VS | 7 | VS | VS |
| RE6 | M | 55 | Trauma | 302 | 6 | VS | 6 | VS | 8 | MCS- | MCS- |
| RE7 | M | 72 | CI | 42 | 5 | MCS- | 5 | MCS- | 9 | MCS+ | MCS+ |
| RE8 | M | 47 | Hemorrhage | 29 | 6 | VS | 6 | VS | 12 | MCS+ | MCS+ |
| RE9 | M | 58 | Trauma | 53 | 9 | MCS- | 9 | MCS- | 10 | MCS- | EMCS |
| RE10 | F | 68 | Hemorrhage | 30 | 8 | MCS- | 9 | MCS+ | 15 | MCS+ | MCS+ |
| RE11 | M | 59 | CI | 68 | 5 | VS | 5 | VS | 6 | MCS+ | MCS+ |
| RE12 | M | 72 | CI | 200 | 2 | VS | 2 | VS | 4 | VS | VS |
| N-RE1 | M | 54 | Hemorrhage | 73 | 6 | VS | 6 | VS | 6 | VS | VS |
| N-RE2 | M | 56 | HIE | 41 | 2 | VS | 2 | VS | 2 | VS | VS |
| N-RE3 | F | 39 | HIE | 128 | 4 | VS | 4 | VS | 4 | VS | VS |
| N-RE4 | M | 18 | Disseminated encephalomyelitis | 48 | 4 | VS | 4 | VS | 4 | VS | MCS- |
| N-RE5 | M | 56 | Hemorrhage | 88 | 3 | VS | 3 | VS | 3 | VS | VS |
| N-RE6 | M | 64 | Hemorrhage | 34 | 10 | MCS- | 10 | MCS- | 10 | MCS- | MCS- |
| N-RE7 | F | 70 | CI | 58 | 4 | VS | 4 | VS | 4 | VS | MCS+ |
| N-RE8 | F | 39 | HIE | 215 | 3 | VS | 3 | VS | 3 | VS | VS |
| N-RE9 | M | 57 | Hemorrhage | 52 | 6 | VS | 6 | VS | 6 | VS | MCS+ |
| RE Group | N-RE Group | |||||
|---|---|---|---|---|---|---|
| Before HD-tDCS | After HD-tDCS | p Value | Before HD-tDCS | After HD-tDCS | p Value | |
| Microstate class A | ||||||
| Duration (ms) | 85.37 ± 14.36 | 91.04 ± 13.05 | 0.323 | 84.34 ± 25.71 | 87.23 ± 33.13 | 0.839 |
| Occurrence (per s) | 2.97 ± 0.65 | 3.09 ± 0.51 | 0.618 | 3.10 ± 1.06 | 3.16 ± 1.18 | 0.91 |
| Coverage (%) | 25.26 ± 7.87 | 27.32 ± 5.77 | 0.473 | 23.63 ± 5.66 | 25.44 ± 8.51 | 0.603 |
| Microstate class B | ||||||
| Duration (ms) | 84.74 ± 15.39 | 87.99 ± 20.50 | 0.665 | 87.57 ± 22.47 | 82.08 ± 23.23 | 0.617 |
| Occurrence (per s) | 2.94 ± 0.61 | 2.64 ± 0.74 | 0.297 | 3.55 ± 1.65 | 3.10 ± 1.47 | 0.555 |
| Coverage (%) | 25.03 ± 8.62 | 23.61 ± 10.54 | 0.722 | 27.83 ± 6.54 | 23.30 ± 7.28 | 0.184 |
| Microstate class C | ||||||
| Duration (ms) | 73.21 ± 10.17 | 90.12 ± 10.43 | 0.001 * | 85.41 ± 25.93 | 85.38 ± 26.29 | 0.999 |
| Occurrence (per s) | 2.50 ± 0.54 | 3.08 ± 0.64 | 0.024 | 3.38 ± 1.14 | 2.96 ± 0.67 | 0.36 |
| Coverage (%) | 18.21 ± 5.38 | 27.13 ± 6.90 | 0.002 * | 26.03 ± 5.851 | 24.33 ± 7.413 | 0.595 |
| Microstate class D | ||||||
| Duration (ms) | 95.05 ± 21.41 | 84.21 ± 19.47 | 0.208 | 80.18 ± 31.20 | 83.52 ± 20.59 | 0.793 |
| Occurrence (per s) | 3.30 ± 0.76 | 2.60 ± 0.82 | 0.042 | 3.11 ± 1.14 | 3.53 ± 1.58 | 0.528 |
| Coverage (%) | 31.50 ± 12.43 | 21.96 ± 10.05 | 0.051 | 22.56 ± 6.65 | 26.92 ± 8.27 | 0.235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Han, J.; Zheng, S.; Zhang, X.; Sun, H.; Zhou, T.; Hu, S.; Yan, X.; Wang, C.; Wang, K.; et al. Dynamic Changes of Brain Activity in Different Responsive Groups of Patients with Prolonged Disorders of Consciousness. Brain Sci. 2023, 13, 5. https://doi.org/10.3390/brainsci13010005
Chen C, Han J, Zheng S, Zhang X, Sun H, Zhou T, Hu S, Yan X, Wang C, Wang K, et al. Dynamic Changes of Brain Activity in Different Responsive Groups of Patients with Prolonged Disorders of Consciousness. Brain Sciences. 2023; 13(1):5. https://doi.org/10.3390/brainsci13010005
Chicago/Turabian StyleChen, Chen, Jinying Han, Shuang Zheng, Xintong Zhang, Haibo Sun, Ting Zhou, Shunyin Hu, Xiaoxiang Yan, Changqing Wang, Kai Wang, and et al. 2023. "Dynamic Changes of Brain Activity in Different Responsive Groups of Patients with Prolonged Disorders of Consciousness" Brain Sciences 13, no. 1: 5. https://doi.org/10.3390/brainsci13010005
APA StyleChen, C., Han, J., Zheng, S., Zhang, X., Sun, H., Zhou, T., Hu, S., Yan, X., Wang, C., Wang, K., & Hu, Y. (2023). Dynamic Changes of Brain Activity in Different Responsive Groups of Patients with Prolonged Disorders of Consciousness. Brain Sciences, 13(1), 5. https://doi.org/10.3390/brainsci13010005






