In Silico Structural Analysis Predicting the Pathogenicity of PLP1 Mutations in Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Summary of Variants
2.2. In Silico Methods for Predicting Mutation Significance
2.3. Protein Stability Correlation Analysis
2.4. Analysis of Protein Structural Conformation and Conservation
2.5. Prediction of the Secondary Structure
2.6. Homology Modeling
2.7. Mutated Structure Prediction
2.8. Protein Three-Dimensional Model Verification
3. Results
3.1. PLP1 Variants Associated with MS
3.2. Variant Functional Analysis
3.3. Conformational Analysis and Alteration of Protein Stability upon Amino Acid Substitution
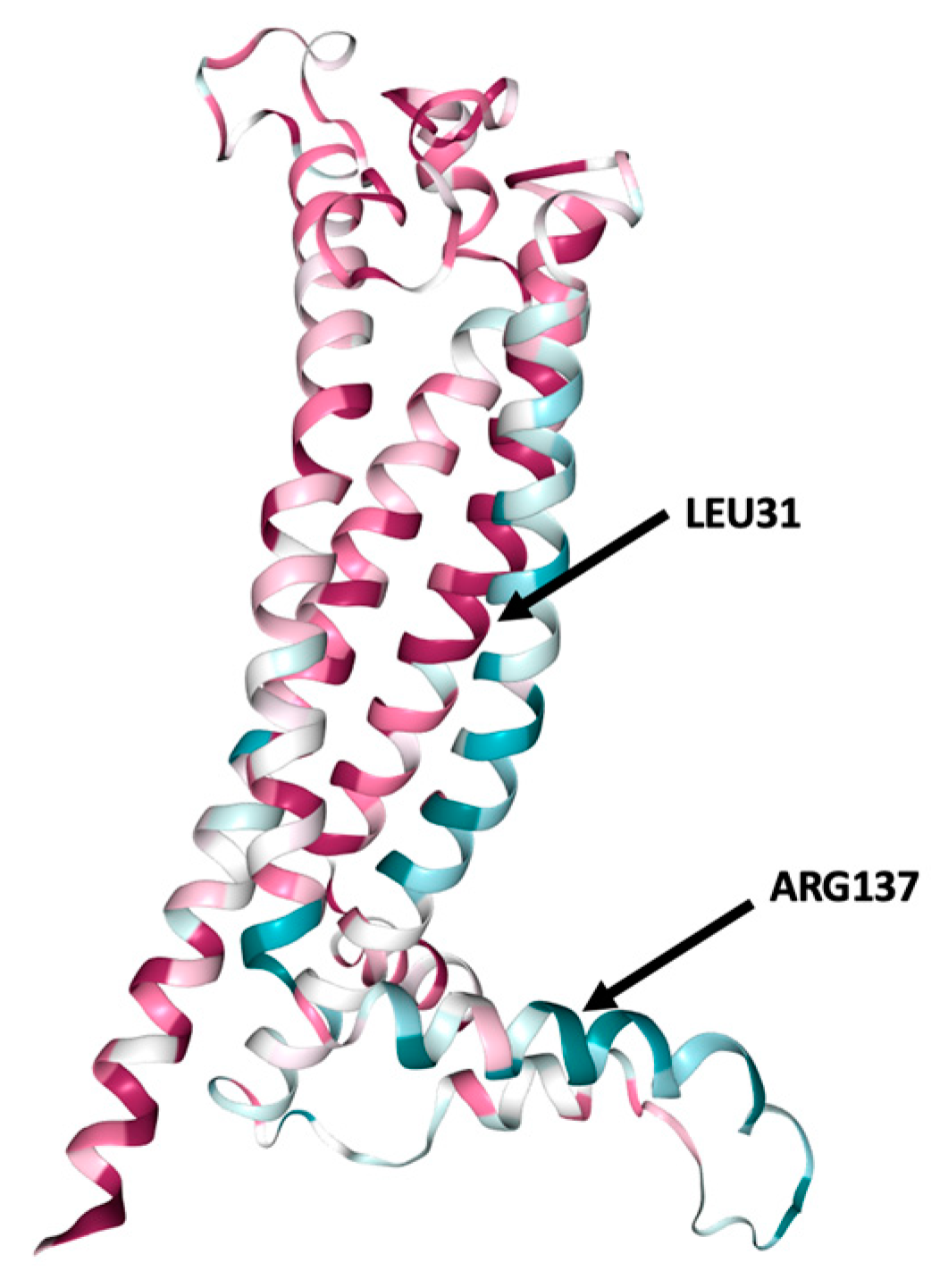
3.4. Analysis of the Structural Conformation and Conservation of PLP1
3.5. PLP1 Protein Secondary Structure Prediction
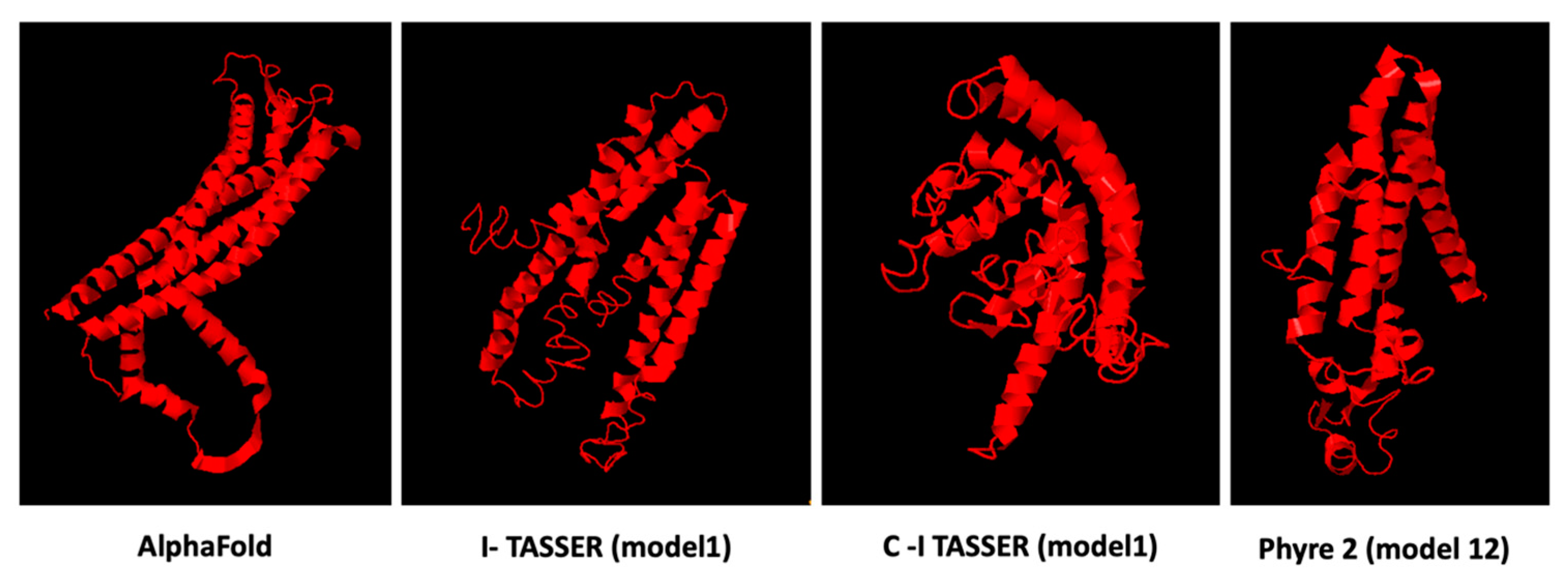
3.6. Prediction Software Benchmarking and Creation of Tertiary Protein Structures
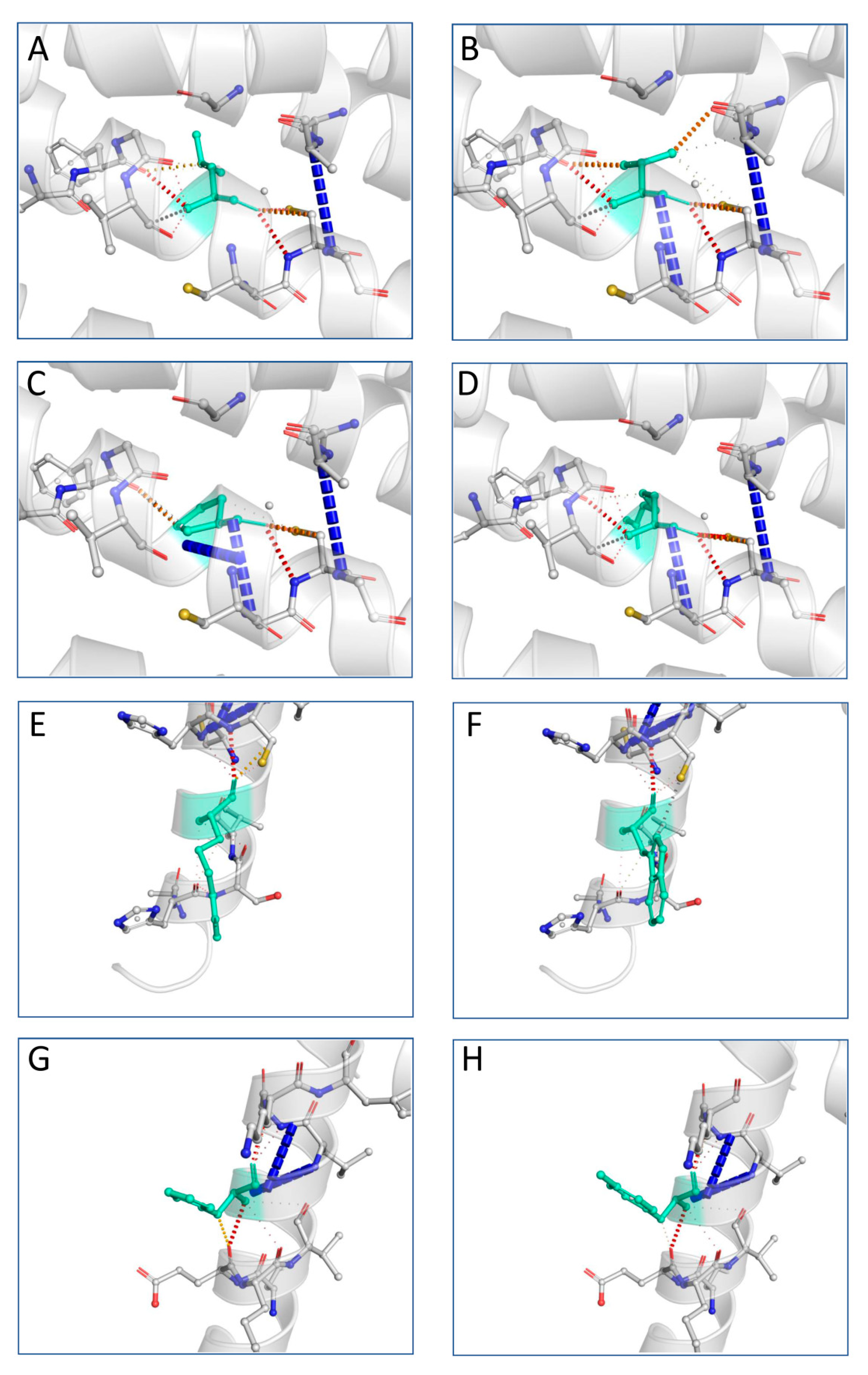
3.7. Variant Tertiary Protein Structures
3.8. Validation of the Predicted Structures
4. Discussion
5. Conclusions
Key Points
- The majority of computational tools indicated that MS-associated PLP1 mutations would have a significant impact on the protein structure, stability and function.
- Loss of protein thermodynamic stability can reduce the ability of its structure to perform normal functions.
- The resulted predictions for L31P, L31V and L31R indicated that these mutations may lead to an altered transmembrane protein.
- The R137W variant may also cause loss of helix, while the H140Y variant may alter the ordered interface of transmembrane protein.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef] [PubMed]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin fat facts: An overview of lipids and fatty acid metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.M.; Pender, M.P. Myelin proteolipid protein: An effective autoantigen and target of autoimmunity in multiple sclerosis. J. Autoimmun. 2008, 31, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.M.; Trifilieff, E.; Pender, M.P. Correlation between anti-myelin proteolipid protein (PLP) antibodies and disease severity in multiple sclerosis patients with PLP response-permissive HLA types. Front. Immunol. 2020, 11, 1891. [Google Scholar] [CrossRef] [PubMed]
- Mangalam, A.K.; Khare, M.; Krco, C.; Rodriguez, M.; David, C. Identification of T cell epitopes on human proteolipid protein and induction of experimental autoimmune encephalomyelitis in HLA class II-transgenic mice. Eur. J. Immunol. 2004, 34, 280–290. [Google Scholar] [CrossRef]
- Martinsen, V.; Kursula, P. Multiple sclerosis and myelin basic protein: Insights into protein disorder and disease. Amino Acids 2021, 54, 99–109. [Google Scholar] [CrossRef]
- Peschl, P.; Bradl, M.; Höftberger, R.; Berger, T.; Reindl, M. Myelin oligodendrocyte glycoprotein: Deciphering a target in inflammatory demyelinating diseases. Front. Immunol. 2017, 8, 529. [Google Scholar] [CrossRef]
- Quarles, R.H. Myelin-associated glycoprotein (MAG): Past, present and beyond. J. Neurochem. 2007, 100, 1431–1448. [Google Scholar] [CrossRef]
- Rothbard, J.B.; Zhao, X.; Sharpe, O.; Strohman, M.J.; Kurnellas, M.; Mellins, E.D.; Robinson, W.H.; Steinman. L. Chaperone activity of α B-crystallin is responsible for its incorrect assignment as an autoantigen in multiple sclerosis. J. Immunol. 2011, 186, 4263–4268. [Google Scholar] [CrossRef]
- Höftberger, R.; Lassmann, H. Inflammatory demyelinating diseases of the central nervous system. Handb. Clin. Neurol. 2018, 145, 263–283. [Google Scholar]
- Mapunda, J.A.; Tibar, H.; Regragui, W.; Engelhardt, B. How Does the Immune System Enter the Brain? Front. Immunol. 2022, 13, 805657. [Google Scholar] [CrossRef] [PubMed]
- Dhaiban, S.; Al-Ani, M.; Elemam, N.M.; Al-Aawad, M.H.; Al-Rawi, Z.; Maghazachi, A.A. Role of peripheral immune cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Sci 2021, 3, 12. [Google Scholar] [CrossRef]
- Cotsapas, C.; Mitrovic, M. Genome-wide association studies of multiple sclerosis. Clin. Transl. Immunol. 2018, 7, e1018. [Google Scholar] [CrossRef] [PubMed]
- Cloake, N.C.; Yan, J.; Aminian, A.; Pender, M.P.; Greer, J.M. PLP1 mutations in patients with multiple sclerosis: Identification of a new mutation and potential pathogenicity of the mutations. J. Clin. Med. 2018, 7, 342. [Google Scholar] [CrossRef]
- Warshawsky, I.; Rudick, R.A.; Staugaitis, S.M.; Natowicz, M.R. Primary progressive multiple sclerosis as a phenotype of a PLP1 gene mutation. Ann. Neurol. 2005, 58, 470–473. [Google Scholar] [CrossRef]
- Rubegni, A.; Battisti, C.; Tessa, A.; Cerase, A.; Doccini, S.; Santorelli, F.M.; Federico, A. SPG2 mimicking multiple sclerosis in a family identified using next generation sequencing. J. Neurol. Sci. 2017, 375, 198–202. [Google Scholar] [CrossRef]
- Cailloux, F.; Gauthier-Barichard, F.; Mimault, C.; Isabelle, V.; Courtois, V.; Giraud, G.; Dastugue, B.; Boespflug-Tanguy, O.; Clinical European Network on Brain Dysmyelinating Disease. Genotype-phenotype correlation in inherited brain myelination defects due to proteolipid protein gene mutations. Eur. J. Hum. Genet. 2000, 8, 837–845. [Google Scholar] [CrossRef]
- Groh, J.; Friedman, H.C.; Orel, N.; Ip, C.W.; Fischer, S.; Spahn, I.; Schäffner, E.; Hörner, M.; Stadler, D.; Buttmann, M.; et al. Pathogenic inflammation in the CNS of mice carrying human PLP1 mutations. Hum. Mol. Genet. 2016, 25, 4686–4702. [Google Scholar]
- Robert, F.; Pelletier, J. Exploring the impact of single-nucleotide polymorphisms on translation. Front. Genet. 2018, 9, 507. [Google Scholar] [CrossRef]
- Flores, S.C.; Alexiou, A.; Glaros, A. Mining the Protein Data Bank to improve prediction of changes in protein-protein binding. PLoS ONE 2021, 16, e0257614. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Achuthan, P.; Akannietal, W. Ensembl 2018. Nucleic Acids Res. 2018, 46, D754–D761. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jian, X.; Boerwinkle, E. dbNSFP: A lightweight database of human non-synonymous SNPs and their functional predictions. Hum. Mutat. 2011, 32, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, C.; Mou, C.; Dong, Y.; Tu, Y. dbNSFP v4: A comprehensive database of transcript-specific functional predictions and annotations for human nonsynonymous and splice-site SNVs. Genome Med. 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Pejaver, V.; Urresti, J.; Lugo-Martinez, J.; Pagel, K.A.; Lin, G.N.; Nam, H.; Mort, M.; Cooper, D.N.; Sebat, J.; Iakoucheva, L.M.; et al. Inferring the molecular and phenotypic impact of amino acid variants with MutPred2. Nat. Commun. 2020, 11, 5918. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, Ν.; Stricher, F.; Serrano, L.; Tawfik, D.S. How protein stability and new functions trade off. PLoS Comput. Biol. 2008, 4, e1000002. [Google Scholar] [CrossRef]
- Rodrigues, C.H.; Pires, D.E.; Ascher, D.B. DynaMut: Predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res. 2018, 46, W350–W355. [Google Scholar] [CrossRef]
- Worth, C.L.; Preissner, R.; Blundell, T.L. SDM—A server for predicting effects of mutations on protein stability and malfunction. Nucleic Acids Res. 2011, 39, 215–222. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef]
- Buchan, D.W.; Minneci, F.; Nugent, T.C.; Bryson, K.; Jones, D.T. Scalable web services for the PSIPRED protein analysis workbench. Nucleic Acids Res. 2013, 41, W349–W357. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homology proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.B.; Skolnick, J. Fr-tm-align: A new protein structural alignment method based on fragment alignments and the tm-score. BMC Bioinform. 2008, 9, 531. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Khalaf, G.; Mattern, C.; Begou, M.; Boespflug-Tanguy, O.; Massaad, C.; Massaad-Massade, L. Mutation of proteolipid protein 1 gene: From severe hypomyelinating leukodystrophy to inherited spastic paraplegia. Biomedicines 2022, 10, 1709. [Google Scholar] [CrossRef]
- Zhang, Y.; Skolnick, J. TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef]
- Sima, A.A.; Pierson, C.R.; Woltjer, R.L.; Hobson, G.M.; Golden, J.A.; Kupsky, W.J.; Schauer, G.M.; Bird, T.D.; Skoff, R.P.; Garbern, J.Y. Neuronal loss in Pelizaeus–Merzbacher disease differs in various mutations of the proteolipid protein 1. Acta Neuropathol. 2009, 118, 531–539. [Google Scholar] [CrossRef]
- Bonnet-Dupeyron, M.N.; Combes, P.; Santander, P.; Cailloux, F.; Boespflug-Tanguy, O.; Vaurs-Barrière, C. PLP1 splicing abnormalities identified in Pelizaeus-Merzbacher disease and SPG2 fibroblasts are associated with different types of mutations. Hum. Mutat. 2008, 29, 1028–1036. [Google Scholar] [CrossRef]
- Grossi, S.; Regis, S.; Biancheri, R.; Mort, M.; Lualdi, S.; Bertini, E.; Uziel, G.; Boespflug-Tanguy, O.; Simonati, A.; Corsolini, F.; et al. Molecular genetic analysis of the PLP1 gene in 38 families with PLP1-related disorders: Identification and functional characterization of 11 novel PLP1 mutations. Orphanet J. Rare Dis. 2011, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Lubetzki, C.; Sol-Foulon, N.; Desmazières, A. Nodes of Ranvier during development and repair in the CNS. Nat. Rev. Neurol. 2020, 16, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Klineova, S.; Lublin, F.D. Clinical course of multiple sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.; Arnold, D.L.; Chataway, J.; Chitnis, T.; Fox, R.J.; Ramajo, A.P.; Murphy, N.; Lassmann, H. Secondary progressive multiple sclerosis: New insights. Neurology 2021, 97, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Oudejans, E.; Luchicchi, A.; Strijbis, E.M.; Geurts, J.J.; van Dam, A.M. Is MS affecting the CNS only? Lessons from clinic to myelin pathophysiology. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e914. [Google Scholar] [CrossRef] [PubMed]
- Patsopoulos, N.A. Genetics of multiple sclerosis: An overview and new directions. Cold Spring Harb. Perspect. Med. 2018, 8, a028951. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Rempe, T.; Whitmire, N.; Dunn-Pirio, A.; Graves, J.S. Therapeutic advances in multiple sclerosis. Front. Neurol. 2022, 13, 824926. [Google Scholar] [CrossRef]
- Spörkel, O.; Uschkureit, T.; Büssow, H.; Stoffel, W. Oligodendrocytes expressing exclusively the DM20 isoform of the proteolipid protein gene: Myelination and development. Glia 2002, 37, 19–30. [Google Scholar] [CrossRef]
- Wang, P.; Sidney, J.; Dow, C.; Mothé, B.; Sette, A.; Peters, B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput. Biol. 2008, 4, e1000048. [Google Scholar] [CrossRef]

| Location | Codon Change | Amino Acid Position | Amino Acid Alteration | Description 1 |
|---|---|---|---|---|
| X:103785668 | CTG/GTG | 31 | L/V | MS- like disease mutation |
| X:103785669 | CTG/CCG | 31 | L/P | Severe PMD mutation |
| X:103785669 | CTG/CGG | 31 | L/R | MS- like disease mutation |
| X 103786682 | CGG/TGG | 137 | R/W | MS- like disease mutation |
| X 103786691 | CAT/TAT | 140 | H/Y | Mild SPG2 mutation |
| Variant | L31P | L31V | L31R | R137W | H140Y | Range (Low to Damaging) |
|---|---|---|---|---|---|---|
| Polyphen2_HDIV_score | 0.999 | 0.997 | 0.999 | 0.999 | 0.015 | 0.03061 to 0.91137 |
| LRT_converted_rankscore | 0.8433 | 0.8433 | 0.8433 | 0.4496 | 0.53742 | 0.00162 to 0.8433 |
| MutationTaster_score | 1 | 0.999999 | 1 | 0.627105 | 0.281663 | 0 to 1 |
| MutationTaster_converted_rankscore | 0.81001 | 0.58761 | 0.81001 | 0.81001 | 0.81001 | 0.08979 to 0.81001 |
| MutationAssessor_score | 2.71 | 2.36 | 2.71 | 1.24 | 0.69 | −5.17 to 6.49 |
| MetaLR_score | 0.9794 | 0.9771 | 0.9794 | 0.9547 | 0.9047 | 0 to 1 |
| MetaRNN_score | 0.988245 | 0.946438 | 0.98141 | 0.892939 | 0.962404 | 0 to 1 |
| MutPred_score | 0.932 | 0.813 | 0.887 | 0.663 | 0.779 | 0 to 1 |
| DEOGEN2_score | 0.994664 | 0.910984 | 0.994756 | 0.707527 | 0.697598 | 0 to 1 |
| ClinPred_score | 0.996376 | 0.982601 | 0.997494 | 0.958003 | 0.509568 | 0 to 1 |
| Variant | ΔΔG (kcal/mol) | Outcome | ΔΔSVibENCoM (kcal.mol−1.K−1) | Outcome 1 |
|---|---|---|---|---|
| L31V | −0.133 | Destabilizing | 0.083 | Increase |
| L31P | −1.011 | Destabilizing | 0.413 | Increase |
| L31R | −0.256 | Destabilizing | 0.231 | Increase |
| R137W | −0.400 | Destabilizing | 0.111 | Increase |
| H140Y | 0.519 | Stabilizing | −0.063 | Decrease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramouli, A.; Krokidis, M.G.; Exarchos, T.P.; Vlamos, P. In Silico Structural Analysis Predicting the Pathogenicity of PLP1 Mutations in Multiple Sclerosis. Brain Sci. 2023, 13, 42. https://doi.org/10.3390/brainsci13010042
Avramouli A, Krokidis MG, Exarchos TP, Vlamos P. In Silico Structural Analysis Predicting the Pathogenicity of PLP1 Mutations in Multiple Sclerosis. Brain Sciences. 2023; 13(1):42. https://doi.org/10.3390/brainsci13010042
Chicago/Turabian StyleAvramouli, Antigoni, Marios G. Krokidis, Themis P. Exarchos, and Panagiotis Vlamos. 2023. "In Silico Structural Analysis Predicting the Pathogenicity of PLP1 Mutations in Multiple Sclerosis" Brain Sciences 13, no. 1: 42. https://doi.org/10.3390/brainsci13010042
APA StyleAvramouli, A., Krokidis, M. G., Exarchos, T. P., & Vlamos, P. (2023). In Silico Structural Analysis Predicting the Pathogenicity of PLP1 Mutations in Multiple Sclerosis. Brain Sciences, 13(1), 42. https://doi.org/10.3390/brainsci13010042










